Abstract
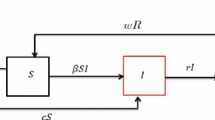
Invasive insects, arthropods, and other invertebrates are of concern due to the role some play in introducing and transmitting pathogens via a pathogen–vector relationship. Indeed, vector-borne diseases represent a significant portion of emerging diseases. We compare and contrast three strategic approaches to managing a vector-borne pathogen: conventional strategies based on disease ecology without regard to economic tradeoffs and cost-effective strategies based on a bioeconomic framework. Conventional strategies entail managing the vector population below a threshold value based on R 0—the basic reproductive ratio of the pathogen, which measures a pathogen’s ability to invade uninfected systems. This does not account for post-infection dynamics, nor does it balance ecological and economic tradeoffs. Thresholds take on a more profound role under a bioeconomic paradigm: rather than unilaterally determining vector control choices, thresholds inform control choices and are influenced by them. Simulation results show cost-effective strategies can lower overall program costs and may be less sensitive to parameter estimation.



Similar content being viewed by others
Notes
A reviewer points out that some may regard management of a post-infected scenario as pest management, but the process of biological invasion involves more than species introduction (Williamson and Fitter 1996). Focusing on post-introduction processes (i.e., establishment and spread) can help to prevent or slow further invasion into surrounding areas.
Implicitly, we have assumed vaccination is not an option, which is often the case for emerging diseases. While the SI framework is a special case of more complex models involving recovered, immune, or exposed population compartments (e.g., SIS, SIR or SEIR models), the basic insights developed for our SI model—that the current state of infection matters and focusing on tradeoffs as opposed to eradication leads to qualitatively different results—are generally applicable to these more complex models. Also note that the vector may also transmit pathogen to sink hosts, causing damages, but these do not affect the basic disease dynamics between the vector and host populations that we model here (Chaves and Hernandez 2004).
See Song et al. (2002) for an approach to combining infection dynamics on multiple time scales.
If there are capacity constraints that only permit a y i (0) < y i *(0), then subsequent vector control pulses may be optimal within the interval t ∈ (0, τ]. We also note that for this problem if τ is large enough, then it is not optimal to engage in any management resulting in W = 0.
Rising marginal costs are also a factor in determining the initial pulse harvest. The costs associated with a pulse harvest are cln(N i /[N i − y i ]) (see Clark 2005). This implies that costs increase as a larger proportion of the vector population is initially harvested.
For a sufficiently large τ it will be important to incorporate discounting and net natural growth within the host population, neither of which are currently included in our model. We hypothesize that including these factors will result in a time-varying positive level of vector control.
Increases in α w effectively result in selective culling of infected host animals. As indicated in footnote 4, selective culling of infected hosts can in some circumstances be detrimental because this increases the probability that an infected vector randomly bites a susceptible (as opposed to infected) host.
References
Asano E, Gross LJ, Lenhart S, Real LA (2008) Optimal control of vaccine distribution in a rabies metapopulation model. Math Biosci Eng 5:219–238
Baly A, Toledo ME, Boelaert M, Reyes A, Vanlerberghe V, Ceballos E, Carvajal M, Maso R, La Rosa M, Denis O, Van der Stuyft P (2007) Cost effectiveness of Aedes aegypti control programmes: participatory versus vertical. Trans R Soc Trop Med Hyg 101:578–586
Barrett S, Hoel M (2007) Optimal disease eradication. Environ Dev Econ 12:627–652
Bicknell KB, Wilen JE, Howitt RE (1999) Public policy an private incentives for livestock disease control. Aust J Agric Resour Econ 43:501–521
Chaves LF, Hernandez M-J (2004) Mathematical modelling of American cutaneous leishmaniasis: incidental hosts and threshold conditions for infection persistence. Acta Trop 92:245–252
Clark CW (2005) Mathematical bioeconomics optimal managment of renewable resources, 2nd edn. Wiley, Hoboken
Cleaveland S, Laurenson MK, Taylor LH (2001) Diseases of humans and their domestic mammals: pathogen characteristics, host range and the risk of emergence. Philos Trans Royal Soc B Biol Sci 356:991–999
Daszak P, Cunningham AA, Hyatt AD (2000) Emerging infectious diseases of wildlife-threats to biodiversity and human health. Science 287:443–449
Diekmann O, Heesterbeek JAP, Metz JAJ (1990) On the definition and the computation of the basic reproduction ratio R 0 in models for infectious diseases in heterogeneous populations. J Math Biol 28:365–382
Dobson A (2004) Population dynamics of pathogens with multiple hosts species. Am Nat 164:s64–s78
Fenichel EP, Horan RD (2007a) Gender-based harvesting in wildlife disease management. Am J Agric Econ 89:904–920
Fenichel EP, Horan RD (2007b) Jointly-determined ecological thresholds and economic trade-offs in wildlife disease management. Nat Resour Model 20:511–547
Gaff H, Joshi HR, Lenhart S (2007) Optimal harvesting during an invasion of a sublethal plant pathogen. Environ Dev Econ 12:673–686
Gersovitz M, Hammer JS (2004) The economical control of infectious diseases. Econ J 114:1–27
Gersovitz M, Hammer JS (2005) Tax/subsidy policies toward vector-borne infectious diseases. J Public Econ 89:647–674
Gubbins S, Carpenter S, Baylis M, Wood JLN, Mellor PS (2008) Assesing the risk of bluetongue to UK livestock: uncertainty and sensitivity analyses of a temperature-dependent model for the basic reproduction number. J Royal Soc Interface 5:363–371
Hagmann R, Charlwood JD, Gil V, Ferreira C, do Rosario V, Smith TA (2003) Malaria and its possible control on the island of principe. Malaria J 2:15
Heesterbeek JAP, Roberts MG (1995) Mathematical models for microparasites of wildlife. In: Grenfell BT, Dobson AP (eds) Ecology of infectious diseases in natural populations. Cambridge University Press, New York
Heesterbeek JAP, Roberts MG (2007) The type-reproduction number T in models for infectious disease control. Math Biosci 206:3–10
Heffernan JM, Smith RJ, Wahl LM (2005) Perspectives on the basic reproductive ratio. J Royal Soc Interface 2:281–293
Holt RD, Dobson AP, Begon M, Bowers RG, Schauber EM (2003) Parasite establishment in host communities. Ecol Lett 6:837–842
Horan RD, Wolf CA (2005) The economics of managing infectious wildlife disease. Am J Agric Econ 87:537–551
Jones KE, Patel NG, Levy MA, Storeygard A, Balk D, Gittleman JL, Daszak P (2008) Global trends in emerging infectious diseases. Nature 451:990–994
Juliano SA, Lounibos LP (2005) Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett 8:558–574
Koonce JF, Eshenroder RL, Christie GC (1993) An economic injury level approach to establishing the intensity of sea lamprey control in the Great Lakes. North Am J Fish Manag 13:1–14
Lord CC, Woolhouse MEJ, Heesterbeek JAP, Mellor PS (1996) Vector-borne diseases and the basic reproduction number: a case study of African horse sickness. Med Vet Entomol 10:19–28
Lounibos LP (2002) Invasions by insect vectors of human disease. Annu Rev Entomol 47:233–266
Lourenço-de-Oliveira R, Castro MG, Braks MAH, Lounibos LP (2004) The invasion of urban forest by dengue vectors in Rio de Janeiro. J Vector Ecol 29:94–100
Martin J, Runge MC, Nichols JD, Lubow BC, Kendall WL (2009) Structured decision making as a conceptual framework to identify thresholds for conservation and management. Ecol Appl 19:1079–1090
McCallum H, Barlow N, Hone J (2001) How should pathogen transmission be modelled. Trends Ecol Evol 16:295–300
Purse BV, Brown HE, Harrup L, Mertens PPC, Rogers DJ (2008) Invasion of bluetongue and other orbivirus infections into Europe: the role of biological and climatic processes. Sci Tech Rev Int Off Epizoot 27:427–442
Roberts MG (1996) The dynamics of bovine tuberculosis in possum populations, an its eradication or control by culling or vaccination. J Anim Ecol 65:451–464
Roberts MG, Heesterbeek JAP (2003) A new method for estimating the effort required to control an infectious disease. Proc Royal Soc B 270:1359–1364
Smith GC, Bennett R, Wilkinson D, Cooke R (2007) A cost—benefit analysis of culling badgers to control bovine tuberculosis. Vet J 173:302–310
Song B, Castillo-Chaves C, Aparicio JP (2002) Tuberculosis models with fast and slow dynamics: the role of close and casual contacts. Math Biosci 180:187–205
Tatem AJ, Hay SI, Rogers DJ (2006) Global traffic and disease vector dispersal. Proc Nat Acad Sci 103:6242–6247
Walker B, Holling CS, Carpenter SR, Kinzig AP (2004) Resilience, adaptability and transformability in social-ecological systems. Ecol Soc 9:5
Wilkinson D, Smith GC, Delahay RJ, Cheeseman CL (2004) A model of bovine tuberculosis in the badger Meles meles: an evaluation of different vaccination strategies. J Appl Ecol 41:492–501
Williamson M, Fitter A (1996) The varying success of invaders. Ecology 77:1661–1666
Wobeser G (2002) Disease management strategies for wildlife. Sci Tech Rev Int Off Epizoot 21:159–178
Wonham MJ, de-Camino-Beck T, Lewis MA (2004) An epidemiological model for West Nile virus: invasion analysis and control applications. Proc Royal Soc Lond B 271:501–507
Acknowledgments
The authors gratefully acknowledge funding provided by the Economic Research Service-USDA cooperative agreement number 58-7000-6-0084 through ERS’ Program of Research on the Economics of Invasive Species Management (PREISM), and by NRI, USDA, CSREES, grant #2006-55204-17459. This work was conducted as part of the SPIDER working group at the National Institute for Mathematical and Biological Synthesis (NIMBioS), sponsored by the National Science Foundation and the U.S. Department of Agriculture through NSF Award #EF-0932858, with additional support from the University of Tennessee, Knoxville. The views expressed here are the authors and should not be attributed to ERS, USDA, or NIMBioS.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
(A) Derivation of condition (7)
Upon imposing the requirement that h i < 1, condition (6) indicates that
Condition (A1) can be re-written as
Write \( \theta_{w} = I_{w} /N_{w} \) and solve for S i :
where the last equality simply comes from multiplying both the numerator and the denominator by \( \alpha_{w} K_{w} \beta_{iw} \). Upon applying the distributive property, condition (A3) becomes
which is condition (7).
(B) Optimal vector management
Problem (12) can be formulated as a linear control problem (i.e., the problem is linear in the control variable y i ). The Hamiltonian for the problem is:
where \( \lambda_{j} \) is the co-state variable associated with the susceptible stock of population j, and \( \mu_{j} \) is the co-state variable associated with the infected stock of population j (j = w,i).The marginal value of y i on the Hamiltonian is:
The marginal value in (B2) vanishes along a singular path. When the value is positive (negative), then h i should be set at its maximum (minimum) value. Generally, the system will not be on the singular path initially, which means an extremal control must be used to move the system as quickly as possible to the singular path (if one exists) (Clark 2005). As there is no upper bound on y i , harvests can only occur at a maximum rate for an instant. Harvests can persist at y i = 0 or at the singular level for a longer time. Hence, except for an initial jump, we have
The remaining necessary conditions for problem (12) are:
The transversality conditions are:
Conditions (B10) and (B11), along with (B2), indicates that h i (τ) = 0. Taking the time derivative of the Hamiltonian yields:
The second row of (B12) comes from condition (B3), while the third row comes from conditions (B4)–(B11). Hence, H is constant after possibly an initial pulse harvest. Define this constant value of H by χ, i.e., H = χ. Using the results H = χ and h i (τ) = 0, along with the transversality conditions (B8)–(B11), we have
which means H(t) < 0 for all t ∈ [0, τ).
As H is a measure of economic welfare, the result H(τ) < 0 means that extending the time horizon to τ reduces welfare. It would be optimal to diminish the time horizon to some value \( \tau^{\prime} < \tau \), such that \( H(\tau^{\prime}) = 0 \), as setting \( H(\tau^{\prime}) = 0 \) means there is no value to extending the time horizon any further (Clark 2005). However, this is not possible since τ is biologically-determined and is therefore exogenously fixed. The implication is that it is optimal to take all actions as soon as possible: an impulse control at time t = 0. The intuition is as follows. First, there is no incentive to delay management because problem (12) is a linear control problem with no discounting. If discounting occurred at a very high rate, then future costs (and benefits) would be worth less and so one would want to wait to invest in vector controls. With no discounting, costs today and in the future are valued equally, so there is no penalty from investing early. But there is a penalty to investing later, as the only stock of concern, S w , can only decrease when the disease problem gets worse. So it is optimal for all vector reduction to take place immediately. The cost associated with an impulse control used for the initial cull is \( c\ln [N_{i0} /(N_{i0} - y_{i} (0))] \) (Clark 2005).To determine the optimal initial harvest, rewrite the problem as
subject to the equations of motion and the initial conditions as before, except that now we have the following initial conditions for the vector populations: \( S_{i} (0^{ + } ) = S_{i0} (1 - y_{i} (0)/N_{i0} ) \) and \( I_{i} (0^{ + } ) = I_{i0} (1 - y_{i} (0)/N_{i0} ) \). The objective function in (B14) now does not depend on time, and the equations of motion no longer depend on human choices. We solve the equations of motion (1)–(4) as functions of the stock levels after the initial cull, and then rewrite problem (B14) as
or, more simply, as
The optimality condition for this problem is:
The left hand side of (B17) is the marginal cost of vector controls. Marginal costs approach infinity as the harvest rate \( h_{i} (0) \) approaches unity; hence it is not optimal to eradicate the vector population assuming the marginal benefits of eradication are finite. The right hand side is the marginal net benefit of vector controls in time period 0. Society benefits from a larger value of \( S_{w} (\tau ) \). This value is increased at the margin as \( I_{i} (0^{ + } ) \) is reduced (i.e., \( - \partial S_{w} /\partial I_{i} (0^{ + } ) > 0 \)), and it may increase or decrease as \( S_{i} (0^{ + } ) \) is reduced (i.e., \( - \partial S_{w} /\partial S_{i} (0^{ + } )_{ < }^{ \ge } 0 \)).
It is worth noting that the impacts of the initial cull will depend on the time horizon τ. The smaller is τ, the larger the impact of a given initial cull on \( S_{w} (\tau ) \), as the vector population will have less time to rebound and infect the host population. In contrast, a larger τ gives infected vectors more time to rebound and infect hosts after the initial cull. Our numerical sensitivity analysis indicates that the less control is required when τ is small, as having only few controls can yield effective protection of the host population in this case while keeping costs low. The optimal level of vector controls is initially increasing in τ, as vector control efforts are initially substituted for reduced protection effectiveness as τ is increased. If τ is too large, however, the level of control is reduced in response to reduced effectiveness; more hosts become infected while society saves from reduced control costs.
Rights and permissions
About this article
Cite this article
Fenichel, E.P., Horan, R.D. & Hickling, G.J. Bioeconomic management of invasive vector-borne diseases. Biol Invasions 12, 2877–2893 (2010). https://doi.org/10.1007/s10530-010-9734-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10530-010-9734-7