Abstract
In breeding Norway spruce, selection for improved growth and survival is performed at age 10–15 years in order to optimize genetic gain per year. We investigated whether a selection based on wood traits such as density and grain angle, measured under bark in the field at the same age would be informative enough with respect to structural quality traits of sawn boards. To achieve this objective, a sawing study was conducted on the butt logs of 401 trees from a 34-year-old Norway spruce progeny trial situated in southern Sweden. Stem discs were excised from the top of the logs and radial profile data of grain angle, and wood density was recorded for specific annual rings. The sawn and dried boards were assessed for structural traits such as twist, board density, bending stiffness (static modulus of elasticity, sMoE) and bending strength (modulus of rupture, MoR). Additive genetic correlations (ra) between single annual ring density measurements and board density, sMoE and MoR were consistently strong (ra> 0.7) for annual rings 5–13. Genetic correlations of similar magnitude between grain angle and board twist were estimated for all investigated annual rings (from 2 to around 26 under bark). Consequently, it was found that indirect selection for wood density and grain angle at the tree age 10–16 years would result in more genetic gain per year than selection at later ages. This makes it feasible to perform simultaneous selection of progeny in the field for both growth and wood traits at similar ages.
Similar content being viewed by others
Introduction
The breeding and genetic improvement of boreal conifer species, such as Norway spruce (Picea abies (L.) Karst), is a challenge due to long rotation ages (50–100 years). The most accurate selection for adaptation, increased growth and improved quality would require extensive and rigorous testing efforts where the traits of interest are assessed at complete rotation age. Naturally such selection would be extremely slow and inefficient. Therefore, breeding programs of boreal conifers are based on selection made earlier in the rotation cycle (e.g. White et al. 2007) thereby enabling a breeding cycle considerably shorter than a full rotation age. The underlying assumption is that early assessments of traits in field trials (selection traits) are highly correlated with the final rotation traits (breeding objective traits).
For growth traits, correlations of early to later assessments (age–age correlations) and correlations of growth measurements to the corresponding breeding objective traits (often projected volume growth) has been extensively studied (e.g. Lambeth 1980; Lee et al. 2002; Jansson et al. 2003; Andersson et al. 2007; Haapanen et al. 2016). By analysing data from repeated growth assessments it is possible to propose a suitable tree age for field trial assessment in order to optimize the genetic gain per unit time. Thus, a profitable balance could be struck between the conflicting desires of inexpensive assessment and fast breeding turnover (early assessments in the field) on one hand, and the desire for improved selection accuracy and relevance with respect to breeding objectives (assessments at full rotation). Isik et al. (2010) studied multiple assessment of height growth in a 17-year-old Norway spruce clone trial and concluded that selection for growth would be most efficient at 13 years of age.
The optimal timing for assessing wood quality traits such as density, grain angle, stiffness (Modulus of elasticity, MoE) and microfibril angle is less well studied. Inexpensive and non-destructive methods for measuring wood traits, such as pilodyn penetration (Cown 1978), density resistography (Isik and Li 2003), under bark assessments for grain angle (Hannrup et al. 2003) and acoustic velocity assessment methods (Auty and Achim 2008; Mora et al. 2009) have slowly but successively been developed. Moreover, it is possible to study age–age correlations of wood properties on a ring-by-ring basis from pith to bark in stem sections or wood cores (e.g. Wu et al. 2007; Lenz et al. 2011; Hong et al. 2015). For Norway spruce a few such studies have recently been published (Steffenrem et al. 2009; Gräns et al. 2009; Chen et al. 2014).
Apart from estimating age–age correlations, an additional difficulty for wood quality traits is their large number, their diverse characteristics and the fact that their relative importance depends heavily on the industry processes and the nature of the end products, for instance the characteristics of structural sawn timber. To date, only a few genetic studies linking wood traits to sawn timber traits have been published for conifer species (e.g. Cown et al. 2004; Beaulieu et al. 2006; Fujimoto et al. 2006; Cherry et al. 2008; Harding et al. 2008; Moore et al. 2009; Tanabe et al. 2014).
For Norway spruce, the potential of improving sawn timber traits by selection and breeding was demonstrated after studying a progeny trial that was harvested and processed through a sawmill (Högberg et al. 2014). For sawn timber traits such as board twist, density and board stiffness measured by acoustic resonance frequency, heritability estimates were moderate to high (0.23–0.44). Furthermore, additive genetic correlations (ra) between these sawn timber traits were also strong. For example, grain angle correlated well with twist of the sawn board (0.93) and pilodyn penetration showed strong correlation with board density (− 0.91) and with stiffness (− 0.75). However, field measurements of grain angle and pilodyn penetration were performed after 18 and 28 years in the field, respectively, which would be late in comparison with the recommended age for selection for increased growth at 10–15 years (Jansson et al. 2003). The single field measurements performed by Högberg et al. (2014) may still not have been performed at the optimal time with respect to efficient breeding. In order to enable time-efficient selection and breeding for improved sawn timber quality, additional research is required to indicate the optimal tree age for wood trait assessment.
The aim of this study was to estimate the optimal timing for field measurements of wood density and grain angle (selection traits) aiming to maximize genetic gain per year for increased board stiffness (modulus of elasticity, MoE), strength (modulus of rupture, MoR) and for decreased board twisting after drying (breeding objective traits). Could wood trait assessment be done as early as growth traits thereby enabling synchronized assessments and saving time? The study aim was achieved by establishing time series of grain angle and density from stem discs and correlating these measurements with the twist, density, stiffness and strength of the previously sawn boards (Högberg et al. 2014). Moreover, as the static stiffness and strength of the boards were measured by bending, it was possible to corroborate the genetic parameters for these traits which previously were indicated only by acoustic assessments.
Materials and methods
Field material
The study trees were sampled from a Norway spruce progeny trial, located at Tönnersjö in south-west Sweden (56.66°N, 13.09°E; altitude, 90 m above sea level). The progenies were generated from 36 parents (plus trees) crossed in a partial diallel design with the intention of representing each parent in seven crosses. A total of 115 full-sib families were thus generated, and progeny seedlings were planted in 1977 in single-tree plots using a randomized complete block design. Each full-sib family was represented by 4 progeny seedlings in each of the 10 blocks (details are given in Högberg et al. 2014). Field measurements of various traits have been carried out at several occasions. In the present context the most important growth traits are height (HT) measured at age HT5, HT6, HT11, HT18 and HT21 in the field, and diameters (DIA, DBH) measured at age DIA11, DBH18, DBH21, DBH29 and DBH34. All field measured diameters were taken at breast height (1.3 m) except for DIA11 which was taken below the fifth branch whorl counted from the top. In this particular branch whorl at age 11, the number of branches were counted (BNO11). From this whorl, the branch growing in the west direction was selected and its branch angle in degrees (BRA11) and branch diameter in mm (BDI11) was measured. Pilodyn penetration was measured at breast height at age 18 (PIL18). Finally, at age 28, the grain angle under bark (GA28) was measured using the wedge grain angle gauge (Hannrup et al. 2003).
Wood processing and characterization
The sawing experiment included 401 trees harvested exclusively from two blocks of the field trial which, historically, had been most intensively investigated and phenotypically assessed. All available trees with a DBH above 140 mm at the time of felling (93%) were included in the study implying that each of the 36 parent plus trees was represented by 22.3 trees on average (std. dev. ± 6.0). From each included tree, one approximately 3.3 m long butt log was cut at age 34 years. Prior to processing, a ~ 6-cm-thick stem disc was extracted from the top of each log. The logs were sawn through the pith, each producing two 50 × 100 mm boards that were 3.24 m long on average. The two boards from each tree were separated into two piles (A-pile and B-pile) and were subsequently air-dried to a moisture content (MC) of 18%. After drying, warp traits (bow, crook and twist, see Högberg et al. 2014) were assessed on the top 3 m of the boards to even out minor variation in board length (std. dev. at ± 15 mm). The boards were subsequently planed on both edges and on the outer face to a dimension of 47 × 95 mm.
The stem discs taken at 3.3 m height were further sawn into ~ 4-cm-wide radial bark-to-bark profiles including the pith. These were then used for measurements of grain angle and wood density data series. The grain angle (rGA) in these radial profiles was assessed from both directions of annual rings 2, 4, 6, 8, 10, 12, 16, 20 from pith and the ring directly under the bark (ranging from ring 22 to 29 from pith). Grain angle was measured using the scribe test method in combination with a precision protractor (Säll 2002). Each ring and side was scribed twice and an overall estimate of grain angle for each ring calculated as the arithmetic mean of the opposite sides thereby cancelling out any inadvertent non-perpendicular orientation of the sawn profile with respect to the stem axis. Care was taken to avoid knots and other grain disturbances and if this was unfeasible the record for that tree and ring was set as missing.
A smaller 1-cm-thick wood sample was excised from the previously sawn radial profiles and used for radial density trend measurements with an X-ray scanner (ITRAX Multiscanner, Cox Analytical Systems) as described by Bergsten et al. (2001), providing two measurements of density for each annual ring. As annual rings of the radial profile were delineated, it was possible to assess the accumulated diameter growth of the stem up to any specified calendar year (rDIA). Due to damage on some samples, only one value could be produced for some annual rings. Prior to scanning, wood samples were reshaped to a longitudinal thickness of approximately 2.2 mm. The samples were scanned in batches with nine samples and one reference calibration wedge of cellulose acetate. Densities were recalculated to green-volume dry-weight densities using equilibrium MC estimated with the Hailwood–Horrobin equation for two hydrates (Hailwood and Horrobin 1946) and measurements of temperature and relative air humidity from each batch-scan. Again, a density record for each annual ring, the mean of the measurements taken at opposite sides (when both present), was used for further analysis (abbreviated as rDEN).
Finally, MC-adjusted board density (bDEN), bending strength (modulus of rupture = MoR) and the static modulus of elasticity (sMoE) for the boards from the A-pile were all assessed according to standards for structural timber EN408 (Anon 2010). First, the actual density of the boards (bDENact) at the measurement time was calculated by measuring the weight and dividing this by the product of the dimensions of the board. The density measure was then adjusted to an MC of 12% as:
where usample = sample MC in per cent. The boards were subjected to four-point loading in edge-wise bending as shown in Fig. 1, at a research facility in Asa, Småland, belonging to the Swedish University of Agricultural Sciences. The static modulus of elasticity sMoE was calculated as:
where \(a\) and \(l_{1}\) are length measurement dependent on the width of the board (h ≈ 95 mm, Fig. 1), \(I\) the second moment of inertia, \(F_{2} - F_{1}\) is an increment of load on the straight line portion of the load deformation curve and \(w_{2} - w_{1}\) is an increment in deformation corresponding to \(F_{2} - F_{1}\). sMoE was then adjusted to correspond to an MC of 12% as below:
Test arrangement for measuring MoR and sMoE in bending according to EN408 (Anon 2010) and a load–deformation curve within the linear elasticity range
In turn, the bending strength at rupture (MoR) was calculated as:
where \(a\) is a length measurement according to Fig. 1, \(F_{ \text{max} }\) = the maximum load and \(W\) = the section modulus.
Statistical analysis
All statistical analyses were performed using ASReml (Gilmour et al. 2009). Data from time series and timber strength measurements were subjected to univariate analyses using the individual tree model:
where \(\varvec{y}\) is a vector of observed values, \(\varvec{b}\) is a vector of fixed block effects, \(\varvec{a}\) is a vector of random additive genetic effects, \(\varvec{f}\) is a vector of random full-sib family effects and \(\varvec{e}\) is a vector of random residuals. \(\varvec{X}\), \(\varvec{Z}_{\varvec{a}}\) and \(\varvec{Z}_{\varvec{f}}\) are design matrices linking phenotypic observations to fixed and random effects, respectively.
To estimate genetic correlations, the trait variance components and covariance components between pairs of traits were estimated by bivariate analysis. The bivariate model was an expansion of the univariate model to include a vector of values for the two traits and matrices corresponding to fixed effect design and random effect design for each trait, respectively.
Variances and covariances of the random effects were modelled as:
where \(\varvec{A}\) is the numerator relationship matrix, \(\varvec{G}\) is the additive genetic (co)variance matrix, \(\varvec{F}\) is the family (co)variance matrix, \(\varvec{R}\) is the residual (co)variance matrix and \(\varvec{I}\) is an identity matrix.
The n × n order symmetric numerator relationship matrix \(\varvec{A}\) in turn was structured as:
where \(\rho_{ij}\) is the additive pedigree-based relationship between any pair of tree individuals i and j of the n individuals included in the analysis. The 2 × 2 order symmetric matrices \(\varvec{G}\), \(\varvec{F}\) and \(\varvec{R}\) are in turn structured as:
where \(\sigma_{a1}^{2}\), \(\sigma_{f1}^{2}\), \(\sigma_{e1}^{2}\) and \(\sigma_{a2}^{2}\), \(\sigma_{f2}^{2}\), \(\sigma_{e2}^{2}\) are additive genetic, full-sib family and residual variances for trait 1 and 2, respectively, whereas \(\sigma_{a12}\), \(\sigma_{f12}\) and \(\sigma_{e12}\) are the corresponding additive genetic, full-sib family and residual covariances between trait 1 and 2.
Genetic interpretation
For any univariate trait analysis, the phenotypic variance (\(\sigma_{p}^{2}\)) and narrow-sense heritabilities (\(h^{2} )\) were estimated as:
Additive genetic correlations between traits 1 and 2 (\(r_{a12}\)) were calculated from bivariate analysis variance components according to Falconer and Mackay (1996) as:
The standard errors of the genetic parameters were estimated using a Taylor series approximation.
Indirect selection efficiency
The efficiency of indirect selection (Seff) is the amount of genetic gain obtained for the breeding objective trait (trait 2) as a result of selection based on an early-measured field trait (trait 1), relative to the genetic gain obtained by direct selection for the breeding objective trait per se. Indirect selection efficiency was calculated as:
where \(h_{1}\) and \(h_{2}\) are the square-root of heritabilities for selection and objective traits, respectively, and \(r_{a12}\) is the additive genetic correlation between them.
Following the work of Lambeth (1980), estimates of indirect selection efficiency can also take into account that the selection trait may be observable at much earlier ages than the breeding objective trait thereby raising an opportunity of speeding up the breeding cycle. The selection efficiency per year (Seff/year) for performing early indirect selection relative to performing direct selection at felling age (34 years) was calculated as:
where \(r_{a12}\), \(h_{1}\) and \(h_{2}\) are as above, \(t_{1}\) = the tree age when indirect selection trait measurement and \(t_{2}\) = the tree age at objective trait measurement (here 34 years). In cases where the selection trait was derived from radial profile measurements, it was assumed that these measurements (taken at 3.3 m height) would be equivalent, in terms of the wood produced, to measurements taken at breast height (1.3 m). Moreover, the tree age (\(t_{1}\)) for such single annual ring measurements was determined as the cambial age of that ring plus the number of years required to reach breast height (tBH) indicated by height growth data, and that the measurement was taken at that height directly under bark (e.g. using the wedge grain angle gauge and/or pilodyn penetration). Furthermore, the equation assumes that an additional time period of 10 years is required in order to complete breeding activities (e.g. archiving clonal material, controlled crossings etc.) of the breeding cycle (see Jansson et al. 2003).
Results
General trends
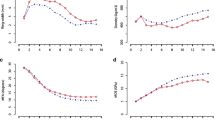
The early height measurements HT5 had an average and a median of 1.46 m (std. dev. ± 0.28 m), and 290 out of 401 trees (72%) had reached breast height at this time (Table 1). In the context of later wood quality assessments, this observation indicated that the cambial age at breast height lagged about 4 years behind the field age of the trees (tBH = 4). The wood samples from stem discs at 3.3 m comprised an average of 26.4 annual rings (std. dev. ± 1.3) indicating that trees reached the height 3.3 m after 7.6 years, about 3–4 years after they reached breast height. The diameter growth at both heights followed the familiar pattern of a rapid initial increase from age 10 to 20 (by 6–7 mm year−1 on average, Fig. 2a) followed by a slower increase from age 20 and onwards (average 4–5 mm year−1). Diameter growth finally reached an average of 185.9 and 151.7 mm at breast height and 3.3 m, respectively, at the time of cutting (Table 1).
Individual measurements of diameter growth (a), wood density (b) and grain angle (c) by increasing tree/cambial age taken from radial profiles extracted at 3.3 m height (red colour diamond) or from field measurements taken at breast height (blue colour diamond). Average trends for radial profiles and field assessments are shown as black lines and squares respectively. The field measurement in subplot c is the grain angle measurements taken under bark at 1.3 m at age 28 (GA28)
The wood density in radial profiles increased slightly with increasing cambial age from 306 kg m−3 close to pith up to 417 kg m−3 in the rings closest to bark (Fig. 2b). The average density of sawn boards was even higher at 472.2 kg m−3 (Table 1) mostly due to the sawn board density being adjusted to an MC of 12% while the radial profile wood density was adjusted to zero MC. The average grain angle in the radial profiles initially increased from left-handed 1.2° in ring 2 to 2.4° annual ring 4 (Fig. 2c). This was subsequently followed by a successive and stable decrease of the grain angle down to a mere 0.7° under bark. For all three traits, a substantial variation around each mean was present. The average values for board density, stiffness and strength were in line with other studies on similar material (Table 1).
Trends of heritability
For height growth, heritabilities (h2) ranged from 0.13 at age 5 to 0.19 at age 21 (Table 1). For DBH, h2-estimates ranged from 0.14 at age 18 to 0.25 at age 28, while the diameter based on the accumulation of annual rings in radial profiles ranged from 0.09 at age 10 to 0.19 at age 34 (Fig. 3a). For the density of single annual rings in the radial profiles, h2-estimates ranged from 0.09 at ring 5 to 0.40 at ring 16 and were consistently higher than 0.10 from ring number 6 and onwards (Fig. 3b). For grain angle in radial profiles, heritability was only 0.08 in the innermost annual rings (Fig. 3c) but increased consistently to the range 0.30–0.33 at a cambial age above 20 years and under bark at both sample heights.
Annual ring heritability estimates (h2) and accompanying estimation errors for accumulated diameter (a), wood density (b) and grain angle (c) in radial profiles extracted at 3.3 m height. Breast height (1.3 m) field assessments of diameter, pilodyn at age 18 and grain angle under bark at age 28 are shown in red in subplots a, b and c respectively. Note that estimates for accumulated diameter in radial profiles at tree ages below 9 years are missing since trees reached the 3.3 m sampling height after 7.6 years on average. Likewise, there are no estimates for wood density above the 26th ring from pith due to unacceptable percentages of missing data (> 50%) at higher ring numbers
When considering density traits based on larger volumes of wood sample, like rDEN and bDEN, h2-estimates were even higher at 0.41 and 0.48, respectively (Table 1) and for other board traits such as twist, stiffness (sMoE), and strength (MoR) heritabilities were moderate in the range 0.21–0.34.
Trends of genetic correlation
The strongest additive genetic correlation (ra) estimated between selection traits measured in the field and objective traits assessed on sawn boards was that between grain angle under bark (GA28) and board twist after drying (0.93, Table 2). But also pilodyn penetration was found to correlate strongly and negatively with board density (− 0.92), mechanically assessed bending stiffness (sMoE, − 0.77) and strength (MoR, − 0.90).
Furthermore, negative ra-estimates, were found between growth traits on the one hand and density (− 0.20 to − 0.64), sMoE (− 0.26 to − 0.73) and MoR (− 0.35 to − 0.78) on the other indicating an adverse relationship. Also ra-estimates between growth traits and board twist tended to be positive and therefore slightly adverse (0.13–0.56). Apart from that, positive genetic correlations were estimated between branch angle and density (0.44), sMoE (0.63) and MoR (0.51), and a negative correlation between the number of branches and MoR (− 0.58), suggesting that branch traits may indeed affect board stiffness and strength.
It was apparent that grain angle measured in the annual rings of radial profiles correlated strongly with board twist (from 0.73 under bark to 0.99 at rings 6 and 10, Fig. 4a). The density of radial profiles also correlated strongly with that of the boards (from 0.37 at ring 1 to 0.99 at rings 5–7 and 10, Fig. 4b). Notably, these strong relationships did not appear to depend much on the annual ring chosen. For instance ra-estimates between radial profile and board density were higher than 0.7 for 21 out of 26 annual rings (Fig. 4b). Radial profile wood density was also positively and consistently correlated with board stiffness (from 0.25 at ring 2 to 0.98 at ring 11, Fig. 4c) and with strength (from 0.25 at ring 2 to 0.96 at ring 6, Fig. 4d). For density ra-estimates with board stiffness and strength, the choice of annual ring mattered somewhat because the strong genetic correlations (> 0.7) appeared to be consistent only within a cambial age span of 5–18 years for board stiffness and 5–13 years for board strength. The genetic correlations between wood traits assessed in radial profiles and boards were generally similar to the corresponding field-to-board trait genetic correlations (Fig. 4). The largest absolute difference observed between radial profile-to-board and field-to-board genetic correlations was only 0.26 in the case of ra between grain angle and MoR (Fig. 4d). This suggests that the impact of the height difference (2 m) between field and radial profile assessments was limited.
Additive genetic correlations (ra) of density (red) and grain angle (blue), measured ring-by-ring on radial profiles extracted at 3.3 m height, with the four sawn timber traits (subplots a, b, c and d). Correlation estimates are also shown for pilodyn at age 18 (red colour circle) and grain angle at age 28 (blue colour circle) both measured at 1.3 m height. Estimation errors are shown as whiskers and please note that the absolute value is shown for pilodyn to facilitate comparisons
Indirect selection responses
With respect to growth, we considered DBH at 34 years to be the objective trait, being assessed at an age when commercial thinning operations are feasible and being essentially half the rotation age common for Norway spruce grown in southern Sweden. Genetic correlations between early field measured growth traits and DBH34 were strong (from 0.85 at age 5 to 0.93 at age 21 for early height and from 0.80 at age 11 to 0.99 at age 28 for DBH, Fig. 5a). The correlation to DBH34 was even high (0.80) for DIA11 despite this trait being assessed under a specific branch whorl rather than exactly at breast height. Only the genetic correlations between accumulated diameter of the radial profiles and DBH34 were below 0.5 for younger tree ages (< 11 years) indicating that diameter growth is sensitive to differences in assessment height and ages (Fig. 5a). The high genetic correlations between early-measured growth traits in the field and DBH34 thus resulted in high indirect selection efficiencies (Seff) for the selection traits (0.80–1.26) with respect to DBH34 (Fig. 5b). As indicated by estimates of Seff/year, selection for height or diameter at the age of 10 years would have resulted in higher genetic gain (by 74 and 96%, respectively, Fig. 5b) than waiting for a direct selection using DBH34 to be achieved. Taking into account the potential to shorten the breeding cycle, it was thus evident that early selection for growth should be advantageous.
Additive genetic correlations—ra (a) between growth traits measured for different tree ages in the field or in radial profiles and the chosen objective growth trait which was diameter at breast height at age 34 (DBH34). Selection efficiency estimates (b) are also shown for the increase of DBH34 when selecting for height growth or DBH measured earlier. Estimates of indirect selection efficiency (Seff) in comparison with direct selection are shown in blue and red for height and DBH, respectively, while corresponding estimates of indirect selection efficiency per year (Seff/year) are shown in cyan and pink. Standard errors for ra-estimates in subplot a are shown as whiskers and the red star indicates DIA11 which was not measured strictly at breast height (1.3 m) but rather under the fifth branch whorl counted from the top (at 1.46 m on average)
The observed strong genetic correlations, between single-ring assessments of wood density and grain angle on one hand and sawn timber traits (twist, board density, sMoE, MoR) on the other hand, suggested that early indirect selection for lower board twist, higher board density, stiffness and strength should be possible. Indeed, estimates of indirect selection efficiency (Seff) of grain angle on board twist were reasonably high across all hypothetical selection ages (0.42–0.80, Fig. 6a). The Seff/year-estimates suggested that a grain angle assessment and selection at the age of 10 would yield 57% greater genetic gains per year than would direct selection on board twist after 34 years.
Selection efficiency estimates in improvement of sawn timber traits when selecting for wood traits assessed in single annual rings. Estimates of indirect selection efficiency in comparison with direct selection (Seff) are shown as solid lines while estimates of indirect efficiency per year (Seff/year) are shown as dashed lines. Selection efficiency estimates for the corresponding field traits (grain angle at age 28 in subplot a and pilodyn at age 18 in b–d) are also shown as filled squares for Seff and filled circles for Seff/year. The calculations underlying this figure assume that trees would reach breast height (1.3 m) after 4 years thus being theoretically possible to assess from age 5
With regards to indirect selection for board density, stiffness and strength, Seff-estimates for the use of single-ring assessed density indicated an efficiency peak in the age span of 14–18 years where Seff even exceeded 1 in the case of stiffness and strength (Fig. 6b–d). The Seff and Seff/year-estimates of PIL18 with respect to board density, stiffness and strength was in fair agreement with the trends for density observed from radial profiles and an early selection based on PIL18 appeared to be considerably more efficient than direct selection for board density (by 16%, Fig. 6b), sMoE (by 38%, Fig. 6c) and MoR (by 71%, Fig. 6d). However, Seff/year-estimates for density measured on radial profiles indicated that earlier selection (at age 8–16) would result in yet more genetic gain per unit time (Fig. 6b–d). For example, selection for density at age 10 would have resulted in extra gains of 39% for board density, 60% for sMoE and 76% for MoR in comparison with gains made by direct selection for these traits. In contrast, Seff/year-estimates also indicated that density assessments performed later than age 25 were considerably less efficient than direct selection. For example, selection on density at age 30 would achieve less gains for board density (− 58%, Fig. 6b), sMoE (− 30%, Fig. 6c) and MoR − 46%, Fig. 6d) than direct selection for the respective trait.
Discussion
The overall aim of the study was to find the optimal timing for selection for sawn timber traits in breeding of Norway spruce. Several other studies on early selection for wood traits have estimated heritability and genetic age–age correlation trends for wood traits assessed as arithmetic or weighted means of annual rings across the radial profiles (e.g. Wu et al. 2007; Lenz et al. 2010, 2011; Chen et al. 2014; Hong et al. 2015). The rationale for using weighted means was to maximize the relevance of the assessment with respect to the whole stem, but this approach would require arduous large-scale sampling and expensive analyses of increment cores. In contrast, both this work and our previous study (Högberg et al. 2014) links wood traits assessed in single annual rings to the performance of sawn structural timber. Högberg et al. (2014) demonstrated that pilodyn and grain angle measurements from a single annual ring, assessed under bark in the field could be informative enough about structural quality characteristics (e.g. twist and MoE) of entire sawn boards of regular dimensions (50 × 100 mm). The present study has further suggested the optimal timing for single annual ring assessments of grain angle and wood density in order to genetically improve quality sawn timber traits such as twist, board density, stiffness and strength.
The rapid diameter growth at ages 10–20 years followed by slower growth later on as well as a slowly increasing wood density by rising cambial age (Fig. 2a) were trends similar to those previously reported for two other 21-year-old Norway spruce progeny trials (Chen et al. 2014; 2015) and were also roughly similar to a 26-year-old clone trial (Gräns et al. 2009). Furthermore, the observed pattern of a positive but decreasing grain angle trend by increasing cambial age has been supported by extensive observations made by Säll (2002). Therefore, it seems likely that the present material fits in as a representative sample of Norway spruce.
Promisingly, non-negligible levels of heritability (h2> 0.1) as well as high and consistent genetic correlations with objective traits (bTWI, bDEN, sMoE and MoR) were estimated even for single annual ring assessments of wood density and grain angle all through rings 6–13 from the pith (Figs. 3, 4). These heritability and genetic correlation estimates also agreed very well with the corresponding parameters estimated for grain angle and pilodyn measured in the field thus indicating some equivalence. In a similar manner, Gräns et al. (2009) found substantial heritability estimates for wood density, microfibril angle and dynamically predicted MoE even though the wood profiles were analysed in sections of 4–5 annual rings. The wood characteristics of these sections all correlated very well with the total weighted increment core averages which agrees with observations that single annual ring assessments of wood traits are very informative with regards to large sections of the stem.
For growth traits in Norway spruce, selection efficiency has already been studied to some extent. Isik et al. (2010) using a 17-year-old clonal test suggested that early selection for height growth could be made after 13 years. Our study material, however, although double the age of that of Isik et al. (2010), exhibited very high genetic age–age correlations and indirect selection efficiency estimates thus suggesting that early selection would be possible even before age 10 (Fig. 5b). Admittedly though, we did not make any growth projections at full rotation age and therefore some caution should be taken when interpreting the efficiency estimates for growth.
The results of this study gave the indication that early selection for increased board density, stiffness and strength could be performed based on pilodyn at the age span 8–16 years (rings 4–12 from the pith) and that later selection would be less efficient when accounting for the extended breeding cycle (Fig. 6). This is roughly similar to the suggestions of Chen et al. (2014) that 6–7 annual rings should be needed although they used weighted area means of increment cores rather than single-ring measurements. In addition, in order to decrease board twisting after drying, we found that selection for lower grain angle based on an assessment at age 10 (ring 6 at breast height) was the optimal strategy. Further delay in assessment would only decrease genetic gains per unit time as a result of breeding cycle extension. It should also be noted that our breeding objective (the structural quality of centrally sawn boards in butt logs) did not require the prediction of wood quality of the whole stem at rotation age as the wooden material underlying the objective traits of this study was already fully developed. In summary, given the breeding objective of this study, assessments and selection for wood density and grain angle that coincide with that of growth traits (~ 10 years) would not only be a possible strategy, but would be 39–76% more time-efficient in comparison with performing direct assessments of breeding objective traits at a later age (Fig. 6).
Apart from determining the optimal tree age (or annual ring) for the genetic evaluation of density and grain angle, we also estimated the heritability for static board stiffness (sMoE) and strength (MoR). This contrasts somewhat to the previous study where board stiffness was only tested dynamically (dMoE) by the acoustic resonance frequency (Högberg et al. 2014). The boards exhibited numerous knots and other grain disturbances that could impair stiffness and strength. There were concerns that a dMoE assessment would be unable to account for such irregularities. Nevertheless, heritability and genetic correlation estimates for sMoE and MoR, assessed by bending (Tables 1, 2) were largely similar to the corresponding parameters previously estimated for dMoE and the additive genetic correlation between dMoE and sMoE was very close to one (0.99 ± 0.04). Although some of the branch traits (BNO11 and BRA11) did appear to exert a genetic influence over sMoE and MoR, this did not diminish the much stronger relationship of PIL18 to sMoE and MoR (Table 2). The negative genetic correlations between growth traits (in particular DBH18) and board density, stiffness and strength were again observed as in many studies of Norway spruce (Gräns et al. 2009; Chen et al. 2014, 2015; Steffenrem et al. 2016), suggesting that index selection and economic weights are needed to properly optimize overall gains of economic value.
One limitation of this study was that only a single field trial (environment) could be investigated. This excludes the possibility of accounting for genotype-by-environment (G × E) interactions and heritabilities estimated in this study could therefore be biased upwards. Nonetheless, for wood density, MoE and grain angle, such biases are still expected to be minor because other studies of Norway spruce have indicated only weak G × E-interactions (ra across sites estimated at 0.74 or above, Hallingbäck et al. 2008; Chen et al. 2014). The foremost complication, however, was the difference in sampling height between the wood trait assessments made in the field at breast height (1.3 m) and the radial profiles extracted from top of the butt log (at 3.3 m). In order to obtain selection efficiency estimates, it was assumed that the trends observed at 3.3 m height would be largely equivalent to unobserved trends lower in the stem at the same range of cambial ages, e.g. at breast height which is routinely used for field assessments. The most likely impact of the difference in assessment heights would be an underestimation of the genetic correlations in comparison with the case where the radial profile and sawn timber tissues coincided. But neither radial profile-based estimates of heritability for the wood traits (grain angle and density) nor their additive genetic correlations with other traits differed much from those breast height assessments that were available (PIL18 and GA28, Figs. 3, 4). Additionally, it should be noted that genetic correlations between the radial profile wood traits and their corresponding objective sawn timber traits were, with few exceptions, very high in spite of the lack of physical overlap between the actual sawn wood and the radial profiles. Therefore, our assumptions about sample height having negligible impact on the wood trait results should be reasonable.
Conclusions
This study has found it possible to make substantial genetic gains in mid-rotation board shape stability, density, stiffness and strength by indirect selection based on grain angle and density measurements (e.g. by pilodyn) on standing trees at an age range of 10–16 years. This raises the possibility of combining the assessments and selection of growth and important wood quality traits in a time-efficient manner. However, negative and adverse genetic correlations between growth and these sawn timber traits, in particular between DBH, and density, stiffness and strength pose a challenge for breeders trying to make simultaneous genetic gains.
References
Andersson B, Elfving B, Persson T, Ericsson T, Kroon J (2007) Characteristics and development of improved Pinus sylvestris in northern Sweden. Can J For Res 37:84–92. https://doi.org/10.1139/X06-224
Anon (2010) EN 408 Timber structures—Structural timber and glued laminated timber—determination of some physical and mechanical properties. European Committee for Standardization
Auty D, Achim A (2008) The relationship between standing tree acoustic assessment and timber quality in Scots pine and the practical implications for assessing timber quality from naturally generated stands. Forestry 81(4):475–487. https://doi.org/10.1093/forestry/cpn015
Beaulieu J, Zhang SY, Yu Q, Rainville A (2006) Comparison between genetic and environmental influences on lumber bending properties in young white spruce. Wood Fiber Sci 38(3):553–564
Bergsten U, Lindeberg J, Rindby A, Evans R (2001) Batch measurements of wood density on intact or prepared drill cores using x-ray microdensitometry. Wood Sci Technol 35(5):435–452. https://doi.org/10.1007/s002260100106
Chen Z-Q, García Gil MR, Karlsson B, Lundqvist S-O, Olsson L, Wu HX (2014) Inheritance of growth and solid wood quality traits in a large Norway spruce population tested at two locations in southern Sweden. Tree Genet Genomes 10:1291–1303. https://doi.org/10.1007/s11295-014-0761-x
Chen Z-Q, Karlsson B, Lundqvist S-O, García Gil MR, Olsson L, Wu HX (2015) Estimating solid wood properties using Pilodyn and acoustic velocity on standing trees of Norway spruce. Ann For Sci 72:499–508. https://doi.org/10.1007/s13595-015-0458-9
Cherry ML, Vikram V, Briggs D, Cress DW, Howe GT (2008) Genetic variation in direct and indirect measures of wood stiffness in coastal Douglas-fir. Can J For Res 38(9):2476–2486. https://doi.org/10.1139/X08-087
Cown DJ (1978) Comparison of the pilodyn and torsiometer methods for the rapid assessment of wood density in living trees. N Z J For Sci 8(3):384–391
Cown DJ, Ball RD, Riddell MJC (2004) Wood density and microfibril angle in 10 Pinus radiata clones: distribution and influence on product performance. N Z J For Sci 34(3):293–315
Falconer DS, Mackay TFC (1996) Introduction to quantitative genetics, 4th edn. Pearson, Prentice Hall, Harlow
Fujimoto T, Akutsu H, Nei M, Kita K, Kuromaru M, Oda K (2006) Genetic variation in wood stiffness and strength properties of hybrid larch (Larix gmelinii var. japonica × L. kaempferi). J For Res 11(5):343–349. https://doi.org/10.1007/s10310-006-0221-z
Gilmour AR, Gogel BJ, Cullis BR, Thompson R (2009) ASReml user guide, 3rd edn. VSN International Ltd, Hemel Hempstead
Gräns D, Hannrup B, Isik F, Lundqvist S-O, McKeand SE (2009) Genetic variation and relationships to growth traits for microfibril angle, wood density and modulus of elasticity in a Picea abies clonal trial in southern Sweden. Scand J For Res 24:494–503. https://doi.org/10.1080/02827580903280061
Haapanen M, Hynynen J, Ruotsalainen S, Kilpeläinen M-L (2016) Realised and projected gains in growth, quality and simulated yield of genetically improved Scots pine in southern Finland. Eur J For Res 135(6):997–1009. https://doi.org/10.1007/s10341-016-0989-0
Hailwood AJ, Horrobin S (1946) Absorption of water by polymers: analysis in terms of a simple model. Trans Faraday Soc 42:B084–B092. https://doi.org/10.1039/TF946420B084
Hallingbäck HR, Jansson G, Hannrup B (2008) Genetic parameters for grain angle in 28-year-old Norway spruce progeny trials and their parent seed orchard. Ann For Sci 65:301p1–301p8. https://doi.org/10.1051/forest:2008005
Hallingbäck HR, Jansson G, Hannrup B (2010) Genetic correlations between spiral grain and growth and quality traits in Picea abies. Can J For Res 40(2):173–183. https://doi.org/10.1139/X09-173
Hannrup B, Säll H, Jansson G (2003) Genetic parameters for spiral grain in Scots pine and Norway spruce. Silvae Genet 52(5–6):215–220
Harding KJ, Copley TR, Peters RF, Dieters MJ, Nester MR, Keys MG, Toon PG (2008) Selecting hybrid pine clones for deployment—the pointy end of wood quality improvement. N Z J For Sci 38(1):120–131
Högberg K-A, Hallingbäck HR, Säll H, Johansson M, Jansson G (2014) The potential for the genetic improvement of sawn timber traits in Picea abies. Can J For Res 44:1–8. https://doi.org/10.1139/cjfr-2013-0382
Hong Z, Fries A, Wu HX (2015) Age trend of heritability, genetic correlation, and efficiency of early selection for wood quality traits in Scots pine. Can J For Res 45:817–825. https://doi.org/10.1139/cjfr-2014-0465
Isik F, Li B (2003) Rapid assessment of wood density of live trees using the Resistograph for selection in tree improvement programs. Can J For Res 33:2426–2435. https://doi.org/10.1139/X03-176
Isik K, Kleinschmit J, Steiner W (2010) Age-age correlations and early selection for height in a clonal genetic test of Norway spruce. For Sci 56(2):212–221
Jansson G, Li B, Hannrup B (2003) Time trends in genetic parameters for height and optimal age for parental selection in Scots pine. For Sci 49(5):696–705
Lambeth CC (1980) Juvenile correlation in Pinaceae and implications for early selection. For Sci 26(4):571–580
Lee SJ, Woolliams J, Samuel CJA, Malcolm DC (2002) A study of population variation and inheritance in Sitka spruce. Silvae Genet 51(2–3):55–65
Lenz P, Cloutier A, MacKay J, Beaulieu J (2010) Genetic control of wood properties in Picea glauca—an analysis of trends with cambial age. Can J For Res 40:703–715. https://doi.org/10.1139/X10-014
Lenz P, MacKay J, Rainville A, Cloutier A, Beaulieu J (2011) The influence of cambial age on breeding for wood properties in Picea glauca. Tree Genet Genomes 7:641–653. https://doi.org/10.1007/s11295-011-0364-8
Moore JR, Mochan SJ, Brüchert F, Hapca AI, Ridley-Ellis DJ, Gardiner BA, Lee SJ (2009) Effects of genetics on the wood properties of Sitka spruce growing in the UK: bending strength and stiffness of structural timber. Forestry 82(5):491–501. https://doi.org/10.1093/forestry/cpp018
Mora CR, Schimleck LR, Isik F, Mahon JM, Clark A, Daniels RF (2009) Relationships between acoustic variables and different measures of stiffness in standing trees. Can J For Res 39(8):1421–1429
Säll H (2002) Spiral grain in Norway spruce. Dissertation, Växjö Universitet
Steffenrem A, Kvaalen H, Høibø OA, Edvardsen ØM, Skrøppa T (2009) Genetic variation of wood quality traits and relationships with growth in Picea abies. Scand J For Res 24(1):15–27. https://doi.org/10.1080/02827580802641215
Steffenrem A, Solhem H, Skrøppa T (2016) Genetic parameters for wood quality traits and resistance to the pathogens Heterobasion parviporum and Endoconidiophora polonica in a Norway spruce breeding population. Eur J For Res 135:815–825. https://doi.org/10.1007/s10342-016-0975-6
Tanabe J, Tamura A, Hamanaka M, Ishiguri F, Takashima Y, Ohshima J, Iizuka K, Yokota S (2014) Wood properties and their among-family variations in 10 open-pollinated families of Picea jezoensis. J Wood Sci 60:297–304. https://doi.org/10.1007/s10086-014-1407-1
White TL, Adams WT, Neal DB (2007) Forest genetics. CABI Publishing, CAB International, Wallingford, Oxfordshire
Wu HX, Powell MB, Yang JL, Ivković M, McRae TA (2007) Efficiency of early selection for rotation-aged wood quality traits in radiata pine. Ann For Sci 64:1–9. https://doi.org/10.1051/forest:2006082
Acknowledgements
We thank Kjell Rosén and Maria Lundgren at Asa field station, Unit for Field-based Forest Research at the University of Agricultural Sciences for assessing the grain angle from radial profiles and for their assistance with static bending tests. We also acknowledge Bertil Enquist, Dept of Building Technology at the Linneaus University, for further assistance with the setup of the static bending tests. The research activities underlying this study were financed by two funding agencies: Föreningen Skogsträdsförädling (2014:293) and The Södra Foundation for Research, Development and Education.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Christian Ammer.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hallingbäck, H.R., Högberg, KA., Säll, H. et al. Optimal timing of early genetic selection for sawn timber traits in Picea abies. Eur J Forest Res 137, 553–564 (2018). https://doi.org/10.1007/s10342-018-1123-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10342-018-1123-2