Abstract
Purpose
To assess the effect of different treatment strategies on the risk of subsequent invasive breast cancer (IBC) in women diagnosed with ductal carcinoma in situ (DCIS).
Methods
Up to 15-year cumulative incidences of ipsilateral IBC (iIBC) and contralateral IBC (cIBC) were assessed among a population-based cohort of 10,090 women treated for DCIS in the Netherlands between 1989 and 2004. Multivariable Cox regression analyses were used to evaluate associations of treatment with iIBC risk.
Results
Fifteen years after DCIS diagnosis, cumulative incidence of iIBC was 1.9 % after mastectomy, 8.8 % after BCS+RT, and 15.4 % after BCS alone. Patients treated with BCS alone had a higher iIBC risk than those treated with BCS+RT during the first 5 years after treatment. This difference was less pronounced for patients <50 years [hazard ratio (HR) 2.11, 95 % confidence interval (CI) 1.35–3.29 for women <50, and HR 4.44, 95 % CI 3.11–6.36 for women ≥50, P interaction < 0.0001]. Beyond 5 years of follow-up, iIBC risk did not differ between patients treated with BCS+RT or BCS alone for women <50. Cumulative incidence of cIBC at 15 years was 6.4 %, compared to 3.4 % in the general population.
Conclusions
We report an interaction of treatment with age and follow-up period on iIBC risk, indicating that the benefit of RT seems to be smaller among younger women, and stressing the importance of clinical studies with long follow-up. Finally, the low cIBC risk does not justify contralateral prophylactic mastectomies for many women with unilateral DCIS.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ductal carcinoma in situ (DCIS) is a potential precursor lesion of invasive breast cancer (IBC) [1]. Most women (80–85 %) diagnosed with DCIS present with a mammographic abnormality without clinical symptoms [2]. Since the introduction of population-based mammographic screening and, more recently, digital mammography, the incidence of DCIS has increased substantially [3–7]. In the Netherlands, the European standardized rate of in situ breast carcinoma—of which DCIS is the most common type (~80 %)—increased fivefold since 1989, up to 25.1 per 100,000 women in 2013 [8]. In the United States, the incidence (age adjusted to the 2000 US standard population) increased even more: from 5.8 per 100,000 in 1975 to 33.8 per 100,000 women in 2010 [9].
The natural course of DCIS is not well known because DCIS has almost always been treated by mastectomy or breast-conserving surgery (BCS) with or without radiotherapy (RT). Between 1988 and 2011, only 2 % of women with DCIS were managed without surgery in the United States [10]. In the Netherlands, the percentage of non-operated DCIS between 1989 and 2004 was 0.8 % [11].
Women with DCIS are treated to prevent the development of IBC, assuming that this may lead to a reduction in breast cancer-specific deaths. Some women with unilateral DCIS even undergo contralateral prophylactic mastectomy. However, the long-term benefit of treating asymptomatic DCIS that may or may not progress to IBC is difficult to quantify [12]. Therefore, screening programs are criticized to be associated with overdiagnosis and resultant overtreatment of DCIS [13, 14].
Considerable uncertainty remains about the likelihood that a treatment strategy will prevent IBC, whether that likelihood will change based on specific patient and DCIS characteristics, and whether the reduction in risk is enough to justify the costs and the potential side effects of that treatment [12]. The effect of different treatment strategies on the risk of subsequent events in women diagnosed with DCIS has been addressed previously in both prospective trials and observational studies [15–27]. However, many of these studies focused on local recurrences, not discriminating between invasive and non-invasive events, or did not have complete information on treatment. Moreover, several studies have analyzed specific subgroups, such as “favorable” and “good-risk” DCIS, or focused on a specific treatment strategy.
Gierisch et al. prioritized research needs for DCIS patients, and pointed out the assessment of the effect of treatment strategies on IBC, using existing observational data [12]. We assessed the effect of DCIS treatment strategies on risk of subsequent ipsilateral invasive breast cancer (iIBC) using a large population-based cohort with complete information on treatment and follow-up. In addition, we analyzed the risk of contralateral IBC (cIBC).
Methods
Patient selection
All women diagnosed with breast carcinoma in situ in the Netherlands between January 1st 1989 and December 31st 2004 were selected from the Netherlands cancer registry (NCR) managed by the Netherlands Comprehensive Cancer Organization. Patients with previous malignancies, except for non-melanoma skin cancer, were not included. This cohort (n = 12,717) was linked to the nationwide network and registry of histology and cytopathology in the Netherlands (PALGA) [28]. The selection criteria for this study were a diagnosis of pure DCIS, i.e., no lobular or other subtype component, and only treated by surgery with or without RT. See Fig. 1 for a detailed list of the excluded cases (n = 2627). The study was approved by the review boards of the NCR and PALGA.
DCIS treatment and other characteristics
Information on treatment, age, date of diagnosis, and grade was derived from data provided by NCR. Guidelines for DCIS treatment in the Netherlands recommend mastectomy or BCS, consisting of microscopic complete tumor excision. From 1999, the addition of RT after BCS is included in the recommendation. Adjuvant (hormonal) treatment is not recommended. Primary DCIS treatment was categorized into (1) BCS+RT; (2) BCS alone; and (3) mastectomy. Initial treatment was defined as the final treatment for the ipsilateral breast within 3 months after DCIS diagnosis. For patients for whom surgery type was not coded by NCR, we retrieved this information from PALGA. We validated whether patients registered by NCR as treated with BCS had indeed undergone BCS using the conclusions of pathology reports within 3 months of DCIS diagnosis. Furthermore, we validated surgical treatment for women who developed subsequent iIBC after mastectomy, using conclusion texts of all available pathology reports. Subsequently, we assessed whether women initially treated with BCS had undergone ipsilateral mastectomy during follow-up, using both NCR and PALGA data.
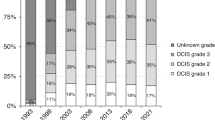
Based on the gradual implementation of the national breast cancer screening program, we categorized year of DCIS diagnosis into two periods: 1989–1998 (implementation phase) and 1999–2004 (full coverage). Age was subdivided into two groups: <50 and ≥50 years. Grade was available for 53 % of the entire cohort. The grading system used in the Netherlands is based on the classification presented by Holland et al. [29].
Follow-up data
The occurrence of iIBC and cIBC was ascertained based on NCR data, and additionally, for patients treated with BCS, through evaluating pathology reports. Follow-up for subsequent IBC and vital status were complete until at least January 1, 2011.
Statistical analyses
Time at risk started at date of DCIS diagnosis and stopped at date of diagnosis of the event of interest (iIBC or cIBC), date of death or emigration, or January 1, 2011, whichever came first. We calculated cumulative incidence of iIBC and cIBC using death as competing risk. P values were based on competing risk regression [30], with time since DCIS diagnosis as time-scale and adjusted for age (continuous). Further, we compared cumulative incidence of cIBC with the expected cumulative incidence of IBC in the general population. Expected cumulative incidence was derived from age- and period-specific cancer incidence and overall mortality in the Dutch female population, estimated using the conditional method [31].
Cox proportional hazards analyses, using age as primary time-scale and time since DCIS diagnosis as secondary time-scale (0–5, 5–10, and ≥10 years), were used to quantify the effects of different treatments on iIBC and cIBC risks. Period of DCIS diagnosis and age group at DCIS diagnosis were added as covariables. Proportional hazard assumptions were verified using graphical and residual-based methods.
To examine whether iIBC risk differed by grade, we performed a subgroup analysis for women with a reported grade. Because the proportion of women with missing data on grade was more than 30 % up to 1998, we performed this subgroup analysis for women diagnosed between 1999 and 2004.
Surgical treatment was either analyzed as initial DCIS treatment (cumulative incidence) or as a time-varying variable including subsequent mastectomies (Cox regression analysis).
All statistical analyses were performed using STATA/SE 13.1 (StataCorp LP, College Station, TX). A two-sided P value less than 0.05 was considered statistically significant.
Results
Patient characteristics
Analyses included 10,090 women (Fig. 1), of whom 7931 (79 %) women were ≥50 years at DCIS diagnosis. Median age at DCIS diagnosis was 57.6 years (interquartile range 50.7–66.3 years). Median follow-up was 10.7 years (interquartile range 7.7–14.3 years). During follow-up, 1856 patients died. Table 1 shows characteristics, events and follow-up of the study population by treatment group.
DCIS treatment
Nearly 48 % (n = 4820) of DCIS patients were initially treated with mastectomy. Of all 5270 women initially treated with BCS, 50 % additionally received RT. Use of BCS increased over time in women <50 years (P trend = 0.010) and ≥50 years (P trend < 0.001). The use of RT after BCS also increased over time in both groups (P trend < 0.001) (Fig. 2). Fifteen years after initial DCIS treatment, cumulative incidence of subsequent ipsilateral mastectomy was 5.2 % in the BCS+RT group, versus 12.0 % in the BCS-alone group.
Ipsilateral invasive breast cancer
During follow-up, 588 women developed an iIBC. The median time to iIBC was 5.8 years (interquartile range 2.8–9.0 years). Fifteen years after DCIS diagnosis, cumulative incidence of iIBC was 1.9 % [95 % confidence interval (95 % CI) 1.5–2.4 %] after mastectomy, 8.8 % (95 % CI 7.1–10.8 %) after BCS+RT, and 15.4 % (95 % CI 13.9–17.0 %) after BCS alone.
When assessing the risk of iIBC by treatment, the proportional hazards assumption was violated. We accounted for time dependency in the treatment effect by addition of an interaction term that involved time and treatment to the model (P interaction < 0.001). Additionally, we found that the effect of treatment was different depending on age group (P interaction < 0.0001). An extra interaction term that involved period of diagnosis and treatment was not significant (P interaction = 0.445). Therefore, Table 2 presents the effect of treatment on iIBC risk by follow-up interval and age group.
Women diagnosed with DCIS between 1999 and 2004 were less likely to develop iIBC than women diagnosed between 1989 and 1998, regardless of treatment and age [hazard ratio (HR) 0.72, 95 % CI 0.59–0.87]. After adjusting for treatment and period, women ≥50 years had lower iIBC risk than <50 women years (HR 0.38, 95 % CI 0.25–0.59). Figure 3 shows the cumulative incidence of iIBC by treatment strategy stratified by period of DCIS diagnosis and age group at DCIS diagnosis.
Cumulative incidence of iIBC by treatment strategy for a women <50 years diagnosed between 1989 and 1998 b women ≥50 years diagnosed between 1989 and 1998 c women <50 years diagnosed between 1999 and 2004 d women ≥50 years diagnosed between 1999 and 2004, with death as competing risk. BCS breast-conserving surgery, RT radiotherapy. P values based on competing risk regression, adjusted for age (continuous) [30]
Both women <50 and ≥50 years treated with BCS alone had a higher risk of developing iIBC than women treated with BCS+RT in the first 5 years after DCIS treatment. However, for women ≥50 years, the difference in iIBC risk after BCS alone compared to BCS+RT was much larger than for women <50 years (HR 2.11, 95 % CI 1.35–3.29 for women <50 years and HR 4.44, 95 % CI 3.11–6.36 for women ≥50 years). While among patients <50 years at DCIS diagnosis, risk of iIBC no longer differed after 5 years following BCS+RT or BCS alone (HR 1.01, 95 % CI 0.66–1.55 for 5–10 years follow-up and HR 0.78, 95 % CI 0.46–1.33 for ≥10 years follow-up), for women ≥50 years, iIBC risk remained increased after BCS alone during subsequent follow-up intervals, although the difference in risks was smaller than in the first 5 years (HR 1.64, 95 % CI 1.01–2.69 for ≥10 years follow-up). A trend in the proportional reduction with age was found when the data were subdivided into three groups according to age: <45, 45–55, and >55 years (data not shown).
Women undergoing mastectomy were less likely to develop iIBC compared to women undergoing BCS (Table 2). The highest absolute iIBC risk after mastectomy was seen for women <50 years treated between 1989 and 1998 (10-year cumulative incidence: 2.9 %, 95 % CI 1.9–4.4 %). For women ≥50 years diagnosed from 1999 to 2004 and treated with mastectomy, the 10-year cumulative incidence was lowest at 0.6 % (95 % CI 0.2–1.2 %).
In a subgroup analysis of women diagnosed with DCIS between 1999 and 2004, the Cox model including grade was comparable to the main model (data not shown). The difference in iIBC risk after BCS alone and BCS+RT was of the same magnitude [e.g., for women ≥50 years in the first 5 years after DCIS treatment: HR 4.78, 95 % CI 2.64–8.65 (model including grade) vs HR 4.57, 95 % CI 2.55–8.22 (main model)]. Additionally, iIBC risk did not differ by grade (adjusted estimate for intermediate vs low grade and high vs low grade: HR 1.25, 95 % CI 0.80–1.97 and HR 1.19, 95 % CI 0.75–1.87, respectively).
Contralateral invasive breast cancer
Contralateral IBC occurred in 536 women. The median time to cIBC was 6.2 years (interquartile range 3.3–9.8 years). Cumulative incidences of cIBC at 15 and 20 years after DCIS diagnosis were 6.4 % (95 % CI 5.9–7.1 %) and 8.9 % (95 % CI 7.7–10.1 %), respectively, reaching a rate of 0.4–0.5 % per annum. The risk of cIBC did not differ by treatment, period of diagnosis, or age group (see Supplemental Table 1, which demonstrates the multivariate Cox proportional hazards analysis for cIBC risk).
The cumulative risk of cIBC is visualized in Fig. 4. The absolute risk of developing cIBC in women treated for DCIS was slightly higher than the risk of IBC in the general population (3.4 % at 15 years).
Cumulative incidence of cIBC by treatment strategy compared with the expected cumulative incidence of IBC in the general population (dashed line) for a women <50 years, and b women ≥50 years, with death as competing risk. BCS breast-conserving surgery, RT radiotherapy. P values based on competing risk regression, adjusted for age (continuous) [30]
Discussion
To the best of our knowledge, this is the largest population-based, nationwide cohort study with accurate and complete long-term outcome data of subsequent invasive breast cancer after DCIS treatment. For women treated with BCS, our study confirms the protective effect of RT with regard to iIBC risk shown by randomized controlled trials (RCTs) [23–27, 32]. Importantly, the benefit of RT regarding iIBC risk may differ by age and follow-up interval. It appeared that the use of RT after BCS in women <50 years reduced the risk of iIBC only in the first years after treatment. In women ≥50 years, iIBC risk remained increased during subsequent follow-up after BCS alone, compared to BCS+RT, but the difference became less pronounced with longer follow-up. Our results suggest that RT is effective in treating microscopic residual disease, but may not prevent de novo IBC in DCIS patients. One of the RCTs also found that the beneficial effect of RT seemed to be restricted to the first 5 years after treatment [24].
Interestingly, the results of our Cox regression analysis point towards less benefit from RT in women <50 years than in older women. This observation could be due to confounding if for example younger women treated with RT were more likely to have DCIS with unfavorable prognostic features. However, a meta-analysis of the RCTs also found age to modify the benefit of RT: women <50 years showed a smaller proportional risk reduction in the rate of local recurrence (either in situ or invasive) than women ≥50. A trend in the proportional reduction with age was also found when the data were subdivided into five age groups and was independent of histological grade, comedonecrosis, nuclear grade, or architecture [32].
Additionally, we found high iIBC risks after BCS—either with or without RT—in women <50 years. Moreover, these young women treated with mastectomy had a higher cumulative iIBC incidence than older women who received this treatment. Prior studies have also reported that local recurrences following mastectomy seem to occur particularly in younger women [33–35]. Data that may explain this higher risk in younger women are limited and inconsistent [35–38]. Despite the increased iIBC risk, young age per se should not be considered a contraindication for BCS, especially because breast cancer-specific mortality has not been shown to differ between mastectomy and BCS [32, 39].
Another clinical relevant observation is that the absolute risk of cIBC was low with a rate of 0.4–0.5 % per annum. This result is comparable to the population-based study by Falk et al. (n = 3,163; median follow-up 5.2 years) [15]. Despite the low cIBC risk, a marked increase in the use of contralateral prophylactic mastectomies among women with DCIS in recent years has been reported [40–42]. Because contralateral prophylactic mastectomies will not likely result in any survival advantage despite the minimization of cIBC risk [43] and are not risk-free [43–45], we advocate that prophylactic contralateral mastectomies for DCIS in women without hereditary breast cancer risk should be discouraged.
One of the strengths of our study was that we differentiated between invasive and non-invasive recurrences. Our 10-year estimates are in line with the 10-year absolute risks reported in other population-based cohort studies and RCTs [15, 17, 32]. However, direct comparison with previous studies, which focused most of their analyses on any local recurrence as outcome, is often difficult. Differences in study design, inclusion criteria, and statistical methods (e.g., cumulative incidence vs Kaplan–Meier estimates) may for example play a role.
Interestingly, the 10-year cumulative incidence and Kaplan–Meier estimates in two, rather small, North American non-randomized prospective studies of women with “favorable” DCIS treated with BCS alone between 1995 and 2002, were only slightly lower than the 10-year cumulative incidence of iIBC for women diagnosed between 1999 and 2004 and treated with BCS alone in our population-based cohort [21, 22]. On the other hand, the estimated 7-year iIBC cumulative incidences in a fifth RCT between BCS+RT (n = 287) and BCS alone (n = 298) in a selected “good-risk” group of women were much lower [23]. Notably, in this RCT in which 62 % of women used tamoxifen, only eight iIBCs occurred in the BCS-alone arm, and only one in the BCS+RT arm (median follow-up 7.2 years). The differences in risk estimates could be explained by differences in selection criteria, and utilization of tamoxifen, although the effect of tamoxifen on iIBC seems to be minimal [46].
A limitation of our study is the potential of confounding by indication. As the allocation of DCIS treatment was not randomized and the indication for treatment may have been related to the risk of IBC, this could have introduced bias. It is plausible to assume that women with less favorable characteristics more often received adjuvant RT after BCS. Therefore, if confounding by indication plays a role, this will probably have resulted in an underestimation of the difference in iIBC risk between BCS+RT and BCS alone. Although grade was associated with treatment strategy in our study, we found that grade was not a confounding factor in our subgroup analysis, as grade was not associated with iIBC risk. We did not have information on several other risk factors associated with local recurrence, such as DCIS size and margin status after excision. However, it is still uncertain to what extent these factors are associated with subsequent invasive breast cancer risk [47, 48] and therefore whether these could be confounding factors in our study.
A last issue concerns the applicability of our results to today’s clinical practice. Our study shows that the risk of developing iIBC was lower for women diagnosed between 1999 and 2004 than for women diagnosed between 1989 and 1998, while risk of cIBC was similar for both periods. The decrease in iIBC risk over the years was independent of treatment strategy and is likely the result of the detection of relatively more harmless DCIS lesions and improvements in preoperative assessment and surgical management. Most likely, the risk found for the latter period reflects the upper boundary of today’s risk of iIBC in women treated for DCIS, as patient evaluation and selection for treatment have evolved further since 2004.
It should be emphasized that the women in our cohort were not treated with tamoxifen for DCIS. In the Netherlands, hormonal treatment for DCIS is not recommended and its use is very limited in current clinical practice [49, 50]. A meta-analysis of RCTs assessing the effect of postoperative tamoxifen showed a reduced rate of cIBC, but no impact on the risk of iIBC or all-cause mortality [46]. The difference in absolute IBC risk between our cohort and a population in which tamoxifen was more common will therefore probably be limited.
In summary, our finding that the reduction in iIBC risk among women treated with BCS + RT, compared to BCS alone, diminishes with longer follow-up, emphasizes the importance of clinical studies with long-term follow-up. Furthermore, the beneficial effect of RT seems to be smaller among younger women and should be investigated further. Finally, the low risk of cIBC does not justify contralateral prophylactic mastectomies for many women with unilateral DCIS.
Abbreviations
- BCS:
-
Breast-conserving surgery
- CI:
-
Confidence interval
- cIBC:
-
Contralateral invasive breast cancer
- DCIS:
-
Ductal carcinoma in situ
- HR:
-
Hazard ratio
- IBC:
-
Invasive breast cancer
- iIBC:
-
Ipsilateral invasive breast cancer
- NCR:
-
Netherlands cancer registry
- PALGA:
-
Nationwide network and registry of histology and cytopathology, the Netherlands
- RCT:
-
Randomized controlled trial
- RT:
-
Radiotherapy
References
Van de Vijver MJ, Peterse H (2003) The diagnosis and management of pre-invasive breast disease: pathological diagnosis–problems with existing classifications. Breast Cancer Res 5:269–275. doi:10.1186/bcr629
Bartlett JMS, Nofech-Moses S, Rakovitch E (2014) Ductal carcinoma in situ of the breast: can biomarkers improve current management? Clin Chem 60:60–67. doi:10.1373/clinchem.2013.207183
Ernster VL, Ballard-Barbash R, Barlow WE et al (2002) Detection of ductal carcinoma in situ in women undergoing screening mammography. J Natl Cancer Inst 94:1546–1554
Sørum R, Hofvind S, Skaane P, Haldorsen T (2010) Trends in incidence of ductal carcinoma in situ: the effect of a population-based screening programme. Breast 19:499–505. doi:10.1016/j.breast.2010.05.014
van Steenbergen LN, Voogd AC, Roukema JA et al (2009) Screening caused rising incidence rates of ductal carcinoma in situ of the breast. Breast Cancer Res Treat 115:181–183. doi:10.1007/s10549-008-0067-5
Vigeland E, Klaasen H, Klingen TA et al (2008) Full-field digital mammography compared to screen film mammography in the prevalent round of a population-based screening programme: the Vestfold County study. Eur Radiol 18:183–191. doi:10.1007/s00330-007-0730-y
Bluekens AMJ, Holland R, Karssemeijer N et al (2012) Comparison of digital screening mammography and screen-film mammography in the early detection of clinically relevant cancers: a multicenter study. Radiology 265:707–714. doi:10.1148/radiol.12111461
Netherlands Comprehensive Cancer Organisation (IKNL). http://www.cijfersoverkanker.nl
Howlader N, Noone AM, Krapcho M, et al. SEER cancer statistics review, 1975–2010, National Cancer Institute
Sagara Y, Mallory MA, Wong S et al (2015) Survival benefit of breast surgery for low-grade ductal carcinoma in situ: a population-based cohort study. JAMA Surg 150:739–745. doi:10.1001/jamasurg.2015.0876
Boekel NB, Schaapveld M, Gietema JA et al (2014) Cardiovascular morbidity and mortality after treatment for ductal carcinoma in situ of the breast. J Natl Cancer Inst 106:156. doi:10.1093/jnci/dju156
Gierisch JM, Myers ER, Schmit KM et al (2014) Prioritization of research addressing management strategies for ductal carcinoma in situ. Ann Intern Med 160:484–491. doi:10.7326/M13-2548
Ripping TM, Verbeek ALM, Fracheboud J et al (2015) Overdiagnosis by mammographic screening for breast cancer studied in birth cohorts in The Netherlands. Int J Cancer 137:921–929. doi:10.1002/ijc.29452
Harding C, Pompei F, Burmistrov D et al (2015) Breast cancer screening, incidence, and mortality across US counties. JAMA Intern Med. doi:10.1001/jamainternmed.2015.3043
Falk RS, Hofvind S, Skaane P, Haldorsen T (2011) Second events following ductal carcinoma in situ of the breast: a register-based cohort study. Breast Cancer Res Treat 129:929–938. doi:10.1007/s10549-011-1531-1
Sprague BL, McLaughlin V, Hampton JM et al (2013) Disease-free survival by treatment after a DCIS diagnosis in a population-based cohort study. Breast Cancer Res Treat 141:145–154. doi:10.1007/s10549-013-2670-3
Rakovitch E, Nofech-Mozes S, Narod SA et al (2013) Can we select individuals with low risk ductal carcinoma in situ (DCIS)? A population-based outcomes analysis. Breast Cancer Res Treat 138:581–590. doi:10.1007/s10549-013-2455-8
Schouten van der Velden AP, van Vugt R, Van Dijck JAAM et al (2007) Local recurrences after different treatment strategies for ductal carcinoma in situ of the breast: a population-based study in the East Netherlands. Int J Radiat Oncol Biol Phys 69:703–710. doi:10.1016/j.ijrobp.2007.03.062
Dick AW, Sorbero MS, Ahrendt GM et al (2011) Comparative effectiveness of ductal carcinoma in situ management and the roles of margins and surgeons. J Natl Cancer Inst 103:92–104. doi:10.1093/jnci/djq499
Kane RL, Virnig BA, Shamliyan T et al (2010) The impact of surgery, radiation, and systemic treatment on outcomes in patients with ductal carcinoma in situ. J Natl Cancer Inst Monographs 2010:130–133. doi:10.1093/jncimonographs/lgq022
Wong JS, Chen Y-H, Gadd MA et al (2014) Eight-year update of a prospective study of wide excision alone for small low- or intermediate-grade ductal carcinoma in situ (DCIS). Breast Cancer Res Treat 143:343–350. doi:10.1007/s10549-013-2813-6
Solin LJ, Gray R, Hughes LL et al (2015) Surgical excision without radiation for ductal carcinoma in situ of the breast: 12-year results from the ECOG-ACRIN E5194 study. J Clin Oncol 33(33):3938–3944. doi:10.1200/JCO.2015.60.8588
McCormick B, Winter K, Hudis C et al (2015) RTOG 9804: a prospective randomized trial for good-risk ductal carcinoma in situ comparing radiotherapy with observation. J Clin Oncol 33:709–715. doi:10.1200/JCO.2014.57.9029
Donker M, Litière S, Werutsky G et al (2013) Breast-conserving treatment with or without radiotherapy in ductal carcinoma in situ: 15-year recurrence rates and outcome after a recurrence, from the EORTC 10853 randomized phase III trial. J Clin Oncol 31:4054–4059. doi:10.1200/JCO.2013.49.5077
Wapnir IL, Dignam JJ, Fisher B et al (2011) Long-term outcomes of invasive ipsilateral breast tumor recurrences after lumpectomy in NSABP B-17 and B-24 randomized clinical trials for DCIS. J Natl Cancer Inst 103:478–488. doi:10.1093/jnci/djr027
Wärnberg F, Garmo H, Emdin S et al (2014) Effect of radiotherapy after breast-conserving surgery for ductal carcinoma in situ: 20 years follow-up in the randomized SweDCIS trial. J Clin Oncol 32(32):3613–3618. doi:10.1200/JCO.2014.56.2595
Cuzick J, Sestak I, Pinder SE et al (2011) Effect of tamoxifen and radiotherapy in women with locally excised ductal carcinoma in situ: long-term results from the UK/ANZ DCIS trial. Lancet Oncol 12:21–29. doi:10.1016/S1470-2045(10)70266-7
Casparie M, Tiebosch ATMG, Burger G et al (2007) Pathology databanking and biobanking in The Netherlands, a central role for PALGA, the nationwide histopathology and cytopathology data network and archive. Cell Oncol 29:19–24
Holland R, Peterse JL, Millis RR et al (1994) Ductal carcinoma in situ: a proposal for a new classification. Semin Diagn Pathol 11:167–180
Fine JPG, Gray RJ (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509
Ederer F, Heise H (1959) Instructions to Ibm 650 programmers in processing survival computations. Technical end results evaluation section. National Cancer Institute, Bethesda
Correa C, McGale P (2010) Overview of the randomized trials of radiotherapy in ductal carcinoma in situ of the breast early breast cancer trialists’ collaborative group (EBCTCG). J Natl Cancer Inst Monogr 2010:162–177. doi:10.1093/jncimonographs/lgq039
Rashtian A, Iganej S, Amy Liu I-L, Natarajan S (2008) Close or positive margins after mastectomy for DCIS: pattern of relapse and potential indications for radiotherapy. Int J Radiat Oncol Biol Phys 72:1016–1020. doi:10.1016/j.ijrobp.2008.06.1954
Carlson GW, Page A, Johnson E et al (2007) Local recurrence of ductal carcinoma in situ after skin-sparing mastectomy. J Am Coll Surg 204:1074–1078. doi:10.1016/j.jamcollsurg.2007.01.063
Vicini FA, Recht A (2002) Age at diagnosis and outcome for women with ductal carcinoma-in situ of the breast: a critical review of the literature. J Clin Oncol 20:2736–2744
Bijker N, van Tienhoven G (2010) Local and systemic outcomes in DCIS based on tumor and patient characteristics: the radiation oncologist’s perspective. J Natl Cancer Inst Monographs 2010:178–180. doi:10.1093/jncimonographs/lgq025
Pradier C, Cornuau M, Norca J et al (2011) Differences in breast carcinoma in situ between menopausal and premenopausal women. Anticancer Res 31:1783–1788
Alvarado R, Lari SA, Roses RE et al (2012) Biology, treatment, and outcome in very young and older women with DCIS. Ann Surg Oncol 19:3777–3784. doi:10.1245/s10434-012-2413-4
Narod SA, Iqbal J, Giannakeas V et al (2015) Breast cancer mortality after a diagnosis of ductal carcinoma in situ. JAMA Oncol. doi:10.1001/jamaoncol.2015.2510
Tuttle TM, Jarosek S, Habermann EB et al (2009) Increasing rates of contralateral prophylactic mastectomy among patients with ductal carcinoma in situ. J Clin Oncol 27:1362–1367. doi:10.1200/JCO.2008.20.1681
Soran A, Kamali Polat A, Johnson R, McGuire KP (2014) Increasing trend of contralateral prophylactic mastectomy: what are the factors behind this phenomenon? Surgeon 12:316–322. doi:10.1016/j.surge.2014.02.005
Rutter CE, Park HS, Killelea BK, Evans SB (2015) Growing use of mastectomy for ductal carcinoma-in situ of the breast among young women in the united states. Ann Surg Oncol 22:2378–2386. doi:10.1245/s10434-014-4334-x
Goldflam K, Hunt KK, Gershenwald JE et al (2004) Contralateral prophylactic mastectomy. Predictors of significant histologic findings. Cancer 101:1977–1986. doi:10.1002/cncr.20617
Montgomery LL, Tran KN, Heelan MC et al (1999) Issues of regret in women with contralateral prophylactic mastectomies. Ann Surg Oncol 6:546–552
Piot-Ziegler C, Sassi M-L, Raffoul W, Delaloye J-F (2010) Mastectomy, body deconstruction, and impact on identity: a qualitative study. Br J Health Psychol 15:479–510. doi:10.1348/135910709X472174
Staley H, McCallum I, Bruce J (2014) Postoperative tamoxifen for ductal carcinoma in situ: cochrane systematic review and meta-analysis. Breast. doi:10.1016/j.breast.2014.06.015
Collins LC, Achacoso N, Haque R et al (2013) Risk factors for non-invasive and invasive local recurrence in patients with ductal carcinoma in situ. Breast Cancer Res Treat 139:453–460. doi:10.1007/s10549-013-2539-5
Kerlikowske K, Molinaro A, Cha I et al (2003) Characteristics associated with recurrence among women with ductal carcinoma in situ treated by lumpectomy. J Natl Cancer Inst 95:1692–1702
Elshof LE, Tryfonidis K, Slaets L et al (2015) Feasibility of a prospective, randomised, open-label, international multicentre, phase III, non-inferiority trial to assess the safety of active surveillance for low risk ductal carcinoma in situ—The LORD study. Eur J Cancer 51:1497–1510. doi:10.1016/j.ejca.2015.05.008
Netherlands Comprehensive Cancer Organisation (IKNL) and the Knowledge institute of Medical Specialists (KiMS) guideline breast cancer (2012). http://www.richtlijnendatabase.nl
Acknowledgments
The authors thank Otto Visser, Annemarie Eeltink and the registration teams of the Netherlands Comprehensive Cancer Organization for the collection of data for the Netherlands Cancer Registry. The authors also thank Lucy Overbeek and PALGA, the nationwide histopathology and cytopathology data network and archive, for providing pathology data. This work was supported by Pink Ribbon (Grant Number 2011.WO19.C88 to J.W.) and the Dutch Cancer Society (Grant Number NKI2009-4363 to M.K.S.).
Funding
This study was funded by Pink Ribbon (Grant Number 2011.WO19.C88 to J.W.) and the Dutch Cancer Society (Grant Number NKI2009-4363 to M.K.S.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
LE Elshof, M Schaapveld, MK Schmidt, EJ Rutgers, FE van Leeuwen, and J Wesseling declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
The study was approved by the review boards of the Netherlands Cancer Regsitry and PALGA, the nationwide histopathology and cytopathology data network and archive. The study used only unidentifiable patient information, and no informed consent was required.
Reasearch involving human and animal rights
This article does not contain any studies with animals performed by any of the authors.
Additional information
An erratum to this article is available at http://dx.doi.org/10.1007/s10549-016-4060-0.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Elshof, L.E., Schaapveld, M., Schmidt, M.K. et al. Subsequent risk of ipsilateral and contralateral invasive breast cancer after treatment for ductal carcinoma in situ: incidence and the effect of radiotherapy in a population-based cohort of 10,090 women. Breast Cancer Res Treat 159, 553–563 (2016). https://doi.org/10.1007/s10549-016-3973-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10549-016-3973-y