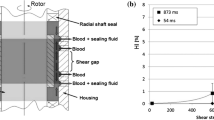
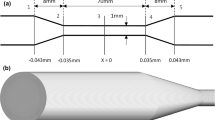
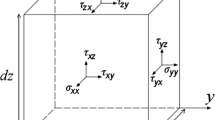
Abstract
Despite decades of research related to hemolysis, the accuracy of prediction algorithms for complex flows leaves much to be desired. Fundamental questions remain about how different types of fluid stresses translate to red cell membrane failure. While cellular- and molecular-level simulations hold promise, spatial resolution to such small scales is computationally intensive. This review summarizes approaches to continuum-level modeling of hemolysis, a method that is likely to be useful well into the future for design of typical cardiovascular devices. Weaknesses are revealed for the Eulerian method of hemolysis prediction and for the linearized damage function. Wide variations in scaling of red cell membrane tension are demonstrated with different types of fluid stresses when the scalar fluid stress is the same, as well as when the energy dissipation rate is the same. New experimental data are needed for red cell damage in simple flows with controlled levels of different types of stresses, including laminar shear, laminar extension (normal), turbulent shear, and turbulent extension. Such data can be curve-fit to create more universal continuum-level models and can serve to validate numerical simulations.


[adopted from Lux and Palek (1995)]















Similar content being viewed by others
Notes
Technically, the distinction between turbulent and laminar flow depends on the stability of the flow. Turbulent flow is unstable to minor disturbances, which produce apparently chaotic motion that is self-perpetuating. “Apparently” is used because turbulent flow is not entirely chaotic. Rather, it can involve coherent structures that evolve with time, for instance, hairpin vortices in flow over flat surfaces, and structures that are not truly steady, but have characteristics with steady, long-term means, such as streaks in turbulent Couette flow. Turbulent flow is inherently unsteady, even though statistically steady means exist, for instance, mean velocity profiles in pipe flow. It is tempting to contrast laminar flow as steady, but this is also not true. Oscillatory (Womersley) flow in a pipe is an example of flow that is unsteady and laminar for certain conditions. While Womersley flow is driven by an oscillatory pressure gradient, unsteadiness can be triggered spontaneously in laminar flow that is steady upstream, for instance, the oscillating Karman vortex street downstream of a cylinder. Neither is laminar flow strictly stable. Laminar flow can switch between multiple states. For instance, for a range of divergence angles, the (bistable) jet in a diverging channel can be attached to either wall. The key feature that identifies turbulent flow is instability that results in velocity and fluid stress fluctuations across a spectrum of length and frequency scales that laminar flows lack. Laminar velocity fluctuations behind a prosthetic valve, for instance, begin decaying immediately downstream, but turbulent fluctuations are continuously reenergized. For steady state, turbulence production equals dissipation. The instantaneous fluctuations of velocity are largely unpredictable, but can be simulated by direct numerical simulation (DNS). The large computational cost of DNS typically leads to the use of statistical measures (e.g., Reynolds decomposition, see Sect. 3.2) to solve turbulent flow problems.
The Deborah number is a ratio of the time constant of the breakup/reformation of the cell layer to the unsteady time constant for the flow, for instance, the time period of oscillatory flow. The Deborah number determines whether the structure reforms within the oscillatory time period. The Weissenberg number is the same time constant for the cell structure divided by a steady time constant for the flow, which is typically a characteristic length divided by the fluid velocity. The characteristic length may be the longitudinal distance between features that substantially change the fluid velocity in a complex geometry, such as the distance between peaks of a wavy wall, or may be the transverse distance in a simple geometry, e.g., the radius in a long straight pipe. In the first case, the Weissenberg number determines whether the structure has time to reform between peaks. In the second case, the characteristic time is the time it takes the flow to move longitudinally a distance equal to the radius, and the Weissenberg number determines how the entrance length for cell layer formation compares to the radius.
References
Abkarian M, Viallat A (2008) Vesicles and red blood cells in shear flow. Soft Matter 4:653–657. https://doi.org/10.1039/B716612E
Abkarian M, Faivre M, Viallat A (2007) Swinging of red blood cells under shear flow. Phys Rev Lett 98:188302
AbouRjaili G, Torbey E, Alsaghir T, Olkovski Y, Costantino T (2012) Hemolytic anemia following mitral valve repair: a case presentation and literature review. Exp Clin Cardiol 17:248–250
Adili N, Melizi M, Belabbas H, Achouri A (2014) Preliminary study of the influence of red blood cells size on the determinism of the breed in cattle. Vet Med Int 2014:429495. https://doi.org/10.1155/2014/429495
Akimov SA, Volynsky PE, Galimzyanov TR, Kuzmin PI, Pavlov KV, Batishchev OV (2017) Pore formation in lipid membrane II: energy landscape under external stress. Sci Rep 7:12509. https://doi.org/10.1038/s41598-017-12749-x
Alemu Y, Bluestein D (2007) Flow-induced platelet activation and damage accumulation in a mechanical heart valve: numerical studies. Artif Organs 31:677–688. https://doi.org/10.1111/j.1525-1594.2007.00446.x
Andersen MN, Gabrieli E, Zizzi JA (1965) Chronic hemolysis in patients with ball-valve prostheses. J Thorac Cardiovasc Surg 50:501–510
Antiga L, Steinman DA (2009) Rethinking turbulence in blood. Biorheology 46:77–81. https://doi.org/10.3233/bir-2009-0538
Arora D, Behr M, Pasquali M (2004) A tensor-based measure for estimating blood damage. Artif Organs 28:1002–1015. https://doi.org/10.1111/j.1525-1594.2004.00072.x
Arwatz G, Smits AJ (2013) A viscoelastic model of shear-induced hemolysis in laminar flow. Biorheology 50:45–55. https://doi.org/10.3233/bir-130626
Bae YB, Jang HK, Shin TH, Phukan G, Tran TT, Lee G, Hwang WR, Kim JM (2015) Microfluidic assessment of mechanical cell damage by extensional stress. Lab Chip 16:96–103. https://doi.org/10.1039/c5lc01006c
Baldwin JT, Deutsch S, Geselowitz DB, Tarbell JM (1990) Estimation of Reynolds stresses within the Penn State left ventricular assist device. ASAIO Trans 36:M274–M278
Baldwin JT, Deutsch S, Petrie HL, Tarbell JM (1993) Determination of principal Reynolds stresses in pulsatile flows after elliptical filtering of discrete velocity measurements. J Biomech Eng 115:396–403. https://doi.org/10.1115/1.2895503
Barbaro V, Grigioni M, Daniele C, D’Avenio G, Boccanera G (1997a) 19 mm sized bileaflet valve prostheses’ flow field investigated by bidimensional laser Doppler anemometry (part I: velocity profiles). Int J Artif Organs 20:622–628
Barbaro V, Grigioni M, Daniele C, D’Avenio G, Boccanera G (1997b) 19 mm sized bileaflet valve prostheses’ flow field investigated by bidimensional laser Doppler anemometry (part II: maximum turbulent shear stresses). Int J Artif Organs 20:629–636
Barns S, Balanant MA, Sauret E, Flower R, Saha S, Gu Y (2017) Investigation of red blood cell mechanical properties using AFM indentation and coarse-grained particle method. Biomed Eng Online 16:140. https://doi.org/10.1186/s12938-017-0429-5
Barthès-Biesel D, Rallison JM (1981) The time-dependent deformation of a capsule freely suspended in a linear shear flow. J Fluid Mech 113:251–267. https://doi.org/10.1017/S0022112081003480
Baskurt OK (1996) Deformability of red blood cells from different species studied by resistive pulse shape analysis technique. Biorheology 33:169–179. https://doi.org/10.1016/0006-355X(96)00014-5
Baskurt OK, Meiselman HJ (2013) Comparative hemorheology. Clin Hemorheol Microcirc 53:61–70. https://doi.org/10.3233/ch-2012-1576
Becker SM, Kuznetsov AV (2015) Heat transfer and fluid flow in biological processes. Academic Press, Raleigh
Bento D, Rodrigues R, Faustino V, Pinho D, Fernandes C, Pereira A, Garcia V, Miranda J, Lima R (2018) Deformation of red blood cells, air bubbles, and droplets in microfluidic devices: flow visualizations and measurements. Micromachines 9:151
Blackshear PLJ, Dorman FD, Steinbach JH, Maybach EJ, Singh A, Collingham RE (1966) Shear, wall interaction and hemolysis. ASAIO J 12:113–120
Bludszuweit C (1995a) Model for a general mechanical blood damage prediction. Artif Organs 19:583–589
Bludszuweit C (1995b) Three-dimensional numerical prediction of stress loading of blood particles in a centrifugal pump. Artif Organs 19:590–596
Bluestein M, Mockros LF (1969) Hemolytic effects of energy dissipation in flowing blood. Med Biol Eng 7:1–16. https://doi.org/10.1007/bf02474665
Boal D (2012) Mechanics of the cell. Cambridge University Press, Cambridge
Boal DH, Seifert U, Zilker A (1992) Dual network model for red blood cell membranes. Phys Rev Lett 69:3405–3408
Boehning F, Mejia T, Schmitz-Rode T, Steinseifer U (2014) Hemolysis in a laminar flow-through Couette shearing device: an experimental study. Artif Organs 38:761–765. https://doi.org/10.1111/aor.12328
Boey SK, Boal DH, Discher DE (1998) Simulations of the erythrocyte cytoskeleton at large deformation. I. Microscopic models. Biophys J 75:1573–1583. https://doi.org/10.1016/s0006-3495(98)74075-5
Brodeur MT, Sutherland DW, Koler RD, Starr A, Kimsey JA, Griswold HE (1965) Red blood cell survival in patients with aortic valvular disease and ball-valve prostheses. Circulation 32:570–581. https://doi.org/10.1161/01.cir.32.4.570
Brown CHI, Lemuth RF, Hellums JD, Leverett LB, Alfrey CP (1975) Response of human platelets to shear stress. ASAIO J 21:35–39
Burton AC (1972) Physiology and biophysics of the circulation; an introductory text. Year Book Medical Publishers, Chicago
Burton NM, Bruce LJ (2011) Modelling the structure of the red cell membrane. Biochem Cell Biol 89:200–215. https://doi.org/10.1139/o10-154
Buys AV, Van Rooy M-J, Soma P, Van Papendorp D, Lipinski B, Pretorius E (2013) Changes in red blood cell membrane structure in type 2 diabetes: a scanning electron and atomic force microscopy study. Cardiovasc Diabetol 12:25. https://doi.org/10.1186/1475-2840-12-25
Cardenas N, Mohanty SK (2012) Investigation of shape memory of red blood cells using optical tweezers and quantitative phase microscopy. Proc SPIE. https://doi.org/10.1117/12.909758
Cardoso C, Cachado P, Garcia T (2013) Hemolytic anemia after mitral valve repair: a case report. BMC Res Notes 6:165. https://doi.org/10.1186/1756-0500-6-165
Çengel YA, Cimbala JM (2006) Fluid mechanics: fundamentals and applications. McGraw-Hill, Boston
Chen Y, Sharp MK (2011) A strain-based flow-induced hemolysis prediction model calibrated by in vitro erythrocyte deformation measurements. Artif Organs 35:145–156. https://doi.org/10.1111/j.1525-1594.2010.01050.x
Chen Y, Kent TL, Sharp MK (2013) Testing of models of flow-induced hemolysis in blood flow through hypodermic needles. Artif Organs 37:256–266. https://doi.org/10.1111/j.1525-1594.2012.01569.x
Coupier G, Kaoui B, Podgorski T, Misbah C (2008) Noninertial lateral migration of vesicles in bounded Poiseuille flow. Phys Fluids 20:111702. https://doi.org/10.1063/1.3023159
Cowger JA, Romano MA, Shah P, Shah N, Mehta V, Haft JW, Aaronson KD, Pagani FD (2014) Hemolysis: a harbinger of adverse outcome after left ventricular assist device implant. J Heart Lung Transplant 33:35–43. https://doi.org/10.1016/j.healun.2013.08.021
Cristini V, Kassab GS (2005) Computer modeling of red blood cell rheology in the microcirculation: a brief overview. Ann Biomed Eng 33:1724–1727. https://doi.org/10.1007/s10439-005-8776-y
Da Q, Teruya M, Guchhait P, Teruya J, Olson JS, Cruz MA (2015) Free hemoglobin increases von Willebrand factor-mediated platelet adhesion in vitro: implications for circulatory devices. Blood 126:2338–2341. https://doi.org/10.1182/blood-2015-05-648030
Danker G, Vlahovska PM, Misbah C (2009) Vesicles in Poiseuille flow. Phys Rev Lett 102:148102
Dewitz TS, Hung TC, Martin RR, McIntire LV (1977) Mechanical trauma in leukocytes. J Lab Clin Med 90:728–736
Ding J, Niu S, Chen Z, Zhang T, Griffith BP, Wu ZJ (2015) Shear-induced hemolysis: species differences. Artif Organs 39:795–802. https://doi.org/10.1111/aor.12459
Discher DE, Boal DH, Boey SK (1998) Simulations of the erythrocyte cytoskeleton at large deformation. II. Micropipette aspiration. Biophys J 75:1584–1597
Dooley PN, Quinlan NJ (2009) Effect of eddy length scale on mechanical loading of blood cells in turbulent flow. Ann Biomed Eng 37:2449–2458. https://doi.org/10.1007/s10439-009-9789-8
Down LA, Papavassiliou DV, O’Rear EA (2011) Significance of extensional stresses to red blood cell lysis in a shearing flow. Ann Biomed Eng 39:1632–1642. https://doi.org/10.1007/s10439-011-0262-0
Dupire J, Socol M, Viallat A (2012) Full dynamics of a red blood cell in shear flow. Proc Natl Acad Sci U S A 109:20808–20813. https://doi.org/10.1073/pnas.1210236109
Ellis JT, Wick TM, Yoganathan AP (1998) Prosthesis-induced hemolysis: mechanisms and quantification of shear stress. J Heart Valve Dis 7:376–386
Erickson HP (2009) Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol Proced Online 11:32–51. https://doi.org/10.1007/s12575-009-9008-x
Evans EA, Waugh R, Melnik L (1976) Elastic area compressibility modulus of red cell membrane. Biophys J 16:585–595
Ezzeldin HM, de Tullio MD, Vanella M, Solares SD, Balaras E (2015) A strain-based model for mechanical hemolysis based on a coarse-grained red blood cell model. Ann Biomed Eng 43:1398–1409. https://doi.org/10.1007/s10439-015-1273-z
Faghih MM, Sharp MK (2016) Extending the power-law hemolysis model to complex flows. J Biomech Eng. https://doi.org/10.1115/1.4034786
Faghih MM, Sharp MK (2018a) Characterization of erythrocyte membrane tension for hemolysis prediction in complex flows. Biomech Model Mechanobiol. https://doi.org/10.1007/s10237-017-0995-2
Faghih MM, Sharp MK (2018b) Evaluation of energy dissipation rate as a predictor of mechanical blood damage. Artif Organs 1:1. https://doi.org/10.1111/aor.13418
Faghih MM, Sharp MK (2018c) Solvent-based bonding of PMMAPMMA for microfluidic applications. Microsyst Technol. https://doi.org/10.1007/s00542-018-4266-7
Faghih MM, Sharp MK (2019) On Eulerian versus Lagrangian models of mechanical blood damage and the linearized damage function. J Artif Organs (accepted)
Farinas MI, Garon A, Lacasse D, N’Dri D (2006) Asymptotically consistent numerical approximation of hemolysis. J Biomech Eng 128:688–696. https://doi.org/10.1115/1.2241663
Faustino V, Pinho D, Yaginuma T, Calhelha RC, Kim GM, Arana S, Ferreira ICFR, Oliveira MSN, Lima R (2014) Flow of red blood cells suspensions through hyperbolic microcontractions. In: Lima R, Imai Y, Ishikawa T, Oliveira NMS (eds) Visualization and simulation of complex flows in biomedical engineering. Springer, Dordrecht, pp 151–163. https://doi.org/10.1007/978-94-007-7769-9_9
Fedosov DA, Caswell B, Karniadakis GE (2010) A multiscale red blood cell model with accurate mechanics, rheology, and dynamics. Biophys J 98:2215–2225. https://doi.org/10.1016/j.bpj.2010.02.002
Fedosov DA, Peltomäki M, Gompper G (2014) Deformation and dynamics of red blood cells in flow through cylindrical microchannels. Soft Matter 10:4258–4267. https://doi.org/10.1039/C4SM00248B
Forstrom RJ (1969) A new measure of erythrocyte membrane strength - the jet fragility test. PhD. thesis, University of Minnesota
Fraser KH, Zhang T, Taskin ME, Griffith BP, Wu ZJ (2012) A quantitative comparison of mechanical blood damage parameters in rotary ventricular assist devices: shear stress, exposure time and hemolysis index. J Biomech Eng 134:081002. https://doi.org/10.1115/1.4007092
Fuller GG, Leal LG (1980) Flow birefringence of dilute polymer solutions in two-dimensional flows. Rheol Acta 19:580–600. https://doi.org/10.1007/BF01517512
Fung YC (1993) Biomechanics: mechanical properties of living tissues. Springer, New York
Galdi GP, Rannacher R, Robertson AM, Turek S (2008) Hemodynamical flows modeling, analysis and simulation. Birkhäuser Verlag AG, Basel
Garon A, Farinas MI (2004) Fast three-dimensional numerical hemolysis approximation. Artif Organs 28:1016–1025. https://doi.org/10.1111/j.1525-1594.2004.00026.x
Ge L, Dasi LP, Sotiropoulos F, Yoganathan AP (2008) Characterization of hemodynamic forces induced by mechanical heart valves: Reynolds vs. viscous stresses. Ann Biomed Eng 36:276–297. https://doi.org/10.1007/s10439-007-9411-x
Gesenhues L, Pauli L, Behr M (2016) Strain-based blood damage estimation for computational design of ventricular assist devices. Int J Artif Organs 39:166–170. https://doi.org/10.5301/ijao.5000484
Giersiepen M, Krause U, Knott E, Reul H, Rau G (1989) Velocity and shear stress distribution downstream of mechanical heart valves in pulsatile flow. Int J Artif Organs 12:261–269
Giersiepen M, Wurzinger LJ, Opitz R, Reul H (1990) Estimation of shear stress-related blood damage in heart valve prostheses–in vitro comparison of 25 aortic valves. Int J Artif Organs 13:300–306
Girdhar G, Bluestein D (2008) Biological effects of dynamic shear stress in cardiovascular pathologies and devices. Expert Rev Med Devices 5:167–181. https://doi.org/10.1586/17434440.5.2.167
Goldsmith HL, Marlow J (1972) Flow behaviour of erythrocytes. I. Rotation and deformation in dilute suspensions. Proc R Soc Lond B Biol Sci 182:351–384. https://doi.org/10.1098/rspb.1972.0084
Gossett DR, Tse HTK, Lee SA, Ying Y, Lindgren AG, Yang OO, Rao J, Clark AT, Di Carlo D (2012) Hydrodynamic stretching of single cells for large population mechanical phenotyping. Proc Natl Acad Sci U S A 109:7630–7635. https://doi.org/10.1073/pnas.1200107109
Goubergrits L (2006) Numerical modeling of blood damage: current status, challenges and future prospects. Expert Rev Med Devices 3:527–531. https://doi.org/10.1586/17434440.3.5.527
Goubergrits L, Osman J, Mevert R, Kertzscher U, Pothkow K, Hege HC (2016) Turbulence in blood damage modeling. Int J Artif Organs 39:160–165. https://doi.org/10.5301/ijao.5000476
Grigioni M, Daniele C, D’Avenio G, Barbaro V (1999) A discussion on the threshold limit for hemolysis related to Reynolds shear stress. J Biomech 32:1107–1112
Grigioni M, Daniele C, Morbiducci U, D’Avenio G, Di Benedetto G, Barbaro V (2004) The power-law mathematical model for blood damage prediction: analytical developments and physical inconsistencies. Artif Organs 28:467–475. https://doi.org/10.1111/j.1525-1594.2004.00015.x
Grigioni M, Morbiducci U, D’Avenio G, Benedetto GD, Gaudio CD (2005) A novel formulation for blood trauma prediction by a modified power-law mathematical model. Biomech Model Mechanobiol 4:249–260. https://doi.org/10.1007/s10237-005-0005-y
Hellem AJ (1960) The adhesiveness of human blood platelets in vitro. Scand J Clin Lab Invest 12(Suppl):1–117
Helms CC, Marvel M, Zhao W, Stahle M, Vest R, Kato GJ, Lee JS, Christ G, Gladwin MT, Hantgan RR, Kim-Shapiro DB (2013) Mechanisms of hemolysis-associated platelet activation. J Thromb Haemost 11:2148–2154. https://doi.org/10.1111/jth.12422
Heuser G, Opitz R (1980) A Couette viscometer for short time shearing of blood. Biorheology 17:17–24
Hiroshi N, Gerhard G (2005) Vesicle dynamics in shear and capillary flows. J Phys Condens Matter 17:S3439
Hochmuth RM, Waugh RE (1987) Erythrocyte membrane elasticity and viscosity. Annu Rev Physiol 49:209–219. https://doi.org/10.1146/annurev.ph.49.030187.001233
Hochmuth RM, Evans CA, Wiles HC, McCown JT (1983) Mechanical measurement of red cell membrane thickness. Science 220:101–102
Houchin DN, Munn JI, Parnell BL (1958) A method for the measurement of red cell dimensions and calculation of mean corpuscular volume and surface area. Blood 13:1185–1191
Hudzik B, Kaczmarski J, Pacholewicz J, Zakliczynski M, Gasior M, Zembala M (2015) Von Willebrand factor in patients on mechanical circulatory support: a double-edged sword between bleeding and thrombosis. Kardiochirurgia i torakochirurgia polska = Pol J Cardio-Thorac Surg 12:233–237. https://doi.org/10.5114/kitp.2015.54459
Hund SJ, Antaki JF, Massoudi M (2010) On the representation of turbulent stresses for computing blood damage. Int J Eng Sci 48:1325–1331. https://doi.org/10.1016/j.ijengsci.2010.09.003
Ishii K, Hosoda K, Nishida M, Isoyama T, Saito I, Ariyoshi K, Inoue Y, Ono T, Nakagawa H, Sato M, Hara S, Lee X, Wu SY, Imachi K, Abe Y (2015) Hydrodynamic characteristics of the helical flow pump. J Artif Organs 18:206–212. https://doi.org/10.1007/s10047-015-0828-y
Iuliano L, Violi F, Pedersen JZ, Pratico D, Rotilio G, Balsano F (1992) Free radical-mediated platelet activation by hemoglobin released from red blood cells. Arch Biochem Biophys 299:220–224
Jhun CS, Stauffer MA, Reibson JD, Yeager EE, Newswanger RK, Taylor JO, Manning KB, Weiss WJ, Rosenberg G (2018) Determination of Reynolds shear stress level for hemolysis. ASAIO J 64:63–69. https://doi.org/10.1097/mat.0000000000000615
Jones SA (1995) A relationship between Reynolds stresses and viscous dissipation: implications to red cell damage. Ann Biomed Eng 23:21–28
Ju M, Ye SS, Namgung B, Cho S, Low HT, Leo HL, Kim S (2015) A review of numerical methods for red blood cell flow simulation. Comput Methods Biomech Biomed Eng 18:130–140. https://doi.org/10.1080/10255842.2013.783574
Kameneva MV, Burgreen GW, Kono K, Repko B, Antaki JF, Umezu M (2004) Effects of turbulent stresses upon mechanical hemolysis: experimental and computational analysis. ASAIO J 50:418–423
Kawase Y, Moo-Young M (1990) Mathematical models for design of bioreactors: applications of: Kolmogoroff’s theory of isotropic turbulence. Chem Eng J 43:B19–B41. https://doi.org/10.1016/0300-9467(90)80048-H
Keller SR, Skalak R (1982) Motion of a tank-treading ellipsoidal particle in a shear flow. J Fluid Mech 120:27–47. https://doi.org/10.1017/S0022112082002651
Keshaviah PR (1976) Hemolysis in the accelerated flow region of an abrupt contraction. PhD Thesis, University of Minnesota, St. Paul, MN
Khoo DP, Cookson AN, Gill HS, Fraser KH (2018) Normal fluid stresses are prevalent in rotary ventricular assist devices: a computational fluid dynamics analysis. Int J Artif Organs 41:738–751. https://doi.org/10.1177/0391398818792757
Klose HJ, Volger E, Brechtelsbauer H, Heinich L, Schmid-Schonbein H (1972) Microrheology and light transmission of blood. I. The photometric effects of red cell aggregation and red cell orientation. Pflugers Arch 333:126–139
Kodippili GC, Spector J, Kang GE, Liu H, Wickrema A, Ritchie K, Low PS (2010) Analysis of the kinetics of band 3 diffusion in human erythroblasts during assembly of the erythrocyte membrane skeleton. Br J Haematol 150:592–600. https://doi.org/10.1111/j.1365-2141.2010.08268.x
Koleva I, Rehage H (2012) Deformation and orientation dynamics of polysiloxane microcapsules in linear shear flow. Soft Matter 8:3681–3693. https://doi.org/10.1039/C2SM07182G
Kolmogorov AN (1991) The local structure of turbulence in incompressible viscous fluid for very large Reynolds numbers. Proc Math Phys Sci 434:9–13
Kon K, Maeda N, Shiga T (1987) Erythrocyte deformation in shear flow: influences of internal viscosity, membrane stiffness, and hematocrit. Blood 69:727–734
Koshiyama K, Wada S (2011) Molecular dynamics simulations of pore formation dynamics during the rupture process of a phospholipid bilayer caused by high-speed equibiaxial stretching. J Biomech 44:2053–2058. https://doi.org/10.1016/j.jbiomech.2011.05.014
Kramer JW (2000) Normal hematology of cattle, sheep and goats. In: Feldman BF, Zinkl JG, Jain NC (eds) Schalm’s veterinary hematology, 5th edn. Lippincott Williams & Wilkins, New York, pp 1075–1084
Ku DN (1997) Blood flow in arteries. Annu Rev Fluid Mech 29:399–434. https://doi.org/10.1146/annurev.fluid.29.1.399
Kunas KT, Papoutsakis ET (1990) Damage mechanisms of suspended animal cells in agitated bioreactors with and without bubble entrainment. Biotechnol Bioeng 36:476–483. https://doi.org/10.1002/bit.260360507
Laugel JF, Beissinger RL (1983) Low stress shear-induced hemolysis in capillary flow. Trans Am Soc Artif Intern Organs 29:158–162
Lee S, Ahn KH, Lee SJ, Sun K, Goedhart PT, Hardeman MR (2004) Shear induced damage of red blood cells monitored by the decrease of their deformability. Korea-Aust Rheol J 16:141–146
Lee SS, Antaki JF, Kameneva MV, Dobbe JG, Hardeman MR, Ahn KH, Lee SJ (2007) Strain hardening of red blood cells by accumulated cyclic supraphysiological stress. Artif Organs 31:80–86. https://doi.org/10.1111/j.1525-1594.2007.00344.x
Lee H, Tatsumi E, Taenaka Y (2009a) Experimental study on the Reynolds and viscous shear stress of bileaflet mechanical heart valves in a pneumatic ventricular assist device. ASAIO J 55:348–354. https://doi.org/10.1097/MAT.0b013e3181a793e0
Lee SS, Yim Y, Ahn KH, Lee SJ (2009b) Extensional flow-based assessment of red blood cell deformability using hyperbolic converging microchannel. Biomed Microdevices 11:1021–1027. https://doi.org/10.1007/s10544-009-9319-3
Leverett LB, Hellums JD, Alfrey CP, Lynch EC (1972) Red blood cell damage by shear stress. Biophys J 12:257–273
Li H, Lykotrafitis G (2014) Erythrocyte membrane model with explicit description of the lipid bilayer and the spectrin network. Biophys J 107:642–653. https://doi.org/10.1016/j.bpj.2014.06.031
Li X, Vlahovska PM, Karniadakis GE (2013) Continuum- and particle-based modeling of shapes and dynamics of red blood cells in health and disease. Soft Matter 9:28–37. https://doi.org/10.1039/C2SM26891D
Lippi G (2012) Hemolysis: an unresolved dispute in laboratory medicine. De Gruyter, Berlin
Liu JS, Lu PC, Chu SH (2000) Turbulence characteristics downstream of bileaflet aortic valve prostheses. J Biomech Eng 122:118–124
Lokhandwalla M, Sturtevant B (2001) Mechanical haemolysis in shock wave lithotripsy (SWL): I. Analysis of cell deformation due to SWL flow-fields. Phys Med Biol 46:413–437
Lu PC, Lai HC, Liu JS (2001) A reevaluation and discussion on the threshold limit for hemolysis in a turbulent shear flow. J Biomech 34:1361–1364
Lux SE, Palek J (1995) Disorders of the red cell membrane. In: Handin RI, Lux SE, Stossel TP (eds) Blood: principles and practice of hematology. Lippincott, Philadelphia, pp 1701–1818
Maffettone PL, Minale M (1998) Equation of change for ellipsoidal drops in viscous flow. J Nonnewton Fluid Mech 78:227–241. https://doi.org/10.1016/S0377-0257(98)00065-2
Maymir JC, Deutsch S, Meyer RS, Geselowitz DB, Tarbell JM (1998) Mean velocity and Reynolds stress measurements in the regurgitant jets of tilting disk heart valves in an artificial heart environment. Ann Biomed Eng 26:146–156. https://doi.org/10.1114/1.86
Mcgraw LA (1992) Blood cell deformability in uniaxial extensional flow. Carnegie Mellon University, Pittsburgh
Misbah C (2006) Vacillating breathing and tumbling of vesicles under shear flow. Phys Rev Lett 96:028104
Misbah C (2012) Vesicles, capsules and red blood cells under flow. J Phys Conf Ser 392:012005
Mitoh A, Yano T, Sekine K, Mitamura Y, Okamoto E, Kim DW, Yozu R, Kawada S (2003) Computational fluid dynamics analysis of an intra-cardiac axial flow pump. Artif Organs 27:34–40
Mizuno T, Tsukiya T, Taenaka Y, Tatsumi E, Nishinaka T, Ohnishi H, Oshikawa M, Sato K, Shioya K, Takewa Y, Takano H (2002) Ultrastructural alterations in red blood cell membranes exposed to shear stress. ASAIO J 48:668–670
Morris DR, Williams AR (1979) The effects of suspending medium viscosity on erythrocyte deformation and haemolysis in vitro. Biochim Biophys Acta 550:288–296. https://doi.org/10.1016/0005-2736(79)90215-3
Morshed KN, Bark D Jr, Forleo M, Dasi LP (2014) Theory to predict shear stress on cells in turbulent blood flow. PLoS ONE 9:e105357. https://doi.org/10.1371/journal.pone.0105357
Moulden TH, Frost W (1977) Handbook of turbulence. Plenum Press, New York
Mueller MR, Schima H, Engelhardt H, Salat A, Olsen DB, Losert U, Wolner E (1993) In vitro hematological testing of rotary blood pumps: remarks on standardization and data interpretation. Artif Organs 17:103–110
Naito K, Mizuguchi K, Nose Y (1994) The need for standardizing the index of hemolysis. Artif Organs 18:7–10
Nakahara T, Yoshida F (1986) Mechanical effects on rates of hemolysis. J Biomed Mater Res 20:363–374. https://doi.org/10.1002/jbm.820200308
Namdee K, Carrasco-Teja M, Fish MB, Charoenphol P, Eniola-Adefeso O (2015) Effect of Variation in hemorheology between human and animal blood on the binding efficacy of vascular-targeted carriers. Sci Rep 5:11631. https://doi.org/10.1038/srep11631
Nevaril CG, Lynch EC, Alfrey CP Jr, Hellums JD (1968) Erythrocyte damage and destruction induced by shearing stress. J Lab Clin Med 71:784–790
Nobili M, Sheriff J, Morbiducci U, Redaelli A, Bluestein D (2008) Platelet activation due to hemodynamic shear stresses: damage accumulation model and comparison to in vitro measurements. ASAIO J 54:64–72. https://doi.org/10.1097/mat.0b013e31815d6898
Nyboe C, Funder JA, Smerup MH, Nygaard H, Hasenkam JM (2006) Turbulent stress measurements downstream of three bileaflet heart valve designs in pigs. Eur J Cardiothorac Surg 29:1008–1013. https://doi.org/10.1016/j.ejcts.2006.03.013
Nygaard H, Giersiepen M, Hasenkam JM, Westphal D, Paulsen PK, Reul H (1990) Estimation of turbulent shear stresses in pulsatile flow immediately downstream of two artificial aortic valves in vitro. J Biomech 23:1231–1238
Nygaard H, Giersiepen M, Hasenkam JM, Reul H, Paulsen PK, Rovsing PE, Westphal D (1992) Two-dimensional color-mapping of turbulent shear stress distribution downstream of two aortic bioprosthetic valves in vitro. J Biomech 25:429–440
Offeman RD, Williams MC (1976) Shear-induced hemolysis: effects of blood chemistry (including aging in storage) and shearing surfaces. Biomater Med Devices Artif Organs 4:49–79
Oliveira M, Alves MA, Pinho FT, McKinley GH (2007) Viscous fluid flow through microfabricated hperbolic contractions. Exp Fluids 43:437–451. https://doi.org/10.1007/s00348-007-0306-2
Omar HR, Mirsaeidi M, Socias S, Sprenker C, Caldeira C, Camporesi EM, Mangar D (2015) Plasma free hemoglobin is an independent predictor of mortality among patients on extracorporeal membrane oxygenation support. PLoS ONE 10:e0124034. https://doi.org/10.1371/journal.pone.0124034
Omori T, Ishikawa T, Barthès-Biesel D, Salsac AV, Imai Y, Yamaguchi T (2012) Tension of red blood cell membrane in simple shear flow. Phys Rev E 86:056321
Ozturk M, O’Rear EA, Papavassiliou DV (2015) Hemolysis related to turbulent eddy size distributions using comparisons of experiments to computations. Artif Organs 39:E227–E239. https://doi.org/10.1111/aor.12572
Ozturk M, O’Rear E, Papavassiliou D (2016) Reynolds stresses and hemolysis in turbulent flow examined by threshold analysis. Fluids 1:42
Paul R, Apel J, Klaus S, Schugner F, Schwindke P, Reul H (2003) Shear stress related blood damage in laminar couette flow. Artif Organs 27:517–529
Pauli L, Nam J, Pasquali M, Behr M (2013) Transient stress-based and strain-based hemolysis estimation in a simplified blood pump. Int J Numer Methods Biomed Eng 29:1148–1160. https://doi.org/10.1002/cnm.2576
Polaschegg HD (2009) Red blood cell damage from extracorporeal circulation in hemodialysis. Semin Dial 22:524–531. https://doi.org/10.1111/j.1525-139X.2009.00616.x
Poorkhalil A, Amoabediny G, Tabesh H, Behbahani M, Mottaghy K (2016) A new approach for semiempirical modeling of mechanical blood trauma. Int J Artif Organs 39:171–177. https://doi.org/10.5301/ijao.5000474
Pope SB (2000) Turbulent flows. Cambridge University Press, Cambridge. https://doi.org/10.1017/cbo9780511840531
Popov EP, Nagarajan S, Lu ZA (1976) Mechanics of materials. Prentice-Hall, Englewood Cliffs
Quinlan NJ (2014) Mechanical loading of blood cells in turbulent flow. In: Doyle B, Miller K, Wittek A, Nielsen MFP (eds) Computational biomechanics for medicine: fundamental science and patient-specific applications. Springer, New York, pp 1–13. https://doi.org/10.1007/978-1-4939-0745-8_1
Quinlan NJ, Dooley PN (2007) Models of flow-induced loading on blood cells in laminar and turbulent flow, with application to cardiovascular device flow. Ann Biomed Eng 35:1347–1356. https://doi.org/10.1007/s10439-007-9308-8
Rand RP (1964) Mechanical properties of the red cell membrane: II. Viscoelastic breakdown of the membrane. Biophys J 4:303–316
Rand RP, Burton AC (1964) Mechanical properties of the red cell membrane: I. Membrane stiffness and intracellular pressure. Biophys J 4:115–135
Richardson E (1974) Deformation and haemolysis of red cells in shear flow. Proc R Soc Lond A Math Phys Eng Sci 338:129–153. https://doi.org/10.1098/rspa.1974.0078
Richardson E (1975) Applications of a theoretical model for haemolysis in shear flow. Biorheology 12:27–37
Rodgers BM, Sabiston DC (1969) Hemolytic anemia following prosthetic valve replacement. Circulation 39:I-155–I-161. https://doi.org/10.1161/01.cir.39.5s1.i-155
Rodrigues RO, Faustino V, Pinto E, Pinho D, Lima R (2013) Red blood cells deformability index assessment in a hyperbolic microchannel: the diamide and glutaraldehyde effect. WebmedCentral Biomed Eng 4(8):WMC004375. https://doi.org/10.9754/journal.wmc.2013.004375
Roland L, Drillich M, Iwersen M (2014) Hematology as a diagnostic tool in bovine medicine. J Vet Diagn Investig 26:592–598. https://doi.org/10.1177/1040638714546490
Rooney JA (1970) Hemolysis near an ultrasonically pulsating gas bubble. Science 169:869–871
Sakota D, Sakamoto R, Sobajima H, Yokoyama N, Waguri S, Ohuchi K, Takatani S (2008) Mechanical damage of red blood cells by rotary blood pumps: selective destruction of aged red blood cells and subhemolytic trauma. Artif Organs 32:785–791. https://doi.org/10.1111/j.1525-1594.2008.00631.x
Sallam AM, Hwang NH (1984) Human red blood cell hemolysis in a turbulent shear flow: contribution of Reynolds shear stresses. Biorheology 21:783–797
Sayed HM, Dacie JV, Handley DA, Lewis SM, Cleland WP (1961) Haemolytic anaemia of mechanical origin after open heart surgery. Thorax 16:356–360
Shadden SC, Hendabadi S (2013) Potential fluid mechanic pathways of platelet activation. Biomech Model Mechanobiol 12:467–474. https://doi.org/10.1007/s10237-012-0417-4
Shakeri M, Khodarahmi I, Sharp MK (2012) Preliminary imaging of red blood cells in turbulent flow. In: ASME 2012 summer bioengineering conference, Puerto Rico, USA, 2012, pp 887–888
Shapira Y, Vaturi M, Sagie A (2009) Hemolysis associated with prosthetic heart valves: a review. Cardiol Rev 17:121–124. https://doi.org/10.1097/CRD.0b013e31819f1a83
Sharp MK, Mohammad SF (1998) Scaling of hemolysis in needles and catheters. Ann Biomed Eng 26:788–797. https://doi.org/10.1114/1.65
Shi L, Pan TW, Glowinski R (2012) Deformation of a single red blood cell in bounded Poiseuille flows. Phys Rev E Stat Nonlinear Soft Matter Phys 85:016307. https://doi.org/10.1103/PhysRevE.85.016307
Shivakumaraswamy T, Mishra P, Radhakrishnan B, Khandekar J, Agrawal N, Patwardhan A, Khandeparkar J (2006) Intravascular hemolysis in patients with normally functioning mechanical heart valves in mitral position. Indian J Thorac Cardiovasc Surg 22:215–218. https://doi.org/10.1007/s12055-006-0005-2
Skalak R, Ozkaya N, Skalak TC (1989) Biofluid mechanics. Annu Rev Fluid Mech 21:167–200. https://doi.org/10.1146/annurev.fl.21.010189.001123
Smith JE (1987) Erythrocyte membrane: structure, function, and pathophysiology. Vet Pathol 24:471–476
Sohrabi S, Liu Y (2017) A cellular model of shear-induced hemolysis. Artif Organs 41:E80–e91. https://doi.org/10.1111/aor.12832
Song X, Throckmorton AL, Wood HG, Antaki JF, Olsen DB (2003) Computational fluid dynamics prediction of blood damage in a centrifugal pump. Artif Organs 27:938–941
Sousa PC, Pinho FT, Oliveira MS, Alves MA (2011) Extensional flow of blood analog solutions in microfluidic devices. Biomicrofluidics 5:14108. https://doi.org/10.1063/1.3567888
Stein PD, Sabbah HN (1976) Turbulent blood flow in the ascending aorta of humans with normal and diseased aortic valves. Circ Res 39:58–65
Surgenor DM, Bishop CW (1974) The red blood cell. Academic Press, New York
Sutera SP (1977) Flow-induced trauma to blood cells. Circ Res 41:2–8
Sutera SP, Mehrjardi MH (1975) Deformation and fragmentation of human red blood cells in turbulent shear flow. Biophys J 15:1–10
Tamagawa M, Akamatsu T, Saitoh K (1996) Prediction of hemolysis in turbulent shear orifice flow. Artif Organs 20:553–559
Taskin ME, Fraser KH, Zhang T, Wu C, Griffith BP, Wu ZJ (2012) Evaluation of Eulerian and Lagrangian models for hemolysis estimation. ASAIO J 58:363–372. https://doi.org/10.1097/MAT.0b013e318254833b
Tennekes H, Lumley JL (1972) A first course in turbulence. MIT Press, Cambridge
Thurston GB (1989) Plasma release-cell layering theory for blood flow. Biorheology 26:199–214
Thurston GB (1990) Light transmission through blood in oscillatory flow. Biorheology 27:685–700
Tomaiuolo G (2014) Biomechanical properties of red blood cells in health and disease towards microfluidics. Biomicrofluidics 8:051501. https://doi.org/10.1063/1.4895755
Tran-Son-Tay R, Sutera SP, Zahalak GI, Rao PR (1987) Membrane stress and internal pressure in a red blood cell freely suspended in a shear flow. Biophys J 51:915–924. https://doi.org/10.1016/s0006-3495(87)83419-7
Travis BR, Leo HL, Shah PA, Frakes DH, Yoganathan AP (2002) An analysis of turbulent shear stresses in leakage flow through a bileaflet mechanical prostheses. J Biomech Eng 124:155–165
Tse WT, Lux SE (1999) Red blood cell membrane disorders. Br J Haematol 104:2–13. https://doi.org/10.1111/j.1365-2141.1999.01130.x
Valladolid C, Yee A, Cruz MA (2018) von Willebrand factor, free hemoglobin and thrombosis in ECMO. Front Med 5:228. https://doi.org/10.3389/fmed.2018.00228
Vallés J, Santos MT, Aznar J, Martı́nez M, Moscardó A, Piñón M, Broekman MJ, Marcus AJ (2002) Platelet-erythrocyte interactions enhance αIIbβ3 integrin receptor activation and P-selectin expression during platelet recruitment: down-regulation by aspirin ex vivo. Blood 99:3978–3984. https://doi.org/10.1182/blood.v99.11.3978
Viallat A, Abkarian M (2014) Red blood cell: from its mechanics to its motion in shear flow. Int J Lab Hematol 36:237–243. https://doi.org/10.1111/ijlh.12233
Villagra J, Shiva S, Hunter LA, Machado RF, Gladwin MT, Kato GJ (2007) Platelet activation in patients with sickle disease, hemolysis-associated pulmonary hypertension, and nitric oxide scavenging by cell-free hemoglobin. Blood 110:2166–2172. https://doi.org/10.1182/blood-2006-12-061697
Vitale F, Nam J, Turchetti L, Behr M, Raphael R, Annesini MC, Pasquali M (2014) A multiscale, biophysical model of flow-induced red blood cell damage. AICHE J 60:1509–1516. https://doi.org/10.1002/aic.14318
Walker HK, Hall WD, Hurst JW (1990) Clinical methods: the history, physical and laboratory examinations. Butterworths, Boston
Williams AR, Hughes DE, Nyborg WL (1970) Hemolysis near a transversely oscillating wire. Science 169:871–873. https://doi.org/10.1126/science.169.3948.871
Wu J, Antaki JF, Snyder TA, Wagner WR, Borovetz HS, Paden BE (2005) Design optimization of blood shearing instrument by computational fluid dynamics. Artif Organs 29:482–489. https://doi.org/10.1111/j.1525-1594.2005.29082.x
Yaginuma T, Pereira AI, Rodrigues PJ, Lima R, Oliveira MSN, Ishikawa T, Yamaguchi T (2011) Flow of red blood cells through a microfluidic extensional device: An image analysis assessment. In: Computational vision and medical image processing: VipIMAGE 2011. CRC Press, pp 217–220. https://doi.org/10.1201/b11570-45
Yaginuma T, Oliveira MSN, Lima R, Ishikawa T, Yamaguchi T (2013) Human red blood cell behavior under homogeneous extensional flow in a hyperbolic-shaped microchannel. Biomicrofluidics 7:054110. https://doi.org/10.1063/1.4820414
Yano T, Sekine K, Mitoh A, Mitamura Y, Okamoto E, Kim DW, Nishimura I, Murabayashi S, Yozu R (2003) An estimation method of hemolysis within an axial flow blood pump by computational fluid dynamics analysis. Artif Organs 27:920–925
Yazdani A, Bagchi P (2012) Three-dimensional numerical simulation of vesicle dynamics using a front-tracking method. Phys Rev E 85:056308
Yeleswarapu KK, Antaki JF, Kameneva MV, Rajagopal KR (1995) A mathematical model for shear-induced hemolysis. Artif Organs 19:576–582
Yen JH, Chen SF, Chern MK, Lu PC (2014) The effect of turbulent viscous shear stress on red blood cell hemolysis. J Artif Organs Off J Jpn Soc Artif Organs 17:178–185. https://doi.org/10.1007/s10047-014-0755-3
Yen J-H, Chen S-F, Chern M-K, Lu P-C (2015) The effects of extensional stress on red blood cell hemolysis. Biomed Eng Appl Basis Commun 27:1550042. https://doi.org/10.4015/S1016237215500428
Yoon YZ, Kotar J, Yoon G, Cicuta P (2008) The nonlinear mechanical response of the red blood cell. Phys Biol 5:036007. https://doi.org/10.1088/1478-3975/5/3/036007
Yu H, Engel S, Janiga G, Thevenin D (2017) A review of hemolysis prediction models for computational fluid dynamics. Artif Organs 41:603–621. https://doi.org/10.1111/aor.12871
Zeng NF, Ristenpart WD (2014) Mechanical response of red blood cells entering a constriction. Biomicrofluidics 8:064123. https://doi.org/10.1063/1.4904058
Zhang Juntao, Gellman B, Koert A, Dasse KA, Gilbert RJ, Griffith BP, Wu ZJ (2006) Computational and experimental evaluation of the fluid dynamics and hemocompatibility of the CentriMag blood pump. Artif Organs 30:168–177. https://doi.org/10.1111/j.1525-1594.2006.00203.x
Zhang Tao, Taskin ME, Fang H-B, Pampori A, Jarvik R, Griffith BP, Wu ZJ (2011) Study of flow-induced hemolysis using novel couette-type blood-shearing devices. Artif Organs 35:1180–1186. https://doi.org/10.1111/j.1525-1594.2011.01243.x
Zhang R, Zhang C, Zhao Q, Li D (2013) Spectrin: structure, function and disease. Sci China Life Sci 56:1076–1085. https://doi.org/10.1007/s11427-013-4575-0
Zhao R, Antaki JF, Naik T, Bachman TN, Kameneva MV, Wu ZJ (2006) Microscopic investigation of erythrocyte deformation dynamics. Biorheology 43:747–765
Zhu A (2000) Introduction to porcine red blood cells: implications for xenotransfusion. Semin Hematol 37:143–149. https://doi.org/10.1016/S0037-1963(00)90039-8
Zimmer R, Steegers A, Paul R, Affeld K, Reul H (2000) Velocities, shear stresses and blood damage potential of the leakage jets of the Medtronic Parallel bileaflet valve. Int J Artif Organs 23:41–48
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Faghih, M.M., Sharp, M.K. Modeling and prediction of flow-induced hemolysis: a review. Biomech Model Mechanobiol 18, 845–881 (2019). https://doi.org/10.1007/s10237-019-01137-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-019-01137-1