Abstract
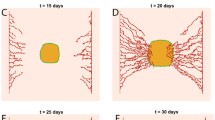
The aim of this article is to show how a tumor can modify energy substrates fluxes in the brain to support its own growth. To address this question we use a modeling approach to explain brain nutrient kinetics. In particular we set up a system of 17 equations for oxygen, lactate, glucose concentrations and cells number in the brain. We prove the existence and uniqueness of nonnegative solutions and give bounds on the solutions. We also provide numerical simulations.







Similar content being viewed by others
References
Adijanto J, Philip N (2012) The SLC16A family of monocarboxylate transporters (MCTs)—physiology and function in cellular metabolism, ph homeostasis, and fluid transport. Curr Top Membr 70:275–312
Aubert A, Costalat R (2005) Interaction between astrocytes and neurons studied using a mathematical model of compartmentalized energy metabolism. J Cereb Blood Flow Metab 25:1476–1490
Aubert A, Costalat R, Valabrgue R (2001) Modelling of the coupling between brain electrical activity and metabolism. Acta Biotheor 49(4):301–326
Aubert A, Costalat R, Magistretti P, Pierre J, Pellerin L (2005) Brain lactate kinetics: modeling evidence for neuronal lactate uptake upon activation. Proc Ntl Acad Sci USA 102:16448–16453
Calvetti D, Rangel GC, Giorda LG, Somersalo E (2018) A computational model integrating brain electrophysiology and metabolism highlights the key role of extracellular potassium and oxygen. J Theor Biol 446:238–258
Colen CB, Shen Y, Ghoddoussi F, Yu P, Francis TB, Koch BJ, Monterey MD, Galloway MP, Sloan AE, Mathupala SP (2011) Metabolic targeting of lactate efflux by malignant glioma inhibits invasiveness and induces necrosis: an in vivo study. Neoplasia 13(7):620–632
Doherty JR, Cleveland JL (2013) Targeting lactate metabolism for cancer therapeutics. J Clin Investig 123(9):3685–3692
Frahm J, Krüger G, Merboldt K, Kleinschmidt A (1996) Dynamic uncoupling and recoupling of perfusion and oxidative metabolism during focal brain activation in man. Magn Reson Med 35:143–148
Griguer CE, Oliva CR, Gillespie GY (2005) Glucose metabolism heterogeneity in human and mouse malignant glioma cell lines. J Neuro-Oncol 74:123–133
Gruetter R, Ugurbil K, Seaquist ER (1998) Steady-state cerebral glucose concentrations and transport in the human brain. J Neurochem 70:397–408
Guillevin R, Menuel C, Vallée J-N, Franoise J-P, Capelle L, Habas C, De Marco G, Chiras J, Costalat R (2011) Mathematical modeling of energy metabolism and hemodynamics of WHO grade II gliomas using in vivo MR data. C R Biol 334:31–38
Guillevin R, Menuel C, Abud L, Costalat R, Capelle L, HoangXuan K, Habas C, Chiras J, Valle J-N (2012) Proton MR spectroscopy in predicting the increase of perfusion MR imaging for WHO grade II gliomas. J Magn Reson Imaging 35(3):543–550
Guillevin R, Herpe G, Verdier M, Guillevin C (2014) Low-grade gliomas: the challenges of imaging. Diagn Interv Imaging 95(10):957–963
Guillevin C, Guillevin R, Miranville A, Perrillat-Mercerot A (2018) Analysis of a mathematical model for brain lactate kinetics. Math Biosci Eng 15(5):1225–1242
Hertz L (2008) Bioenergetics of cerebral ischemia: a cellular perspective. Neuropharmacology 55:289–309
Jolivet R, Coggan JS, Allaman I, Magistretti PJ (2015) Multi-timescale modeling of activitydependent metabolic coupling in the neuron-glia-vasculature ensemble. PLoS Comput Biol 11:e1004036
Mangiardi JR, Yodice P (1990) Metabolism of the malignant astrocytoma. Neurosurgery 26:1–19
Mendoza-Juez B, Martínez-González A, Calvo GF, Pérez-García VM (2012) A mathematical model for the glucose-lactate metabolism of in vitro cancer cells. Bull Math Biol 74:1125–1142
Pellerin L, Magistretti PJ (1994) Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Ntl Acad Sci 91:10625–10629
Romero-Garcia S, Moreno-Altamirano MMB, Prado-Garcia H, Snchez- Garca FJ (2016) Lactate contribution to the tumor microenvironment: mechanisms, effects on immune cells and therapeutic relevance. Front Immunol 7:52
Siesjo BK (1978) Brain energy metabolism. Pharmazie in unserer Zeit, Wiley
Sonveaux P, Vgran F, Schroeder T, Wergin MC, Verrax J, Rabbani ZN, De Saedeleer CJ, Kennedy KM, Diepart C, Jordan BF, Kelly MJ, Gallez B, Wahl ML, Feron O, Dewhirst MW (2008) Targeting lactate-fueled respiration selectively kills hypoxic tumor cells in mice. J Clin Investig 118(12):3930–3942
Tomida A, Yun J, Tsuruo T (1996) Glucoseregulated stresses induce resistance to camptothecin in human cancer cells. Int J Cancer 68(3):391–396
Vafaee MS, Gjedde A (2000) Model of blood-brain transfer of oxygen explains nonlinear flow-metabolism coupling during stimulation of visual cortex. J Cereb Blood Flow Metab 20:747–754
Valabrégue R, Aubert A, Burger J, Bittoun J, Costalat R (2003) Relation between cerebral blood flow and metabolism explained by a model of oxygen exchange. J Cereb Blood Flow Metab 23:536–545
Valvona CJ, Fillmore HL, Nunn PB, Pilkington GJ (2016) The regulation and function of lactate dehydrogenase a: therapeutic potential in brain tumor. Brain Pathol 26(1):3–17
Walenta S, Mueller-Klieser WF (2004) Lactate: mirror and motor of tumor malignancy. Semin Radiat Oncol 14(3):267–274
Yaromina A, Quennet V, Zips D, Meyer S, Shakirin G, Walenta S, Mueller-Klieser W, Baumann M (2009) Co-localisation of hypoxia and perfusion markers with parameters of glucose metabolism in human squamous cell carcinoma (hSCC) xenografts. Int J Radiat Biol 85(11):972–980
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Parameters Value for the Simulations
Appendix: Parameters Value for the Simulations
Initial values chosen for this simulation are given in Aubert et al. works. We refer the interested reader to Aubert and Costalat (2005) to get an explanation of these relevant values.
We give in following Tables 4, 5, 6 and 7 the main parameter values. The function F is a positive square wave function with a maximum value of \(0.048\hbox { s}^{-1}\) (during \(10\hbox { s}\)) and a minimum value of \(0.036\hbox { s}^{-1}\) (during \(100\hbox { s}\)). It reports the cerebral blood flow (Aubert et al. 2001; Aubert and Costalat 2005; Jolivet et al. 2015; Guillevin et al. 2018). It can be taken as follows:
with parameters from Table 4.
Oxygen parameter values are based on Valabrégue et al. (2003) and Aubert and Costalat (2005) works. The quadratic adjustement we have done is explained in the main text.
Glucose parameter values are taken up from Jolivet et al. (2015) and Aubert and Costalat (2005) models. Chosen values are supported by the related literature (Gruetter et al. 1998).
Lactate parameter values are also taken up from Jolivet et al. (2015) and Aubert and Costalat (2005) models. To the best of our knowledge there has been no measure of the lactate maximal transport rate between astrocyte and neuron yet. Therefore its value has been chosen arbitrarily here.
Moreover every tumor shows up a different mechanism concerning substrate use (Romero-Garcia et al. 2016). Gliomas are ad initio formed by degenarated astrocytic cells. Therefore we arbitrarily chose here to keep the same maximal transport rate for both astrocytes and tumor cells. We have:
Knowing that glioma cells are more efficient to take up substrates we arbitrarily chose for this simulation tumor Michaelis–Mentens constant times higher than astrocytic Michaelis–Mentens constant.
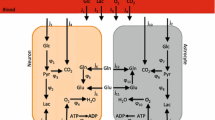
Finally, glucose, lactate and oxygen consumption rate of each compartment are arbitrarily chosen keeping in mind that astrocytes show higher abilities to take glucose and convert it into lactate than neurons. Contrariwise, neurons have better abilities to take lactate and convert it into energy.
Rights and permissions
About this article
Cite this article
Perrillat-Mercerot, A., Bourmeyster, N., Guillevin, C. et al. Mathematical Modeling of Substrates Fluxes and Tumor Growth in the Brain. Acta Biotheor 67, 149–175 (2019). https://doi.org/10.1007/s10441-019-09343-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10441-019-09343-1
Keywords
- Brain lactate kinetics
- Fast–slow system
- Well-posedness
- Regularity
- Simulations
- Glioma
- ANLS
- Lactate
- Brain energy substrates