Abstract
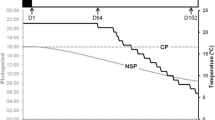
The aim of the present study was to investigate the effect of photoperiod regime combined with thermal treatment (PTT) on maturation of three-year-old pikeperch (Sander lucioperca). To induce the onset of maturation, all treatment groups underwent a wintering phase of 3 months at 12 °C and 12-h light-to-12-h dark. Controls were maintained at 23 °C and 12-h light-to-12-h dark throughout the trial. After the wintering phase, the PTT groups experienced regimes with 8-, 10-, 12-, and 14-h light for 4 months at 14 °C referred to as photo-thermal treatment (PTT). The four PTT groups as well as the control were studied in triplicates, with 12 fish per tank. We assessed gonad development and maturation by histological analysis, sex steroid plasma concentrations (17β-estradiol, testosterone, and 11-ketotestosterone) and, at the end of the experiment, mRNA expression of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) in the pituitary. As expected, both male and female pikeperch initiated reproductive maturation during the wintering phase, which was confirmed through histological analysis and sex steroid measurements. During wintering, the onset of maturation in females was confirmed by the increasing diameter of ovarian follicles (217 ± 65 to 800 ± 97 µm), the developmental stages (50 % mid and 21 % late vitellogenic females), concomitant with elevated 17β-estradiol (E2) plasma concentrations reaching 2600 ± 1500 pg/mL, compared to 380 ± 230 pg/mL at the onset of the experiment. Similarly in males, maturation was indicated by peak concentrations of the androgens observed within the first 2 months of wintering (testosterone 21 ± 12 ng/mL first month, 11-ketotestosterone 5.7 ± 3 ng/mL second month). Subsequently, the photoperiod treatments after the wintering phase influenced the progression of reproductive maturation. During the PTT, follicle diameter increased irrespective of the light regime from 800 to more than 1100 µm, but the progression of the vitellogenesis was differentially modulated by the photoperiod. Already after 1 month of the PTT, 92 and 86 % of females reared at 12- and 14-h light per day were in a late vitellogenic stage. After 2 months, females with mainly atretic follicles were observed under long light conditions (14 h of light), indicating overripeness and spawning. In contrast, 82 and 72 % of the females reared at 8- and 10-h light per day were still in the final stage of vitellogenesis at the end of the experiment and thus ready to undergo final maturation. Concomitant to the histological outcomes, highest E2 concentrations were observed under long-day conditions (12, 14 h) in females with peaks of 4200 ± 3300 (12 h of light) and 6800 ± 4200 pg/mL (14 h of light). In male pikeperch, ongoing spermatogenesis was indicated by rising androgen levels especially under long-day conditions (14 h of light) reaching peak levels of 27 ± 21 ng/mL testosterone and 39 ± 91 ng/mL of 11-ketotestosterone at the end of the experiment. In all PTT, the mRNA expression of FSH and LH was significantly elevated compared to the control, confirming the activation of the hypothalamus–pituitary–gonad axis. Here, no effect between the different light regimes was detected, neither in males nor females. Thus, photoperiod revealed a slight influence on male and female pikeperch maturation when combined with an effective thermal treatment and therefore can be explored as a cheap and easy-to-handle fine-tuning tool for artificial pikeperch reproduction.









Similar content being viewed by others
Abbreviations
- 11-KT:
-
11-Ketotestosterone
- 17,20-P:
-
17,20β-Progesterone
- E2:
-
17β-Estradiol
- FSHβ:
-
Follicle-stimulating hormone β-subunit
- HPG axis:
-
Hypothalamus–pituitary–gonad axis
- LV:
-
Late vitellogenic follicles
- LHβ:
-
Luteinizing hormone β-subunit
- n. c.:
-
Natural conditions
- n. s.:
-
Not specified
- PTT:
-
Photo-thermal treatment
- RAS:
-
Recirculating aquaculture system
- T:
-
Testosterone
- us.:
-
Unspecified
References
Abdulfatah A, Fontaine P, Kestemont P, Gardeur JN, Marie M (2011) Effects of photothermal kinetics and amplitude of photoperiod decrease on the induction of the reproduction cycle in female Eurasian perch Perca fluviatilis. Aquaculture 322:169–176
Abdulfatah A, Fontaine P, Kestemont P, Milla S, Marie M (2013) Effects of the thermal threshold and the timing of temperature reduction on the initiation and course of oocyte development in cultured female of Eurasian perch Perca fluviatilis. Aquaculture 376:90–96
Barron JM, Jensen NR, Anders PJ, Egan JP, Ireland SC, Cain KD (2012) Effects of temperature on the intensive culture performance of larval and juvenile North American burbot (Lota lota maculosa). Aquaculture 364:67–73
Blecha M, Kristan J, Samarin AM, Rodina M, Policar T (2015) Quality and quantity of pikeperch (Sander lucioperca) spermatozoa after varying cold water treatments. J Appl Ichthyol 31:75–78
Bromage N, Porter M, Randall C (2001) The environmental regulation of maturation in farmed finfish with special reference to the role of photoperiod and melatonin. Aquaculture 197:63–98
Ciereszko RE, Dabrowski K, Ciereszko A, Ottobre JS (1998) Plasma concentrations of steroid hormones in male yellow perch, Perca flavescens: the effect of age and photothermal manipulation. Environ Biol Fish 51:97–105
Dabrowski K, Ciereszko RE, Ciereszko A, Toth GP, Christ SA, El-Saidy D, Ottobre JS (1996) Reproductive physiology of yellow perch (Perca flavescens): environmental and endocrinological cues. J Appl Ichthyol 12:139–148
Fleige S, Pfaffl MW (2006) RNA integrity and the effect on the real-time qRT-PCR performance. Mol Asp Med 27:126–139
Hassin S, Holland MCH, Zohar Y (1999) Ontogeny of follicle-stimulating hormone and luteinizing hormone gene expression during pubertal development in the female striped bass, Morone saxatilis (Teleostei). Biol Reprod 61:1608–1615
Hermelink B, Wuertz S, Trubiroha A, Rennert B, Kloas W, Schulz C (2011) Influence of temperature on puberty and maturation of pikeperch, Sander lucioperca. Gen Comp Endocrinol 172:282–292
Hermelink B, Wuertz S, Rennert B, Kloas W, Schulz C (2013) Temperature control of pikeperch (Sander lucioperca) maturation in recirculating aquaculture systems: induction of puberty and course of gametogenesis. Aquaculture 400:36–45
Jackson LF, Sullivan CV (1995) Reproduction of white perch: the annual gametogenic cycle. Trans Am Fish Soc 124:563–577
Kristan J, Alavi SMH, Stejskal V, Policar T (2013) Hormonal induction of ovulation in pikeperch (Sander lucioperca L.) using human chorionic gonadotropin (hCG) and mammalian GnRH analogue. Aquac Int 21:811–818
Ljubobratovic U, Kucska B, Feledi T, Poleksic V, Markovic Z, Lenhadt M, Ronyai A (2013) Combined methods for artificial reproduction of pikeperch, Sander lucioperca. In: Conference proceedings VI international conference “Water and Fish”, Faculty of Agriculture, University of Belgrade – Serbia, Belgrade, pp 261–266. 12–14 June 2011
Lubzens E, Young G, Bobe J, Cerda J (2010) Oogenesis in teleosts: how fish eggs are formed. Gen Comp Endocrinol 165:367–389
Malison JA, Procarione LS, Barry TP, Kapuscinski AR, Kayes TB (1994) Endocrine and gonadal changes during the annual reproductive-cycle of the fresh-water teleost, Stizostedion vitreum. Fish Physiol Biochem 13:473–484
Mazon MJ, Moles G, Rocha A, Crespo B, Lan-Chow-Wing O, Espigares F, Munoz I, Felip A, Carrillo M, Zanuy S, Gomez A (2015) Gonadotropins in European sea bass: endocrine roles and biotechnological applications. Gen Comp Endocrinol 221:31–41
Migaud H, Fontaine P, Sulistyo I, Kestemont P, Gardeur JN (2002) Induction of out-of-season spawning in Eurasian perch Perca fluviatilis: effects of rates of cooling and cooling durations on female gametogenesis and spawning. Aquaculture 205:253–267
Migaud H, Gardeur JN, Kestemont P, Fontaine P (2004a) Off-season spawning of Eurasian perch Perca fluviatilis. Aquac Int 12:87–102
Migaud H, Fontaine P, Kestemont P, Wang N, Brun-Bellut J (2004b) Influence of photoperiod on the onset of gonadogenesis in Eurasian perch Perca fluviatilis. Aquaculture 241:561–574
Migaud H, Wang N, Gardeur JN, Fontaine P (2006) Influence of photoperiod on reproductive performances in Eurasian perch Perca fluviatilis. Aquaculture 252:385–393
Migaud H, Davie A, Taylor JF (2010) Current knowledge on the photoneuroendocrine regulation of reproduction in temperate fish species. J Fish Biol 76:27–68
Mueller-Belecke A, Zienert S (2008) Out-of-season spawning of pike perch (Sander lucicoperca L.) without the need for hormonal treatments. Aquac Res 39:1279–1285
Mylonas CC, Zohar Y (2001) Use of GnRHa-delivery systems for the control of reproduction in fish. Rev Fish Biol Fish 10:463–491
Okuzawa K (2002) Puberty in teleosts. Fish Physiol Biochem 26:31–41
Palinska-Zarska K, Zarski D, Krejszeff S, Nowosad J, Bilas M, Trejchel K, Brylewski A, Targonska K, Kucharczyk D (2014) The effect of age, size and digestive tract development on burbot, Lota lota (L.) larvae weaning effectiveness. Aquac Nutr 20:281–290
Palinska-Zarska K, Zarski D, Krejszeff S, Kupren K, Laczynska B, Kucharczyk D (2015) Optimal feeding level of burbot larvae fed Artemia spp. and reared under controlled conditions. N Am J Aquac 77:295–301
Pankhurst NW, Porter MJR (2003) Cold and dark or warm and light: variations on the theme of environmental control of reproduction. Fish Physiol Biochem 28:385–389
Pfaffl MW (2001) A new mathematical model for relative quantification in real-time RT-PCR. Nucl Acid Res 29:e45
Pimakhin A, Kouril J, Stejskal V, Zak J (2015) The effect of geographical origin of perch (Perca fluviatilis L. 1758) populations on growth rates under natural and aquaculture conditions: a review. J Appl Ichthyol 31:56–63
Rocha A, Zanuy S, Carrillo M, Gomez A (2009) Seasonal changes in gonadal expression of gonadotropin receptors, steroidogenic acute regulatory protein and steroidogenic enzymes in the European sea bass. Gen Comp Endocrinol 162:265–275
Ronyai A (2007) Induced out-of-season and seasonal tank spawning and stripping of pike perch (Sander lucioperca L.). Aquac Res 38:1144–1151
Schaefer F, Overton J, Wuertz S (2016) Pikeperch Sander lucioperca egg quality cannot be predicted by total antioxidant capacity and mtDNA fragmentation. Anim Reprod Sci. doi:10.1016/j.anireprosci.2016.02.016
Selman K, Wallace RA, Sarka A, Qi XP (1993) Stages of oocyte development in the Zebrafish, Brachydanio rerio. J Morphol 218:203–224
Stacey NE (1984) Control of the timing of ovulation by exogenous and endogenous factors. In: Potts GW, Wootton RJ (eds) Fish reproduction: strategies and tactics. Academic Press, London, pp 207–222
Steffens W, Geldhauser F, Gerstner P, Hilge V (1996) German experiences in the propagation and rearing of fingerling pikeperch (Stizostedion lucioperca). Ann Zool Fenn 33:627–634
Sulistyo I, Rinchard J, Fontaine P, Gardeur JN, Capdeville B, Kestemont P (1998) Reproductive cycle and plasma levels of sex steroids in female Eurasian perch Perca fluviatilis. Aquat Living Resour 11:101–110
Sulistyo I, Fontaine P, Rinchard J, Gardeur JN, Migaud H, Capdeville B, Kestemont P (2000) Reproductive cycle and plasma levels of steroids in male Eurasian perch Perca fluviatilis. Aquat Living Resour 13:99–106
Szczepkowski M, Zakes Z, Szczepkowska B, Piotrowska I (2011) Effect of size sorting on the survival, growth and cannibalism in pikeperch (Sander lucioperca.) larvae during intensive culture in RAS. Czech J Anim Sci 56:483–489
Taranger GL, Carrillo M, Schulz RW, Fontaine P, Zanuy S, Felip A, Weltzien FA, Dufour S, Karlsen O, Norberg B, Andersson E, Hausen T (2010) Control of puberty in farmed fish. Gen Comp Endocrinol 165:483–515
Trubiroha A, Wuertz S, Frank SN, Sures B, Kloas W (2009) Expression of gonadotropin subunits in roach (Rutilus rutilus, Cyprinidae) infected with plerocercoids of the tapeworm Ligula intestinalis (Cestoda). Int J Parasitol 39:1465–1473
Wang N, Gardeur JN, Henrotte E, Marie M, Kestemont P, Fontaine P (2006) Determinism of the induction of the reproductive cycle in female Eurasian perch, Perca fluviatilis: identification of environmental cues and permissive factors. Aquaculture 261:706–714
Wang N, Teletchea F, Kestemont P, Milla S, Fontaine P (2010) Photothermal control of the reproductive cycle in temperate fishes. Rev Aquac 2:209–222
Wocher H, Harsanyi A, Schwarz FJ (2012) Larviculture of burbot (Lota lota L.): larval rearing using Artemia and weaning onto dry feed. Aquac Res 44:106–113
Wuertz S, Nitsche A, Jastroch M, Gessner J, Klingenspor M, Kirschbaum F, Kloas W (2007) The role of the IGF-I system for vitellogenesis in maturing female sterlet, Acipenser ruthenus Linnaeus, 1758. Gen Comp Endocrinol 150:140–150
Zakes Z (2007) Out-of-season spawning of cultured pikeperch [Sander lucioperca (L.)]. Aquac Res 38:1419–1427
Zakes Z, Demska-Zakes K (2009) Controlled reproduction of pikeperch Sander lucioperca (L.): a review. Arch Pol Fish 17:153–170
Zakes Z, Szczepkowski M, Partyka K, Wunderlich K (2013) Effect of gonadotropin hormonal stimulation on out-of-season propagation success of different year classes of indoor-reared pikeperch (Sander lucioperca (L.)). Aquac Int 21:801–810
Zarski D, Kucharczyk D, Targonska K, Palinska K, Kupren K, Fontaine P, Kestemont P (2012) A new classification of pre-ovulatory oocyte maturation stages in pikeperch, Sander lucioperca (L.), and its application during artificial reproduction. Aquac Res 43:713–721
Zarski D, Targonska K, Kaszubowski R, Kestemont P, Fontaine P, Krejszeff S, Kupren K, Kucharczyk D (2013) Effect of different commercial spawning agents and thermal regime on the effectiveness of pikeperch, Sander lucioperca (L.), reproduction under controlled conditions. Aquac Int 21:819–828
Acknowledgments
This study was supported by the German research funding DFG as part of the project “Influence of exogenous factors on the endocrine regulation of gonad maturation in pikeperch S. lucioperca” (SCHU 2308/1-1 AOBJ: 530719). The authors acknowledge AquaPri (Egved Denmark), Landesforschungsanstalt Landwirtschaft & Fischerei (Mecklenburg-West Pomerania, Germany), Institute of Inland Fisheries Potsdam-Sacrow (Brandenburg, Germany), Müritz AquaArt GmbH (Mecklenburg-West Pomerania, Germany), and the Fischzucht Rietschen (Saxonia, Germany) for the information on the reproductive management of pikeperch (Table 4).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Hermelink, B., Kleiner, W., Schulz, C. et al. Photo-thermal manipulation for the reproductive management of pikeperch Sander lucioperca . Aquacult Int 25, 1–20 (2017). https://doi.org/10.1007/s10499-016-0009-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-016-0009-x