Abstract
Endotoxin is known to cause acute lung injury (ALI). Here, we investigated the effects and mechanisms of recombinant human bone morphogenetic protein-2 (rhBMP-2) on pulmonary arteriole remodeling in endotoxin-induced ALI in rats. Sixty Wistar rats were randomly divided into 3 groups (n = 20). Control group was infused with normal saline. Lipopolysaccharides (LPS) alone group was infused with LPS. LPS plus rhBMP2 group was infused with rhBMP-2 and LPS. Lung tissue was harvested. The tunica media of the pulmonary arterioles was measured. The expression levels of survivin, p21, cyclin D1, and activated caspase-3 were examined. The proliferation and apoptosis of pulmonary artery smooth muscle cells were evaluated. The tunica media of pulmonary arterioles in LPS alone group was significantly thicker than both that in control and that in LPS plus rhBMP2 groups (P < 0.01). The multiplication rate in LPS alone group was also significantly higher than both that in control and that in LPS plus rhBMP2 groups (P < 0.01). The apoptotic rate in LPS alone group was lower than that in LPS plus rhBMP2 group (P < 0.01). Compared with the control and LPS plus rhBMP2 groups, the expression levels of mRNA and proteins of survivin and cyclin D1 were increased in LPS alone group (P < 0.01), while the expression levels of p21 and activated caspase-3 were decreased (P < 0.01). RhBMP-2 inhibits the remodeling of pulmonary arterioles in endotoxin-induced ALI, which is achieved by enhancing apoptosis and inhibiting proliferation of pulmonary artery smooth muscle cells.
Similar content being viewed by others
Introduction
The remodeling of blood vessels refers to the migration and proliferation of smooth muscle cells in the tunica media following endothelial injury. This causes an abnormal accumulation of extracellular matrix. Abnormal proliferation and apoptosis of pulmonary artery smooth muscle cells (PASMC) prompt thickening of the intima and tunica media and result in stricture of the blood vessels.
Bone morphogenetic protein (BMP) belongs to transforming growth factor superfamily and participates in regulating and controlling the generation and formation of bone, cartilage, and embryo. It additionally plays an important role in the regulation and control of the growth, proliferation, apoptosis, migration and differentiation of PASMC and the synthesis of matrix, and consequently regulates the remodeling of pulmonary blood vessels [1]. Endotoxin, which is an intrinsic component of the outer membrane of Gram-negative bacteria, is composed of lipopolysaccharide (LPS) and is known to cause acute lung injury (ALI) [2]. Our previous study has shown that there was remodeling of the pulmonary arteries in LPS-induced ALI [3]. Then, the pulmonary arterial pressure was increased, and respiratory and circulatory failure followed [3].
Recently, it was reported that BMP signaling regulated regeneration of the airways following acute injury [4]. However, the effects of recombinant human bone morphogenetic protein-2 (rhBMP-2), one of important recombinant BMPs and purified BMP extracts, have not yet been known on the remodeling of pulmonary arteries after LPS-induced ALI. This study was designed to observe the effects of rhBMP-2 on LPS-induced remodeling of pulmonary arteries in rats with ALI and further explore the possible mechanisms.
Materials and methods
Experimental animals and design
Sixty Wistar rats (body weight 220–260 g, male and female) were obtained from the Center for Experimental Animals at China Medical University (Shenyang, China), with a National Animal Use License number of LN-034. All experiments and surgical procedures were approved by the Institutional Animal Care and Use Committee at China Medical University, which complied with the National Institute of Health Guide for the Care and Use of Laboratory Animals, and all efforts were made to minimize animal suffering. Animals were randomly divided into 3 groups (n = 20): control group (group C), LPS group (group L), and rhBMP-2 + LPS group (group R). Anesthesia was induced by intraperitoneal injection of 50 mg/kg of 1 % pentobarbital sodium. In group C, 5 ml of normal saline was infused into the femoral vein using a micro pump in 1.5 h. In group L, 3 ml of normal saline (NS) was infused into the femoral vein in 1 h, and then, 1 mg/kg of LPS (055: B5, Sigma Company, USA, dissolved in 2 ml NS) was infused in 30 min. After successful model preparation, the rats were observed for signs of ALI. Appreciable signs of ALI included: gray and violet lips, followed by listlessness and erect hair after reviving from anesthesia. In group R, 10 μg/kg of rhBMP-2 (production code number: 120–02, Shanghai Zewei Biological Company, dissolved in 3 ml of 0.9 % NS) was infused into the femoral vein in 1 h, and LPS was infused as above. At 24 and 48 h after infusion of LPS, 4 μg/kg of rhBMP-2 was injected separately via the indwelling venous catheter. All the rats were killed 72 h after the infusion of LPS or NS. The lung was harvested for pathology.
Histology
The tissue of the superior lobe of the right lung (3 mm × 3 mm × 3 mm) was put into 4 % polyoxymethylene and fixed for 24 h. Paraffin embedding and sectioning were performed. Five-micrometer-thick sections from the level of the porta of lung were prepared for each specimen. Hematoxylin and eosin (H&E) staining was performed, followed by observation and photography. The micro image analysis system (Olympus Company, Japan) was used to measure the thickness of the media in the pulmonary arterioles with outside diameters between 65 and 75 μm in each section. Only pulmonary arterioles accompanying respiratory bronchi were chosen. The sections perpendicular to the long axis of blood vessels were then selected, and 8 values were measured around the circle (one value was read at each 45° angle around the circle). The mean value was calculated. This was repeated for all 5 sections for each case. The percentage of the thickness of the media was then calculated (thickness of media 2/outside diameter of blood vessels × 100 %). This value is the indicator for the degree of the thickening of the media of pulmonary arterioles.
Proliferation and apoptosis of pulmonary arterioles
The proliferation of pulmonary arterioles was determined by immunofluorescence staining of proliferating cell nuclear antigen (PCNA). In detail, the lung tissues were first fixed with 4 % polyoxymethylene. Five-micrometer-thick sections were prepared for each case from the level of porta of lung. Fluorescent staining of the paraffin sections was performed according to the manufacturer’s instructions. Monoclonal antibodies and FITC-conjugated biotinylated goat anti-rabbit IgG (Wuhan Boster Biological Technology Co., Ltd.) were used to label the proliferative cell nuclear antigens. The average value of the 5 sections was calculated. Then, observation and photography were performed using a fluorescence microscope at 400 × magnification. Proliferation index was determined by the following formula: number of PCNA immunoreactive cells/number of the cells observed × 100 %.
The TUNEL kit was used to detect the apoptosis of PASMC in pulmonary arterioles according to the manufacturer’s instructions (Boehringer Mannheim, Germany). Specimens partially degraded with DNase were used to prepare positive control sections. Negative controls were prepared by performing the same steps as the experimental group, except without the addition of TdT enzyme. The cross sections of 5 blood vessels were chosen in each slide to calculate apoptotic index of the PASMC. Apoptosis index was determined by the following formula: number of apoptotic cells/number of the cells observed × 100 %.
RT-PCR
Tissue homogenate of the lung was harvested for RT-PCR test. RNA was extracted using Trireagent kit [Takara Biotechnology (Dalian) Co., Ltd.] through one-step method. The purity and concentration of the RNA were measured at 260/280 nm. Oligotex mRNA Purification Kit (Qiagen Company, USA) was used to purify RNA, yielding PolyA mRNA. Ten-U DNaseI was used to avoid potential pollution of genome DNA before synthesis of cDNA. The primer was 275 ng Oligo (dT) 30, and the reverse transcriptase was 200 u Moloney Murine Leukemia Virus. The reaction system was incubated for 50 min at 42 °C and then 5 min at 95 °C for inactivation of the reverse transcriptase. The cDNA was stored at −20 °C for future use.
The primers for survivin, p21, and cyclin D1 were synthesized by Union Star (Dalian) Bioengineering Company. The sequences of the primers and the length of the corresponding product were listed in the Table 1. Β-actin was used as the internal reference. The integrated density value (IDV) of each band was measured using the Gel Imaging System (KADAKID, USA). The ratio of target band and internal reference was used for statistical analysis.
Immunoblotting
Lung tissues were homogenized. The protein concentration was measured using the Coomassie Brilliant Blue method. Proteins were separated by SDS–PAGE. Twenty-five microliters of loading solution including 50 μg of protein extract was loaded. Electrophoresis was performed in a 10 % SDS–PAGE bath at 150 V for 1 h, followed by 50 V with 100 mA for 1.5 h. Then, the proteins were transferred to nitrocellulose membranes. The membranes were blocked with TBS containing 5 % of evaporated skim milk overnight and probed with a primary antibody, in the blocking buffer, then incubated with the appropriate secondary antibodies (goat anti-rabbit IgG, Wuhan Boster Biological Technology Co., Ltd.). Membrane was also incubated with anti-β-actin antibodies. The primary antibodies used were monoclonal rabbit anti-survivin, anti-p21, anti-cyclin D1, and anti-caspase-3 (1:400, Wuhan Boster Biological Technology Co., Ltd.).
The bands were visualized by the Automatic Electrophoresis Gel Imaging System (ChemiImager5500, AlPhaInnCh), and the integrated density values were collected by FluorChen V.2.0 system. To control for protein quality and loading, the intensity of each target band was normalized against β-actin in the same sample.
Statistics
The SPSS software (version 12.0, SPSS Inc., Chicago, IL, USA) was used for statistical analysis. Measurement data were expressed as the mean ± standard deviation (SD). One-way analysis of variance was used for comparison among groups. When the F value indicated significance, least-significant difference post hoc comparisons were made as appropriate to correct for multiple comparisons. All P values were two-tailed and P < 0.05 was considered to be statistically significant.
Results
Histological examination
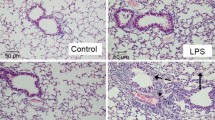
The lung tissues of the rats in group C exhibited an intact structure (Fig. 1a). The alveolar space was clear, and there was no edema in the alveolar septum. The capillary vessels were slightly dilated and there was mild inflammatory cell infiltration. There was no hyperplasia of smooth muscle cells in the media of pulmonary arterioles. The structure of arterial wall was complete, and the surface of intima was smooth and composed of one layer of endothelium. The smooth muscle cells of media were shuttle-shaped and in regular arrangement. There was no constriction of the lumen.
Changes of lung morphology in different groups. Representative images of H&E staining of lung tissues in group C (a), group L (b), and group R (c). The thickness of media in the pulmonary arterioles (labeled with arrow) in group L was obviously higher than that in group C and group R. Scale bar = 10 μm in (a)
In group L (Fig. 1b), there were increased exudates, congestion in pulmonary alveolus, and collapse, rupture and fusion of the pulmonary alveoli. The thickness of the alveolar septum was increased. The number of inflammatory cells, monocytes, and neutrophils in the lung tissues was increased. There was endotheliocytic swelling and necrosis in the pulmonary arterioles. In addition, there was rupture in the elastic fibers of the tunica media and loss of tunica intima. The intima was ruptured and new intima formed, some of the new intima protruded into the lumen. The shape of the new smooth muscle cells was irregular and disordered. Hyperplasia of smooth muscle cells in the media of pulmonary arterioles was observed and the thickness of the media was increased. This resulted in stricture and even occlusion of the lumen of the blood vessels.
In group R (Fig. 1c), there was inflammatory cell infiltration around the blood vessels. Telangiectasias and hemorrhage into alveolar spaces were decreased. The impairment of the intima of the pulmonary arterioles was alleviated, and the inflammatory cell infiltration reduced compared with group L. The thickness of the tunica media was decreased when compared with group L.
Measurement of the tunica media of the pulmonary arterioles
The percentage thickness of the tunica media in the pulmonary arterioles in group L was significantly higher than that in group C and group R (P < 0.01). However, there was no significant difference in the thickness of the tunica media in the pulmonary arterioles between group R and group C (P > 0.05) (Table 2).
Proliferation and apoptosis of PASMC
The multiplication rate of PASMC in the tunica media of pulmonary arterioles in group L was significantly increased than that of group C and group R (P < 0.01), but the apoptotic rate of group L was lower than that of group R (P < 0.01). However, there was no significant difference in the multiplication rate and apoptotic rate between group R and group C (P > 0.05) (Table 2).
The expression levels of survivin, p21 and cyclin D1 and activated caspase-3
To further characterize the molecular difference between the groups, markers of cell cycle regulation, such as cyclin D1 and p21, were analyzed, along with apoptosis markers, such as survivin and active caspase-3. In group L, the expression levels of mRNA and proteins of survivin and cyclin D1 increased (P < 0.01), while the expression levels of p21 and activated caspase-3 decreased (P < 0.01) compared with group C and group R. There was no significant difference in the expression levels of proteins and mRNA of survivin, cyclin D1, p21, and activated caspase-3 between group R and group C (P > 0.05) (Figs. 2, 3).
Expression levels of survivin mRNA, cyclin D1 mRNA, and P21 mRNA in different groups. C, group C; L, group L; R, group R. a The representative expression levels of survivin mRNA, cyclin D1 mRNA, and P21 mRNA determined by RT-PCR in group C, group L, and group R. b The bar graph is the results of semiquantitative measurement of survivin mRNA, cyclin D1 mRNA, and P21 mRNA. The height of each bar represents the mean ± SD of group averages. * Compared with group C, P < 0.01; # compared with group L, P < 0.01
Expression levels of survivin, cyclin D1, P21, and caspase-3 in different groups. C, group C; L, group L; R, group R. a The representative expression levels of survivin, cyclin D1, P21, and caspase-3 determined by immunoblotting in group C, group L, and group R. b The bar graph is the results of semiquantitative measurement of survivin, cyclin D1, P21, and caspase-3. The height of each bar represents the mean ± SD of group averages. * Compared with group C, P < 0.01; # compared with group L, P < 0.01
Discussion
ALI is a syndrome consisting of acute hypoxemic respiratory failure with bilateral pulmonary infiltrates that is associated with both pulmonary and nonpulmonary risk factors and that is not primarily due to left atrial hypertension [5]. Acute lung injury, or its severe form, acute respiratory distress syndrome, is still a major cause of morbidity and mortality in children [6]. Despite current clinical and experimental research on the treatment of lung injury is aimed at inhibiting different stages of ALI with drugs or therapy, the outcome has, however, not been improved significantly. Mortality in ALI is still as high as 18–27 %, and the mortality rate of acute respiratory distress syndrome is even higher, which is 29–50 % [7]. Therefore, clarified potential mechanism is needed to improve the treatment and minimize the mortality associated with ALI.
The administration route and dose of LPS to induce acute lung injury model vary with the requirement and design of individual experiment [8]. In our study, a small dose at a constant rate infusion method was used to prepare the acute lung injury models and obtained a 72 h survival rate of 100 % in rats. The pathological results of group L are characterized by disruption of the alveolar–capillary interface, widespread capillary leakage into the interstitium and alveolar space, and extensive release of cytokines and migration of neutrophils. It has been indicated that the acute lung injury was induced successfully in our experiments. In addition, it was reported that injection with normal saline solution alone could elicit a marked inflammatory response, manifested by increased leukocyte count [9]. This inflammatory response came to normal until 7 days after injection [9]. Our results were in agreement with the experimental observation. There were a fairly pronounced hypercellularity and mild inflammatory cell infiltration in the control group.
In the present study, the administration time and dose of rhBMP-2 were determined based on its half-life [10]. Our findings indicated that rhBMP-2 alleviated the inflammatory responses during LPS-induced acute lung injury. Our previous study showed that the proliferation occurred obviously at 24 h, if PASMC was cultured in an endotoxin-endothelial cell culture media [3]. Therefore, tissues were harvested at 72 h in order to assure that significant remodeling of the pulmonary blood vessels would be detected.
The results from PCNA immunostaining and TUNEL staining showed that the proliferation of PASMC was significantly inhibited and the number of apoptotic cells was increased after rhBMP-2 treatment, indicating that rhBMP-2 might prevent the increase of thickness of the tunica media of pulmonary arterioles and the remodeling of pulmonary blood vessels after ALI by affecting the equilibrium of the proliferation and apoptosis of PASMC. It was reported that rhBMP-2 could combine with BMP Type II receptor (BMPR-II) and form a heterodimer with BMPR I receptor, then activated Type I receptor kinase field. The kinase field can activate the phosphorylation of intracytoplasmic signal transducin (R-Smad: Smad-1,5). These Smad proteins bind with common Smad-4 (Co-Smad), form a complex, and are transported into the nuclei. Once in the nuclei, this complex can directly regulate and control the genetic transcription at the target location [10, 11]. Previous studies showed that BMP-2 could inhibit the proliferation of PASMC, facilitate apoptosis, and maintain the normal structural form of pulmonary arteries [12–14].
Survivin is a member of the inhibitor of apoptosis proteins family. It is overexpressed in tumor cells, but not expressed in most of normal adult cells (including PASMC). It has been shown that in arterial impairment model and the PASMC cultured in high concentration of serum, the expression of survivin can be significantly higher, then it inhibits apoptosis and facilitates the remodeling of blood vessels [15]. The anti-apoptosis effects of survivin are realized by suppressing the release of cytochrome C from mitochondria and reducing the activation of caspase-3. Caspase-3 is a type of endonuclease, and the hydrolyzed fragments of caspase-3 have the activity of endonuclease. The decreased activation of caspase-3 inhibits the apoptosis of cells. As the most important positive regulatory gene for the regulation and control of G1 phase, its gene expression products appear temporarily in specific phases in normal cell cycle. The half-life of caspase-3 is about 20 min. It is often negative or weakly positive in normal tissues. The overexpression of caspase-3 may result in the abnormal regulation and control on cell cycle, leading to abnormal cell proliferation, and even tumors [16]. P21 is a member of cyclin-dependent kinase inhibitors, the important regulating and controlling factors for cell cycle. It can prevent cells from entering S phase and suppress the proliferation of cells by inhibiting the activity of cyclin-dependent kinase or inhibiting the binding of cyclin-dependent kinase with cyclin D1. Our results showed that as compared to the group C and group R, the expression levels of survivin and cyclin D1 and the proliferation index of PASMC were increased, while the expression levels of activated caspase-3 and the apoptosis index were decreased in group L. There was no significant difference between group R and group C. These results indicated that rhBMP-2 may upregulate p21 and suppress the transcription and expression levels of proteins survivin and cyclin D1, resulting in the overexpression of caspase-3 which can inhibit the proliferation of PASMC and facilitate its apoptosis.
Conclusions
After rhBMP-2 administration, inflammatory cell infiltration around the blood vessels, and telangiectasias and hemorrhage in the alveolar spaces were significantly decreased. The injury to the intima of the pulmonary arterioles was alleviated, and the inflammatory cell infiltration was decreased when compared with group L, indicating that rhBMP-2 can prevent the remodeling of pulmonary artery in LPS-induced acute lung injury. The mechanisms may involve the upregulation of p21 protein by activating caspase-3, resulting in the inhibition of the expression levels of survivin and cyclin D1 proteins, which can facilitate the apoptosis of PASMC and restrain its proliferation.
References
Rudarakanchana N, Trembath RC, Morrell NW (2001) New insights into the pathogenesis and treatment of primary pulmonary hypertension. Thorax 56:888–890
Stephens KE, Ishizaka A, Larrick JW, Raffin TA (1988) Tumor necrosis factor causes increased pulmonary permeability and edema. Comparison to septic acute lung injury. Am Rev Respir Dis 137:1364–1370
Pei L, Wang J, Fu W, Sheng ZR (2000) Effects of stress reactions on the plasma TNF-α concentration, oxygen partial pressure in blood and pulmonary arterial pressure in the rats with infectious shock. Nat Med J China 80:66–67
Masterson JC, Molloy EL, Gilbert JL, McCormack N, Adams A, O’Dea S (2011) Bone morphogenetic protein signalling in airway epithelial cells during regeneration. Cell Signal 23:398–406
Ware LB, Matthay MA (2000) The acute respiratory distress syndrome. N Engl J Med 342:1334–1349
Kneyber MC, Markhorst DG (2009) Management of acute lung injury and acute respiratory distress syndrome in children: a different perspective. Crit Care Med 37:3192–3193
Wheeler AP, Bernard GR (2007) Acute lung injury and the acute respiratory distress syndrome: a clinical review. Lancet 369:1553–1564
Ma H, Zhu L, Liu Q, Zhang Z (2003) Discussion on the animal model of endotoxin shock. J Emerg Tradit Chin Med 12:260–261
Wagner AE, McIlwraith CW, Martin GS (1982) Effect of intra-articular injection of orgotein and saline solution on equine synovia. Am J Vet Res 43:594–597
Lefer AM, Tsao PS, Ma XL, Sampath TK (1992) Anti-ischaemic and endothelial protective actions of recombinant human osteogenic protein (hOP-1). J Mol Cell Cardiol 24:585–593
Morrell NW, Yang X, Upton PD, Jourdan KB, Morgan N, Sheares KK, Trembath RC (2001) Altered growth responses of pulmonary artery smooth muscle cells from patients with primary pulmonary hypertension to transforming growth factor-beta(1) and bone morphogenetic proteins. Circulation 104:790–795
Zhang S, Fantozzi I, Tigno DD, Yi ES, Platoshyn O, Thistlethwaite PA, Kriett JM, Yung G, Rubin LJ, Yuan JX (2003) Bone morphogenetic proteins induce apoptosis in human pulmonary vascular smooth muscle cells. Am J Physiol Lung Cell Mol Physiol 285:L740–L754
Brindle NP, Saharinen P, Alitalo K (2006) Signaling and functions of angiopoietin-1 in vascular protection. Circ Res 98:1014–1023
Wong WK, Knowles JA, Morse JH (2005) Bone morphogenetic protein receptor type II c-terminus interacts with c-Src: implication for a role in pulmonary arterial hypertension. Am J Respir Cell Mol Biol 33:438–446
Dohi T, Beltrami E, Wall NR, Plllescia J, Altieri DC (2004) Mitochondrial survivin inhibits apoptosis and promotes tumorigenesis. J Clin Invest 114:1117–1127
Marx J (1994) How cells cycle toward cancer. Science 263:319–321
Acknowledgments
The authors are very grateful for the excellent theoretical and technical assistance from the staff of JianWen Center for Medical Research and Advisory (Shenyang, China).
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Kunpeng Wang and Jian Gong equally contributed to this manuscript.
Rights and permissions
About this article
Cite this article
Wang, K., Gong, J., Pei, L. et al. The effect of rhBMP-2 on pulmonary arterioles remodeling in endotoxin-induced acute lung injury in rats. Clin Exp Med 13, 187–192 (2013). https://doi.org/10.1007/s10238-012-0197-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10238-012-0197-2