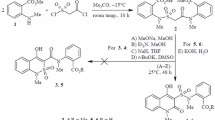
A targeted synthesis of a series of halo-substituted 6-hydroxy-2-methyl-4-oxo-1,2-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline-5-carboxylic acid anilides have been carried out. Peculiarites of the 1H NMR spectra and the results of investigating the diuretic activity of these compounds are discussed.
Similar content being viewed by others
References
I. V. Ukrainets, E. V. Mospanova, A. V. Turov, and V. A. Parshikov, Khim. Geterotsikl. Soedin., 885 (2011).
J. M. Pascual, E. Rodilla, J. A. Costa, F. Perez-Lahiguera, C. Gonzalez, E. Lurbe, and J. Redón, Blood Pressure, 18, 247 (2009).
E. Rodilla, J. A. Costa, F. Pérez-Lahiguera, C. González, and J. M. Pascual, Med. Clin. (Barcelona), 131, 406 (2008).
L. Ghiadoni, Expert Opin. Pharmacother., 11, 1647 (2010).
T. Ninomiya, S. Zoungas, B. Neal, M. Woodward, A. Patel, V. Perkovic, A. Cass, M. Cooper, D. Grobbee, P. Hamet, S. Harrap, L. Liu, G. Mancia, C.-E. Mogensen, N. Poulter, A. Rodgers, B. Williams, S. MacMahon, and J. Chalmers, J. Hypertens., 28, 1141 (2010).
H. Yamada, Y. Mishiro, K. Kusunose, and M. Sata, J. Cardiovasc. Pharmacol. Ther., 15, 145 (2010).
Y. Hasegawa, K. Shimada, and T. Yamaguchi, Blood Pressure, Suppl., 19, 10 (2010).
S. A. Brandão, M. C. Izar, S. M. Fischer, A. O. Santos, C. M. Monteiro, R. M. Póvoa, T. Helfenstein, A. C. Carvalho, A. M. Monteiro, E. Ramos, M. Gidlund, A. M. Figueiredo Neto, and F. A. Fonseca, Am. J. Hypertens., 23, 208 (2010).
H. Arima, C. Anderson, T. Omae, L. Liu, C. Tzourio, M. Woodward, S. Macmahon, B. Neal, A. Rodgers, and J. Chalmers, J. Hypertens., 28, 395 (2010).
A. Kleemann and J. Engel, Pharmaceutical Substances. Synthesis, Patents, Applications, Multimedia Viewer, Version 2.00, Georg Thime Verlag, Stuttgart (2001).
E. V. Mospanova, Diss. Cand. Pharmaceut. Sci., Kharkiv (2008).
I. V. Ukrainets, N. Yu. Golik, K. V. Andreeva, and O. V. Gorokhova, Khim. Geterotsikl. Soedin., 1806 (2010). [Chem. Heterocycl. Comp., 46, 1459 (2010)].
M. Karplus, J. Chem. Phys., 30, 11 (1959).
L. N. Sernov and V. V. Gatsura, Elements of Experimental Pharmacology [in Russian], Moscow, 2000, p. 103.
M. D. Mashkovskii, Drugs, RIA, Novaya Volna, Umerenkov Publishing House, Moscow, 2009, p. 499.
Author information
Authors and Affiliations
Corresponding author
Additional information
*For Communication 196, see [1].
Translated from Khimiya Geterotsiklicheskikh Soedinenii, No. 7, pp. 1009–1017, July, 2011. Original article submitted June 22, 2010.
Rights and permissions
About this article
Cite this article
Ukrainets, I.V., Golik, N.Y., Shemchuk, A.L. et al. 4-hydroxy-2-quinolones. 197*. The search for novel diuretics amongst halo-substituted 6-hydroxy-2-methyl-4-oxo-1,2-dihydro-4H-pyrrolo-[3,2,1-ij]quinoline-5-carboxylic acid anilides. Chem Heterocycl Comp 47, 826–832 (2011). https://doi.org/10.1007/s10593-011-0842-9
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-011-0842-9