Abstract
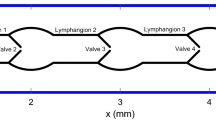
The paper describes the extension of a previously developed model of pressure-dependent contraction rate to the case of multiple lymphangions. Mechanical factors are key modulators of active lymphatic pumping. As part of the evolution of our lumped-parameter model to match experimental findings, we have designed an algorithm whereby the time until the next contraction depends on lymphangion transmural pressure in the contraction just completed. The functional dependence of frequency on pressure is quantitatively matched to isobaric contraction experiments on isolated lymphatic segments. When each of several lymphangions is given this ability, a scheme for their coordination must be instituted to match the observed synchronization. Accordingly, and in line with an experiment on an isolated lymphatic vessel segment in which we measured contraction sequence and conduction delay, we took the fundamental principle to be that local timing can be overridden by signals to initiate contraction that start in adjacent lymphangions, conducted with a short delay. The scheme leads to retrograde conduction when the lymphangion chain is pumping against an adverse pressure difference, but antegrade conduction when contractions occur with no or a favourable pressure difference. Abolition of these conducted signals leads to chaotic variation of cycle-mean flow-rate from the chain, diastolic duration in each lymphangion, and inter-lymphangion delays. Chaotic rhythm is also seen under other circumstances. Because the model responds to increasing adverse pressure difference by increasing the repetition rate of contractions, it maintains time-average output flow-rate better than one with fixed repetition rate.














Similar content being viewed by others
Notes
The relatively small number of cycles computed here does not permit formal verification of dynamical chaos, but the recurrence plot clearly shows that while \(\bar{Q}\) varies irregularly by a factor of 5.5, the system is following a trajectory which limits the returns to an attractor—they are not uniformly distributed. This is strongly indicative of chaos.
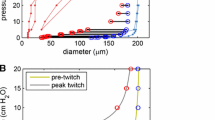
An example of pre-chaotic behaviour occurred with \(p_{\mathrm{a}} = 3\), \(p_{\mathrm{b}} = 4\,\hbox {cmH}_{2}\hbox {O}\); inter-lymphangion delays 3–4, and 4–5, were locked to + \(t_{\mathrm{p}}\), while delay 1–2 varied between 0.09 s and \(t_{\mathrm{p}}\), and delay 2–3 between \(-\) 0.11 and + 0.23 s, in a stable period-5 rhythm which was also seen in all the cycle-mean flow-rates and diastolic durations. Similarly, a post-transient period-3 rhythm associated with V2 staying open in one of every three contraction cycles occurred at \(p_{\mathrm{a}}=p_{\mathrm{b}} = 6\,\hbox {cmH}_{2}\hbox {O}\) (see Figs. 9, 10), and period-2 rhythm resulted from V2 staying open in alternate cycles at \(p_{\mathrm{a}} = 6\), \(p_{\mathrm{b}} = 3\,\hbox {cmH}_{2}\hbox {O}\) (see Figs. 13, 14).
Only slightly greater than 60 s, as was proved in a separate run (not shown).
This term is used to distinguish these behaviours from the steady chaos exhibited in Fig. 8.
Relative to our previous papers on this model, the suffix order has been changed; thus for instance what was previously \(p_{i\mathrm{m}}\) is now \(p_{\mathrm{m},i}\).
The definition of \({\mathrm{x}_i}\) here corrects an error in the equivalent Appendix eq. 8 of Bertram et al. (2017).
References
Aukland K, Reed RK (1993) Interstitial-lymphatic mechanisms in the control of extracellular fluid volume. Physiol Rev 73(1):1–78
Baish JW, Kunert C, Padera TP, Munn LL (2016) Synchronization and random triggering of lymphatic vessel contractions. PLoS Comput Biol 12(12):e1005231. https://doi.org/10.1371/journal.pcbi.1005231
Bertram CD, Macaskill C, Davis MJ, Moore JE Jr (2014b) Development of a model of a multi-lymphangion lymphatic vessel incorporating realistic and measured parameter values. Biomech Model Mechanobiol 13(2):401–416. https://doi.org/10.1007/s10237-013-0505-0
Bertram CD, Macaskill C, Davis MJ, Moore JE Jr (2016b) Consequences of intravascular lymphatic valve properties: a study of contraction timing in a multi-lymphangion model. Am J Physiol Heart Circ Physiol 310(7):H847–H860. https://doi.org/10.1152/ajpheart.00669.2015
Bertram CD, Macaskill C, Davis MJ, Moore JE Jr (2017) Valve-related modes of pump failure in collecting lymphatics: numerical and experimental investigation. Biomech Model Mechanobiol 16(6):1987–2003. https://doi.org/10.1007/s10237-017-0933-3
Bertram CD, Macaskill C, Moore JE Jr (2014a) Incorporating measured valve properties into a numerical model of a lymphatic vessel. Comput Methods Biomech Biomed Eng 17(14):1519–1534. https://doi.org/10.1080/10255842.2012.753066
Bertram CD, Macaskill C, Moore JE Jr (2016a) Pump function curve shape for a model lymphatic vessel. Med Eng Phys 38(7):656–663. https://doi.org/10.1016/j.medengphy.2016.04.009
Browse NL, Doig RL, Sizeland D (1984) The resistance of a lymph node to lymph flow. Br J Surg 71:192–196
Caulk AW, Dixon JB, Gleason RL Jr (2016) A lumped parameter model of mechanically mediated acute and long-term adaptations of contractility and geometry in lymphatics for characterization of lymphedema. Biomech Model Mechanobiol 15(6):1601–1618. https://doi.org/10.1007/s10237-016-0785-2
Contarino C, Toro EF (2017) Modelling the lymphatics: a 1D lymph flow model coupled to an electro-fluid-mechanical contraction model. Paper presented at the 5th international conference on computational and mathematical biomedical engineering-CMBE2017, Pittsburgh, 10–12 April
Crowe MJ, von der Weid P-Y, Brock JA, Van Helden DF (1997) Co-ordination of contractile activity in guinea-pig mesenteric lymphatics. J Physiol 500(1):235–244
Davis MJ (2005) An improved, computer-based method to automatically track internal and external diameter of isolated microvessels. Microcirculation 12(4):361–372. https://doi.org/10.1080/10739680590934772
Davis MJ, Davis AM, Ku CW, Gashev AA (2009a) Myogenic constriction and dilation of isolated lymphatic vessels. Am J Physiol Heart Circ Physiol 296(2):H293–H302
Davis MJ, Davis AM, Lane MM, Ku CW, Gashev AA (2009b) Rate-sensitive contractile responses of lymphatic vessels to circumferential stretch. J Physiol 587(1):165–182. https://doi.org/10.1113/jphysiol.2008.162438
Davis MJ, Rahbar E, Gashev AA, Zawieja DC, Moore JE Jr (2011) Determinants of valve gating in collecting lymphatic vessels from rat mesentery. Am J Physiol Heart Circ Physiol 301(1):H48–H60. https://doi.org/10.1152/ajpheart.00133.2011
Davis MJ, Scallan JP, Wolpers JH, Muthuchamy M, Gashev AA, Zawieja DC (2012) Intrinsic increase in lymphangion muscle contractility in response to elevated afterload. Am J Phys Heart Circ Physiol 303(7):H795–H808. https://doi.org/10.1152/ajpheart.01097.2011
Gashev AA, Davis MJ, Delp MD, Zawieja DC (2004) Regional variations of contractile activity in isolated rat lymphatics. Microcirculation 11(6):477–492
Gashev AA, Davis MJ, Zawieja DC (2002) Inhibition of the active lymph pump by flow in rat mesenteric lymphatics and thoracic duct. J Physiol 540(Pt 3):1023–1037. https://doi.org/10.1113/jphysiol.2002.016642
Jamalian S, Davis MJ, Zawieja DC, Moore JE Jr (2016) Network scale modeling of lymph transport and its effective pumping parameters. PLoS ONE 11(2):e0148384. https://doi.org/10.1371/journal.pone.0148384
Jamalian S, Jafarnejad M, Zawieja SD, Bertram CD, Gashev AA, Zawieja DC, Davis MJ, Moore JE Jr (2017) Demonstration and analysis of the suction effect for pumping lymph from tissue beds at subatmospheric pressure. Sci Rep 7:1–17. https://doi.org/10.1038/s41598-017-11599-x (article 12080)
Kornuta JA, Nepiyushchikh ZV, Gasheva OY, Mukherjee A, Zawieja DC, Dixon JB (2015) Effects of dynamic shear and transmural pressure on wall shear stress sensitivity in collecting lymphatic vessels. Am J Physiol Regul Integr Comput Physiol 309(9):R1122–R1134. https://doi.org/10.1152/ajpregu.00342.2014
Kunert C, Baish JW, Liao S, Padera TP, Munn LL (2015) Mechanobiological oscillators control lymph flow. Proc Natl Acad Sci USA 112(35):10938–10943. https://doi.org/10.1073/pnas.1508330112
Kunert C, Baish JW, Liao S, Padera TP, Munn LL (2016) Letter: Reply to Davis: nitric oxide regulates lymphatic contractions. PNAS 113(2):E106. https://doi.org/10.1073/pnas.1522233113
McGeown JG, McHale NG, Roddie IC, Thornbury K (1987) Peripheral lymphatic responses to outflow pressure in anaesthetized sheep. J Physiol 383:527–536
McHale NG, Meharg MK (1992) Co-ordination of pumping in isolated bovine lymphatic vessels. J Physiol 450:503–512
McHale NG, Roddie IC (1976) The effect of transmural pressure on pumping activity in isolated bovine lymphatic vessels. J Physiol 261(2):255–269
Mohanakumar S, Majgaard J, Telinius N, Katballe N, Pahle E, Hjortdal VE, Boedtkjer DMB (2018) Spontaneous and \(\alpha \)-adrenoceptor-induced contractility in human collecting lymphatic vessels require chloride. Am J Physiol Heart Circ Physiol. https://doi.org/10.1152/ajpheart.00551.2017 (in press)
Scallan JP, Zawieja SD, Castorena-Gonzalez JA, Davis MJ (2016) Lymphatic pumping: mechanics, mechanisms and malfunction. J Physiol 594(20):5749–5768. https://doi.org/10.1113/JP272088
Telinius N, Majgaard J, Mohanakumar S, Pahle E, Nielsen J, Hjortdal V, Aalkjær C, Boedtkjer DB (2017) Spontaneous and evoked contractility of human intestinal lymphatic vessels. Lymphat Res Biol 15(1):17–22. https://doi.org/10.1089/lrb.2016.0039
Zawieja DC (2009) Contractile physiology of lymphatics. Lymphat Res Biol 7(2):87–96. https://doi.org/10.1089/lrb.2009.0007
Zawieja DC, Davis KL, Schuster R, Hinds WM, Granger HJ (1993) Distribution, propagation, and coordination of contractile activity in lymphatics. Am J Physiol Heart Circ Physiol 264(4, Pt 2):H1283–H1291
Zawieja SD, Castorena-Gonzalez JA, Dixon JB, Davis MJ (2017) Experimental models used to assess lymphatic contractile function. Lymphat Res Biol 15(4):331–342. https://doi.org/10.1089/lrb.2017.0052
Zawieja SD, Castorena-Gonzalez JA, Scallan JP, Davis MJ (2018) Differences in L-type \(\text{ Ca }^{2+}\) channel activity partially underlie the regional dichotomy in pumping behavior by murine peripheral and visceral lymphatic vessels. Am J Physiol Heart Circ Physiol 314(5):H991–H1010. https://doi.org/10.1152/ajpheart.00499.2017
Acknowledgements
All authors acknowledge support from U.S. National Institutes of Health (NIH) Grant U01-HL-123420. MJD’s laboratory was supported by NIH Grant R01-HL-120867.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Appendices
Appendix 1: Equations for n lymphangions with inlet/outlet pipette resistance

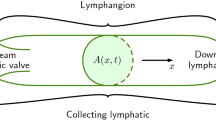
Mass conservation: \(\frac{dD_i }{dt}=\frac{D_i ^{3}}{32\,\upmu L^{2}}\left( {p_{1,i} -2p_{{\mathrm{m},}i} +p_{2,i} } \right) \), for \(i=1\) to n; D, diameter; t, time; \(\mu \), viscosity; L, lymphangion length; p, pressure; and suffices 1, m and 2 denote the upstream end, mid–point, and downstream end of a lymphangion.Footnote 5
Momentum conservation: \(p_{1,i} -p_{{\mathrm{m},}i} =\frac{64\,\upmu L}{\pi D_i ^{4}} \frac{p_{2,i-1} -p_{1,i} }{R_{\mathrm{V}} \left( {p_{2,i-1} ,p_{1,i} ;p_{\mathrm{e}} } \right) }\),
and \(p_{{\mathrm{m},}i} -p_{2,i} =\frac{64\upmu L}{\pi D_i ^{4}}\frac{p_{2,i} -p_{1,i+1} }{R_{\mathrm{V}} \left( {p_{2,i} ,p_{1,i+1} ;p_{\mathrm{e}} } \right) }\), where
\(R_{{\mathrm{V},}i} =R_{{\mathrm{Vn}}} +\frac{R_{{\mathrm{Vx}}} }{1+\exp \left[ {-s_{\mathrm{o}} \left( {\Delta p_{{\mathrm{V},}i} -\Delta p_{{\mathrm{o},}i} } \right) } \right] },\) with \(\Delta p_{{\mathrm{V},}i} =p_{2,i-1} -p_{1,i} \);
\(R_{\mathrm{V}}\), valve resistance; \(R_{\mathrm{Vn}}\), minimum valve resistance; \(R_{\mathrm{Vx}}+R_{\mathrm{Vn}}\), maximum valve resistance; \(s_{\mathrm{o}}\), a constant defining the rate of transition. The open/close threshold \(\Delta p_{{\mathrm{o},}i} \) is a nonlinear function of \(p_{2,i-1} -p_{\mathrm{e}} \) when closed, and of \(p_{1,i} -p_{\mathrm{e}}\) when open; see Bertram et al. (2014b).
For \(i=1\) only, \(p_{2,i-1} =p_{2,0} =p_{\mathrm{a}} -R_{\mathrm{a}} \frac{p_{2,0} -p_{1,1} }{R_{{\mathrm{V},}1} }\); for \(i=n\) only, \(p_{1,i+1} =p_{\mathrm{b}} +R_{\mathrm{b}} \frac{p_{2,n} -p_{1,n+1} }{R_{{\mathrm{V},}n+1} }\); \(p_{\mathrm{a}}\) and \(p_{\mathrm{b}}\) are the pressures in the up- and downstream reservoirs, and \(R_{\mathrm{a}}\) and \(R_{\mathrm{b}}\) are the resistances of the up- and downstream micropipettes cannulating the vessel segment, respectively.
Constitutive relations: the passive and maximally contracted states are defined by the curves \(D_{i,{\mathrm{psv}}} =f_{\mathrm{psv}} \left( {\Delta p_{\mathrm{tm,}i} } \right) \) and \(D_{i,\mathrm{act}} =f_{\mathrm{act}} \left( {\Delta p_{\mathrm{tm,}i} } \right) \), respectively, where \(\Delta p_{\mathrm{tm,}i} =p_{\mathrm{m,}i} -p_{\mathrm{e}} \); see Bertram et al. (2017). A contraction consists of smooth evolution of the \(D_i \left( {\Delta p_{\mathrm{tm,}i} } \right) \)-relation between these extremes governed by \(D_i =D_{i,\mathrm{psv}} -M\left( t \right) \left[ {D_{i,\mathrm{psv}} -D_{i,\mathrm{act}} } \right] \), where the time-course of active contraction \(M\left( t \right) \) is calculated as detailed by Bertram et al. (2017); at its simplest, it is an inverted cosine wave with frequency f and duration 1 / f.
Pressure-dependent diastolic duration: the period of relaxation between systoles (in the absence of activation signals conducted from other lymphangions) is calculated at each instant of end-systole as \(\Delta t_{\mathrm{r,}i} =\frac{1}{f_{\mathrm{tw,}i} }-\frac{1}{f}\), where \(f_{\mathrm{tw,}i} =60y_i \), with \(y_i =-\,1.39q_i ^{2}+12.6q_i +0.647\), \(q_i =\ln \left( {1+x_i } \right) \), and \(x=f\int _{t_\mathrm{c} }^{t_\mathrm{c} +1/f} {\Delta p_{\mathrm{tm},i} } dt\) is the average transmural pressure (in \(\mathrm{cmH}_{2}\hbox {O}\)) over the preceding systole starting at time \({{ t}}_{\mathrm{c}}\); see Bertram et al. (2017).Footnote 6
Appendix 2: Numerical scheme for organizing contraction times
Two vectors are maintained throughout the simulation, each of length equal to the number of lymphangions plus two (i.e. including the two part-lymphangions at the ends).
The first vector, say \(s_{\mathrm{old}}\), contains the times at which each lymphangion last began contraction. The second, say \(s_{\mathrm{new}}\), contains the most up-to-date information about the times at which each lymphangion will next start contraction. The algorithm is as follows:
-
1.
Initialize: at \(t = t_{0}\), all lymphangions start contracting, so set the vector elements \(s_{\mathrm{old}}(i)= t_{0}\) for all i. Set \(s_{\mathrm{new}}(i)=t_{\mathrm{large}}\) for all i, where \(t_{\mathrm{large}}\) is some large positive value greater than the maximum time in the full simulation.
-
2.
At the conclusion of each (full) timestep dt in the Runge-Kutta integration, at time t:
-
(a)
For each lymphangion \(L_{i}\), if \(s_{\mathrm{new}}(i)\) lies in the interval [t - dt, t], initiate contraction of lymphangion \(L_{i}\), update \(s_{\mathrm{old}}(i)=s_{\mathrm{new}}(i)\), and propagate the signals to nearest-neighbour lymphangions \(L_{i-1}\) and \(L_{i+1}\). These lymphangions may then initialize contraction after a delay \(t_{\mathrm{p}}\), by updating \(s_{\mathrm{new}}(i + 1) = \mathrm{min}(\hbox {s}_{\mathrm{new}}({i}+ 1)\), \(t+t_{\mathrm{p}})\) and similarly for \(s_{\mathrm{new}}(i - 1)\), but the updates can only occur if they are outside the current relevant refractory period, e.g. for the case of the neighbouring lymphangion \(L_{i+1}\), set \(s_{\mathrm{new}}(i + 1) = t+t_{\mathrm{p}}\) only if \(t+t_{\mathrm{p}} > s_{\mathrm{old}}(i + 1)\) + \(t_{\mathrm{n}}\).
-
(b)
For each lymphangion \(L_{i}\), if the time of conclusion of a contraction lies in the interval [\(t - dt, t\)], update \(s_{\mathrm{new}}(i) = \hbox {min}(s_{\mathrm{old}}(i)+ t_{\mathrm{n}}\), \(t+t_{\mathrm{r}})\), where \(t_{\mathrm{r}}\) is calculated according to the average of \(\Delta p_{\mathrm{tm}}(t)\) over the just-concluded contraction.
-
(a)
Rights and permissions
About this article
Cite this article
Bertram, C.D., Macaskill, C., Davis, M.J. et al. Contraction of collecting lymphatics: organization of pressure-dependent rate for multiple lymphangions. Biomech Model Mechanobiol 17, 1513–1532 (2018). https://doi.org/10.1007/s10237-018-1042-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10237-018-1042-7