Abstract
In this review, we explore the roles of macrophages both in vessel development and in vascularization of tissue engineered constructs. Upon the implantation of tissue engineered constructs into the body, macrophages respond, invade and orchestrate the host’s immune response. By altering their phenotype, macrophages can adopt a variety of roles. They can promote inflammation at the site of the implanted construct; they can also promote tissue repair. Macrophages support tissue repair by promoting angiogenesis through the secretion of pro-angiogenic cytokines and by behaving as support cells for nascent vasculature. Thus, the ability to manipulate the macrophage phenotype may yield macrophages capable of supporting vessel development. Moreover, macrophages are an easily isolated autologous cell source. For the generation of vascularized constructs outside of the body, these isolated macrophages can also be skewed to adopt a pro-angiogenic phenotype and enhance blood vessel development in the presence of endothelial cells. To assess the influence of macrophages on vessel development, both in vivo and in vitro models have been developed. Additionally, several groups have demonstrated the pro-angiogenic roles of macrophages in vascularization of tissue engineered constructs through the manipulation of macrophage phenotypes. This review comments on the roles of macrophages in promoting vascularization within these contexts.



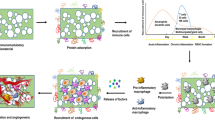
Reprint permission from: Moore et al. Ref. 39.

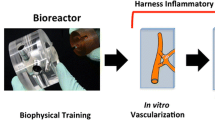
Reprint permission from: Ogle et al.29

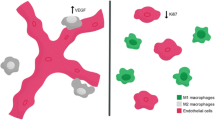
Adapted from: Hsu et al.25
Similar content being viewed by others
References
Alvarez, M. M., J. C. Liu, G. Trujillo-de Santiago, et al. Delivery strategies to control inflammatory response: modulating M1-M2 polarization in tissue engineering applications. J. Control Release. 1:1–10, 2015. https://doi.org/10.1016/j.jconrel.2016.01.026.
Ambati, B. K., M. Nozaki, N. Singh, et al. Corneal avascularity is due to soluble VEGF receptor-1. Nature 443(7114):993–997, 2006. https://doi.org/10.1038/nature05249.Corneal.
Armulik, A., A. Abramsson, and C. Betsholtz. Endothelial/pericyte interactions. Circ. Res. 97(6):512–523, 2005. https://doi.org/10.1161/01.RES.0000182903.16652.d7.
Arras, M., W. D. Ito, D. Scholz, B. Winkler, J. Schaper, and W. Schaper. Monocyte activation in angiogenesis and collateral growth in the rabbit hindlimb. J. Clin. Invest. 101(1):40–50, 1998. https://doi.org/10.1172/JCI119877.
Awojoodu, A. O., M. E. Ogle, L. S. Sefcik, et al. Sphingosine 1-phosphate receptor 3 regulates recruitment of anti-inflammatory monocytes to microvessels during implant arteriogenesis. Proc. Natl. Acad. Sci. USA 110(34):13785–13790, 2013. https://doi.org/10.1073/pnas.1221309110.
Barnett, F. H., M. Rosenfeld, M. Wood, et al. Macrophages form functional vascular mimicry channels in vivo. Sci. Rep. 6:36659, 2016. https://doi.org/10.1038/srep36659.
Brown, B. N., B. D. Ratner, S. B. Goodman, S. Amar, and S. F. Badylak. Macrophage polarization: an opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials 33(15):3792–3802, 2012. https://doi.org/10.1016/j.biomaterials.2012.02.034.
Cao, R., E. Brakenhielm, R. Pawliuk, et al. Angiogenic synergism, vascular stability and improvement of hind-limb ischemia by a combination of PDGF-BB and FGF-2. Nat. Med. 9(5):548–553, 2003.
Daley, J. M., S. K. Brancato, A. A. Thomay, J. S. Reichner, and J. E. Albina. The phenotype of murine wound macrophages. J. Leukoc. Biol. 87(1):59–67, 2010. https://doi.org/10.1189/jlb.0409236.
Das, A., M. Sinha, S. Datta, et al. Monocyte and macrophage plasticity in tissue repair and regeneration. Am. J. Pathol. 185(10):2596–2606, 2015. https://doi.org/10.1016/j.ajpath.2015.06.001.
DeFalco, T., I. Bhattacharya, A. V. Williams, D. M. Sams, and B. Capel. Yolk-sac-derived macrophages regulate fetal testis vascularization and morphogenesis. Proc. Natl. Acad. Sci. 111(23):E2384–E2393, 2014. https://doi.org/10.1073/pnas.1400057111.
DiPietro, L. A. Wound healing: the role of the macrophage and other immune cells. Shock. 4(4):233–240, 1995.
Dondossola, E., B. M. Holzapfel, S. Alexander, S. Filippini, D. W. Hutmacher, and P. Friedl. Examination of the foreign body response to biomaterials by nonlinear intravital microscopy. Nat. Biomed. Eng. 1(1):1–20, 2017. https://doi.org/10.1038/s41551-016-0007.
Du, R., K. V. Lu, C. Petritsch, et al. HIF1α induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell 13(3):206–220, 2008. https://doi.org/10.1016/j.ccr.2008.01.034.
Fantin, A., J. M. Vieira, G. Gestri, et al. Tissue macrophages act as cellular chaperones for vascular anastomosis downstream of VEGF-mediated endothelial tip cell induction. Blood 116(5):829–840, 2010. https://doi.org/10.1182/blood-2009-12-257832.
Fournier, G. A., G. A. Lutty, S. Watt, A. Fenselau, and A. Patz. A corneal micropocket assay for angiogenesis in the rat eye. Investig. Ophthalmol. Vis. Sci. 21(2):351–354, 1981.
Garash, R., A. Bajpai, B. M. Marcinkiewicz, and K. L. Spiller. Drug delivery strategies to control macrophages for tissue repair and regeneration. Exp. Biol. Med. 2016. https://doi.org/10.1177/1535370216649444.
Geissmann, F., S. Jung, and D. R. Littman. Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19(1):71–82, 2003. https://doi.org/10.1016/S1074-7613(03)00174-2.
Gerri, C., R. Marín-Juez, M. Marass, A. Marks, H.-M. Maischein, and D. Y. R. Stainier. Hif-1α regulates macrophage-endothelial interactions during blood vessel development in zebrafish. Nat. Commun. 8(May):15492, 2017. https://doi.org/10.1038/ncomms15492.
Gordon, S., and P. R. Taylor. Monocyte and macrophage heterogeneity. Nat. Rev. Immunol. 5(12):953–964, 2005. https://doi.org/10.1038/nri1733.
Griffith, L. G., and G. Naughton. Tissue engineering–current challenges and expanding opportunities. Science 295(5557):1009–1014, 2002. https://doi.org/10.1126/science.1069210.
Hasan, A., A. Paul, N. E. Vrana, et al. Microfluidic techniques for development of 3D vacularized tissue. Biomaterials 35(1):7308–7325, 2014. https://doi.org/10.1088/1367-2630/15/1/015008.Fluid.
Hibino, N., T. Yi, D. R. Duncan, et al. A critical role for macrophages in neovessel formation and the development of stenosis in tissue-engineered vascular grafts. Faseb J. 25(12):4253–4263, 2011. https://doi.org/10.1096/fj.11-186585.
Hsieh, J., T. Smith, V. Meli, T. Tran, E. Botvinick, and W. F. Liu. Differential regulation of macrophage inflammatory activation by fibrin and fibrinogen. Acta Biomater. 47:14–24, 2016. https://doi.org/10.1016/j.cyto.2014.10.031.Interleukin-10.
Hsu, C. W., R. A. Poché, J. E. Saik, et al. Improved angiogenesis in response to localized delivery of macrophage-recruiting molecules. PLoS ONE 10(7):1–27, 2015. https://doi.org/10.1371/journal.pone.0131643.
Jetten, N., S. Verbruggen, M. J. Gijbels, M. J. Post, M. P. J. De Winther, and M. M. P. C. Donners. Anti-inflammatory M2, but not pro-inflammatory M1 macrophages promote angiogenesis in vivo. Angiogenesis 17(1):109–118, 2014. https://doi.org/10.1007/s10456-013-9381-6.
Kappel, D. F. Organ donation in the United States—2014. J. Leg. Med. 36(1):7–16, 2015. https://doi.org/10.1080/01947648.2015.1047299.
Koh, T. J., and L. A. DiPietro. Inflammation and wound healing: the role of the macrophage. Expert Rev. Mol. Med. 13:e23, 2011. https://doi.org/10.1017/S1462399411001943.
Krieger, J. R., M. E. Ogle, J. McFaline-Figueroa, C. E. Segar, J. S. Temenoff, and E. A. Botchwey. Spatially localized recruitment of anti-inflammatory monocytes by SDF-1α-releasing hydrogels enhances microvascular network remodeling. Biomaterials 77:280–290, 2016. https://doi.org/10.1016/j.biomaterials.2015.10.045.
Kumar, A. H. S., K. Martin, E. C. Turner, et al. Role of CX3CR1 receptor in monocyte/macrophage driven neovascularization. PLoS ONE. 2013. https://doi.org/10.1371/journal.pone.0057230.
Lee, K. Y. Alginate: properties and biomedical applications. Prog. Polym. Sci. 37(1):106–126, 2012. https://doi.org/10.1016/j.progpolymsci.2011.06.003.Alginate.
Leibovich, S. J., P. J. Polverini, H. M. Shepard, D. M. Wiseman, V. Shively, and N. Nuseir. Macrophage-induced angiogenesis is mediated by tumour necrosis factor-alpha. Nature 329(6140):630–632, 1987. https://doi.org/10.1038/329630a0.
Mantovani, A., S. K. Biswas, M. R. Galdiero, A. Sica, and M. Locati. Macrophage plasticity and polarization in tissue repair and remodelling. J. Pathol. 229(2):176–185, 2013. https://doi.org/10.1002/path.4133.
Mantovani, A., S. Sozzani, M. Locati, P. Allavena, and A. Sica. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M 2 mononuclear phagocytes. Trends Immunol. 23(11):549–555, 2002.
Martinez, F. O., and S. Gordon. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 6:13, 2014. https://doi.org/10.12703/p6-13.
Moldovan, N. I., P. J. Goldschmidt-Clermont, J. Parker-Thornburg, S. D. Shapiro, and P. E. Kolattukudy. Contribution of monocytes/macrophages to compensatory neovascularization: the drilling of metalloelastase-positive tunnels in ischemic myocardium. Circ. Res. 87(5):378–384, 2000. https://doi.org/10.1161/01.RES.87.5.378.
Moon, J. J., and J. L. West. Vascularization of engineered tissues: approaches to promote angio-genesis in biomaterials. Curr. Top. Med. Chem. 8(4):300–310, 2008. https://doi.org/10.2174/156802608783790983.
Moore, E. M., V. Suresh, G. Ying, and J. L. West. M0 and M2 macrophages enhance vascularization of tissue engineering scaffolds. Regen. Eng. Transl. Med. 2018. https://doi.org/10.1007/s40883-018-0048-0.
Moore, E. M., G. Ying, and J. L. West. Macrophages influence vessel formation in 3D bioactive hydrogels. Adv. Biosyst. 2017. https://doi.org/10.1002/adbi.201600021.
Murray, P. J., J. E. Allen, S. K. Biswas, et al. Macrophage activation and polarization: nomenclature and experimental guidelines. Immunity 41(1):14–20, 2014. https://doi.org/10.1016/j.immuni.2014.06.008.
Nathan, C. F. Secretory products of macrophage. J. Clin. Invest. 79(February):319–326, 1987. https://doi.org/10.1172/JCI112815.
Nomi, M., A. Atala, P. De Coppi, and S. Soker. Principals of neovascularization for tissue engineering. Mol. Aspects Med. 23(6):463–483, 2002. https://doi.org/10.1016/S0098-2997(02)00008-0.
Novak, M. L., and T. J. Koh. Phenotypic transitions of macrophages orchestrate tissue repair. Am. J. Pathol. 183(5):1352–1363, 2013. https://doi.org/10.1016/j.ajpath.2013.06.034.
Nsiah, B. A., E. M. Moore, L. C. Roudsari, N. K. Virdone, and J. L. West. Angiogenesis in Hydrogel Biomaterials. Durham: Duke University, 2015. https://doi.org/10.1016/b978-1-78242-105-4.00008-0.
Nucera, S., D. Biziato, and M. de Palma. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int. J. Dev. Biol. 55(4–5):495–503, 2011. https://doi.org/10.1387/ijdb.103227sn.
Okuno, Y., A. Nakamura-Ishizu, K. Kishi, T. Suda, and Y. Kubota. Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood 117(19):5264–5272, 2011. https://doi.org/10.1182/blood-2011-01-330720.
Peters, E. B., N. Christoforou, E. Moore, J. L. West, and G. A. Truskey. CD45 + cells present within mesenchymal stem cell populations affect network formation of blood-derived endothelial outgrowth cells. Biores. Open Access. 4:75–88, 2015. https://doi.org/10.1089/biores.2014.0029.
Phelps, E. A., N. Landazuri, P. M. Thule, W. R. Taylor, and A. J. Garcia. Bioartificial matrices for therapeutic vascularization. Proc. Natl. Acad. Sci. 107(8):3323–3328, 2010. https://doi.org/10.1073/pnas.0905447107.
Poché, R. A., J. E. Saik, J. L. West, and M. E. Dickinson. The mouse cornea as a transplantation site for live imaging of engineered tissue constructs. Cold Spring Harb. Protoc. 5(4):1–11, 2010. https://doi.org/10.1101/pdb.prot5416.
Polverini, P. J., P. S. Cotran, M. A. Gimbrone, and E. R. Unanue. Activated macrophages induce vascular proliferation. Nature 269(5631):804–806, 1977. https://doi.org/10.1038/269804a0.
Przaeres, P., V. Almeida, L. Lousado, et al. Macrophages generate pericytes in the developing brain macrophages generate pericytes in the developing brain. Cell Mol. Neurobiol. 1:1–10, 2017. https://doi.org/10.1007/s10571-017-0549-2.
Rehman, J., J. Li, C. M. Orschell, and K. L. March. Peripheral blood “endothelial progenitor cells” are derived from monocyte/macrophages and secrete angiogenic growth factors. Circulation 107(8):1164–1169, 2003. https://doi.org/10.1161/01.CIR.0000058702.69484.A0.
Richardson, T. P., M. C. Peters, A. B. Ennett, and D. J. Mooney. Access polymeric system for dual growth factor delivery. Nat. Biotechnol. 19:1029–1034, 2001.
Rohde, E., C. Malischnik, D. Thaler, et al. Blood monocytes mimic endothelial progenitor cells. Stem Cells. 24(2):357–367, 2006. https://doi.org/10.1634/stemcells.2005-0072.
Rouwkema, J., N. C. Rivron, and C. A. van Blitterswijk. Vascularization in tissue engineering. Trends Biotechnol. 26(8):434–441, 2008. https://doi.org/10.1016/j.tibtech.2008.04.009.
Rymo, S. F., H. Gerhardt, F. W. Sand, R. Lang, A. Uv, and C. Betsholtz. A two-way communication between microglial cells and angiogenic sprouts regulates angiogenesis in aortic ring cultures. PLoS ONE. 2011. https://doi.org/10.1371/journal.pone.0015846.
Sidky, Y. A., and E. C. Borden. Inhibition of angiogenesis by interferons: effects on tumor-and lymphocyte-induced vascular responses. Cancer Res. 47(19):5155–5161, 1987.
Spiller, K. L., R. R. Anfang, K. J. Spiller, et al. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 35(15):4477–4488, 2014. https://doi.org/10.1016/j.biomaterials.2014.02.012.
Spiller, K. L., S. Nassiri, C. E. Witherel, et al. Sequential delivery of immunomodulatory cytokines to facilitate the M1-to-M2 transition of macrophages and enhance vascularization of bone scaffolds. Biomaterials 37:194–207, 2015. https://doi.org/10.1016/j.biomaterials.2014.10.017.
Sunderkötter, C., M. Goebeler, K. Schulze-Osthoff, R. Bhardwaj, and C. Sorg. Macrophage-derived angiogenesis factors. Pharmacol. Ther. 51(2):195–216, 1991. https://doi.org/10.1016/0163-7258(91)90077-Y.
Takemura, R., and Z. Werb. Secretory products of macrophages and their physiological functions. Am. J. Physiol. 246(1 Pt 1):C1–C9, 1984.
Tang, N., L. Wang, J. Esko, et al. Loss of HIF-1α in endothelial cells disrupts a hypoxia-driven VEGF autocrine loop necessary for tumorigenesis. Cancer Cell 6(5):485–495, 2004. https://doi.org/10.1016/j.ccr.2004.09.026.
Tattersall, I. W., J. Du, Z. Cong, et al. In vitro modeling of endothelial interaction with macrophages and pericytes demonstrates Notch signaling function in the vascular microenvironment. Angiogenesis 19(2):201–215, 2016. https://doi.org/10.1007/s10456-016-9501-1.
Von Tell, D., A. Armulik, and C. Betsholtz. Pericytes and vascular stability. Exp. Cell Res. 312(5):623–629, 2006. https://doi.org/10.1016/j.yexcr.2005.10.019.
Wynn, T. A. A., and K. M. M. Vannella. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 44(3):450–462, 2016. https://doi.org/10.1016/j.immuni.2016.02.015.
Yamamoto, S., M. Muramatsu, E. Azuma, et al. A subset of cerebrovascular pericytes originates from mature macrophages in the very early phase of vascular development in CNS. Sci. Rep. 1:1–16, 2017. https://doi.org/10.1038/s41598-017-03994-1.
Acknowledgments
The authors would like to acknowledge Chih-Wei Hsu for the use of his image (Fig. 5). The authors would also like to thank Dr. Botchwey for the use of Fig. 4: Reprinted from Ref. 29. Figure 3, Reprinted from Ref. 39. Funding was provided by National Science Foundation (Grant No. DGE-1644868), Foundation for the National Institutes of Health (Grant No. R01 HL097520).
Conflict of interest
The authors declare no conflicts of interest with regards to this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Rights and permissions
About this article
Cite this article
Moore, E.M., West, J.L. Harnessing Macrophages for Vascularization in Tissue Engineering. Ann Biomed Eng 47, 354–365 (2019). https://doi.org/10.1007/s10439-018-02170-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-018-02170-4