Abstract
Background
Comparisons between surgical cases of mixed poorly differentiated adenocarcinoma and pure signet ring cell carcinoma have revealed higher frequencies of lymph node metastasis and submucosal invasion in the former. However, this comparison has not been reported for endoscopically treated cases. Therefore, we compared cases of curative and noncurative resection in patients who underwent endoscopic submucosal dissection to determine what factors could influence the curative resection rate.
Methods
We analyzed 268 undifferentiated-type early gastric cancers in 264 patients treated with endoscopic submucosal dissection in our hospital between March 2005 and March 2017, involving 229 and 39 cases of curative and noncurative resection, respectively. Treatment results were compared between 129 lesions of pure signet ring cell carcinoma and 139 lesions of mixed poorly differentiated adenocarcinoma.
Results
The overall curative resection rate was 85.4% (229/268). On comparing noncurative and curative resection cases, after excluding factors that determine curative resection (e.g., tumor diameter), we found that the former was associated with older age and significantly more mixed poorly differentiated adenocarcinomas, with odds ratios of 1.052 [95% confidence interval (CI), 1.017–1.089] and 2.746 (95% CI, 1.162–6.485), respectively, on multivariate analysis. The curative resection rate was significantly higher in pure signet ring cell carcinoma than in mixed poorly differentiated adenocarcinoma (93.8% vs. 77.7%, respectively).
Conclusion
Advanced age and mixed poorly differentiated adenocarcinoma predicted endoscopic noncurative resection.
Similar content being viewed by others
Introduction
According to reports by Gotoda et al. [1] and Hirasawa et al. [2], undifferentiated-type early gastric cancers (UD-type EGCs) have a negligible risk of lymph node metastasis so long as they measure 20 mm or less, are without ulcer scars, and have not invaded the lymph or blood vessels; consequently, they fall into the category of extended indications for endoscopic submucosal dissection (ESD), as proposed by the Japanese Gastric Cancer Association (JGCA) [3]. However, it should be noted that these studies used surgical specimens.
UD-type EGCs mainly comprise poorly differentiated adenocarcinoma (PDA) and signet ring cell carcinoma (SRC). Differences between these carcinomas have occasionally been reported with regard to surgical cases. For instance, comparisons between surgical specimens of mixed PDA and pure SRC have revealed higher frequencies of lymph node metastasis and submucosal invasion in the former [4,5,6]. Treatment outcomes and prognoses of UD-type EGCs after endoscopic treatment have also been studied, and the suitability of endoscopic treatment in these cases has been discussed [7,8,9]. However, mixed PDA and pure SRC have not been studied separately. Although there have been some reports on the endoscopic treatment of UD-type EGCs, none has addressed what differences exist between mixed PDA and pure SRC as histological factors and whether UD-type EGC with mixed PDA or with pure SRC predicts curative or noncurative resection.
Previous reports on surgical cases [4,5,6] suggest that pure SRC manifests a lesser degree of malignancy than mixed PDA, but the results may differ between histological preparations of surgical specimens that contain the full thickness of the gastric wall (sectioned at 5-mm intervals) and those from endoscopic resection, which are limited to the submucosal layer of the stomach (sectioned at 2-mm intervals).
In this study, we compared UD-type EGCs treated with curative resection with those treated with noncurative resection and examined whether histological type would have an influence on curative or noncurative resection, as has been observed in surgical cases. In addition, only tumors conforming to the following criteria specified by the JGCA guidelines [3] were studied: diameter of 20 mm or less, intramucosal carcinoma, negative lymphovascular invasion, negative ulcer scars, negative vertical margin, negative horizontal margin, and R0 resection. These cases were also examined for other factors that might predict curative or noncurative resection. In addition, the lesions were divided into mixed PDAs and pure SRCs, and the difference in the rate of curative resection between these two groups was analyzed.
Patients and methods
Patients
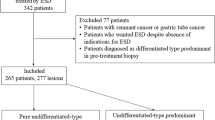
A total of 3419 patients with early gastric cancers were treated with ESD in our hospital between March 2005 and March 2017. Among these patients, there were 268 pure UD-type EGCs in 264 (7.3%) patients, excluding remnant cancers (because of difficulty in technique), differentiated carcinomas, and mixed histological-type carcinomas (including predominantly undifferentiated mixed type). The 268 UD-type EGCs comprised 229 lesions treated with curative resection and 39 treated with noncurative resection. In addition, there were 129 pure SRCs and 139 mixed PDAs. The cases of mixed PDA included 8 cases of pure PDA devoid of SRC. Pure PDAs were included with mixed PDAs in our analysis because the purpose of this study was to compare lesions with and without PDA in accordance with the aforementioned previous studies of surgical cases [4,5,6].
Methods
This was a single-center retrospective study. First, all undifferentiated carcinomas were categorized by patient age, sex, lesion location, macroscopic type, and treatment outcomes of ESD (maximum tumor diameter, tumor depth, histological type, lymphovascular invasion, horizontal margin, vertical margin, ulcerative findings, en bloc resection, R0 resection, rate of curative resection, and adverse events). Following this, cases of curative and noncurative resection were compared. Cases of curative and noncurative resection were examined separately for pure SRC and mixed PDA to identify any differences in the rate of curative resection between the two groups. All patients with UD-type EGCs treated with ESD underwent preoperative conventional endoscopy, dye-spraying endoscopy, and narrow-band imaging (NBI)–combined magnifying endoscopy to diagnose the extent and depth of the tumor. According to the guidelines issued by the JGCA [3], cases with intramucosal carcinomas measuring 20 mm or less and without ulcerative findings were preoperatively determined as meeting the criteria for expanded indications for ESD, which was performed accordingly. For each lesion, biopsies from four sites surrounding the lesion were confirmed to be negative. For each resected ESD specimen, sections were prepared at 2-mm intervals for pathological evaluation. Pathological examination was always performed by pathologists specialized in gastroenterology. In accordance with previous studies in surgical cases [4,5,6], specimens consisting of SRC alone were defined as pure SRCs, and SRC lesions were defined as mixed PDAs if even a minimal PDA component was present. Endpoints were maximum tumor diameter, invasion depth, histological type, ulcerative findings, lymphovascular invasion, horizontal margin, and vertical margin. Curative resection was defined as the presence of intramucosal UD-type EGCs measuring 20 mm or less in diameter without ulcerative findings, and with negative horizontal margins, negative vertical margins, and no lymphovascular invasion, in accordance with the JGCA gastric cancer treatment guidelines [3].
All patients undergoing treatment for UD-type EGCs were given a detailed explanation of the advantages and disadvantages of ESD before providing their informed consent. Consensus has already been established in our institution regarding the expanded indication of ESD to UD-type EGCs.
Statistical analysis
Fisher’s exact test was used for comparison of the two groups. The mean values and standard deviations of age and tumor diameter were analyzed using the t test and the F test. The Mann–Whitney U test was used for unequal variances with median. When comparing noncurative and curative cases, factors that were found to have significant differences on univariate analysis were subjected to multivariate analysis (logistic regression analysis).
Statistical significance was set at p < 0.05. StatView software, version 5.0 (SAS Institute, Cary, NC, USA), was used for statistical processing.
Results
Table 1 shows the overall patient characteristics and treatment results in cases with UD-type EGCs. The overall rate of curative resection was 85.4% (229/268). A comparison of patient characteristics between cases of curative resection and noncurative resection (Table 2) revealed that in cases of curative resection the patients were of significantly younger age. Flat-, depressed-, or elevated-type lesions accounted for macroscopic type in the majority of cases, and the flat type was significantly more common in cases of curative resection. A comparison of the treatment results (Table 3) showed that factors determining curative and noncurative resection, as specified in the JGCA guidelines (i.e., tumor diameter, submucosal invasion, lymphovascular invasion, vertical margin, and horizontal margin), were all lower in cases of curative resection than in cases of noncurative resection, and the rates of en bloc resection and R0 resection were significantly higher in cases of curative resection. With regard to histological type, mixed PDA and pure SRC were significantly more frequent in cases of noncurative and curative resection, respectively. To identify factors that predict noncurative resection, other than those specified by the JGCA guidelines for determining curative and noncurative resection, multivariate analysis was performed for those factors that showed a significant difference on univariate analysis (age, flat-type lesion, number of patients with mixed PDA) (Table 4). Compared with cases of curative resection, cases of noncurative resection showed a significant difference for the number of cases with patient age, and the number of mixed PDAs, with odds ratios of 1.052 (95% CI, 1.017–1.089), and 2.746 (95% CI, 1.162–6.485), respectively. When the actual rates of curative resection for mixed PDA and pure SRC were compared, the rate was 77.7% for mixed PDA whereas it was significantly higher, at 93.8%, for pure SRC (Table 5). Thus, among cases of noncurative resection of UD-type EGC, cases with advanced patient age and mixed PDA were significantly more frequent.
Discussion
The rate of curative resection with ESD for UD-type EGCs varies widely among different institutions, ranging from 63.9% to 82.5% [7, 8, 10,11,12,13,14,15]. In this study, overall the rate of curative resection of UD-type EGCs was 85.4%. This study compared cases of curative and noncurative resection in relationship to their histological type (pure SRC or mixed PDA) to identify factors predictive of curative or noncurative resection, in addition to known predictive factors (tumor diameter, invasion depth, ulcerative findings, lymphovascular invasion, horizontal margin, vertical margin, and R0 resection).
In a comparison of cases of curative and noncurative resection, multivariate analysis revealed a significant difference in age, flat-type lesions, and presence of mixed PDA. Thus, flat-type lesions were identified as a predictor of curative resection, and advanced age and the presence of PDA as predictors of noncurative resection.
The patients with noncurative resection were significantly older. Patients of advanced age often have more underlying diseases than younger patients and are in poorer general condition [16, 17]. It has also been reported that the 5-year survival rate after surgical gastrectomy is lower in patients aged 75 years or older than in young or middle-aged patients [18]. Therefore, it is sometimes undesirable to proceed with aggressive surgical treatment in elderly patients, and it is suggested that complete endoscopic biopsy be initially attempted as a means of diagnostic therapy, particularly when it is difficult to make a definitive judgment in diagnosing the depth of invasion. This detail may explain why patients with noncurative resection were of significantly higher ages.
As for the significantly higher percentage of mixed PDA, previous studies comparing pure SRCs and mixed PDAs in surgical specimens have shown that the rates of lymph node metastasis and biological malignancy were higher in mixed PDAs [4,5,6]. In contrast, studies using surgical specimens of early gastric cancer have found that SRC has a better prognosis [6, 19,20,21,22,23]. It has also been reported that SRC progresses from intramucosal carcinoma to PDA at the site of invasion [24]. A recent report has also documented that submucosal invasion was more frequent in PDAs than in SRCs treated with endoscopy [25]. A higher frequency of positive vertical margins in PDAs has also been reported [26]. However, in these reports of endoscopic treatment, there was no detailed analysis concerning whether SRCs were pure SRCs or whether they were SRCs with mixed PDA components. In this study, we defined the SRC group as pure SRCs alone, and cases that were predominantly SRC but contained a mixed PDA component were included in the mixed PDA group. Our results suggested that the presence of a PDA component was generally a predictor of noncurative resection. When the actual rates of curative resection were compared, the percentage was 93.8% among pure SRCs, whereas for mixed PDAs it was significantly lower at 77.7%. Among the factors determining noncurative resection, tumor diameter was significantly greater and submucosal invasion and positive vertical margin were more frequent, consistent with findings from previous reports [25, 26]. Additionally, ulcerative findings were also more common. Noncurative resection is defined as the risk of local recurrence or lymph node recurrence at a later stage, unless additional surgical resection is performed. Therefore, specimens from endoscopic resection also predicted the high biological malignancy of mixed PDA.
In this study, there were 129 pure SRCs, accounting for 48.1% of all UD-type cancers, and the rate of curative resection was 93.8%. The rate of curative resection is reported to be 91.1% among cases of differentiated-type early gastric cancer measuring 20 mm or less [27], which is higher than the reported rates of curative resection of UD-type EGCs (63.9–82.5%) [7, 8, 10,11,12,13,14,15]. Preoperative biopsy involves obtaining a small portion of the lesion. Therefore, even if the preoperative biopsy specimen is indicative of SRC, this does not mean the whole tumor is SRC. However, if a preoperative biopsy specimen is indicative of mixed PDA, the lesion is classified as mixed PDA. Mixed PDA has a lower curative resection rate than SRC.
In the future, it will be helpful to distinguish between mixed PDA and SRC before endoscopic treatment for UD-type EGCs. It will be important to explain the differences to patients to allow them to choose endoscopic treatment based on a sufficient understanding of the possibility of additional surgical resections.
However, the results of this study suggest that the rate of curative resection of pure SRCs is comparable to that of differentiated cancer. To date, there have been no reports stating that pure SRC is distinguishable from mixed PDA before endoscopic treatment. Further studies are necessary to investigate differences in endoscopic findings between pure SRCs and mixed PDAs.
The limitations of this study are its retrospective nature and single-nstitution setting. However, the subjects of this study represent an accumulation of clinical cases treated in a cancer-specialized hospital over a period of approximately 12 years, enabling a sufficiently meaningful investigation. Because our institution is a cancer-specialized hospital, there might have been a selection bias when comparing with the general prevalence rate, taking into consideration the frequency of patient referral. However, as the study included all patients who underwent endoscopic treatment, the bias is likely to be minimal. When patients are referred to our hospital, a preoperative biopsy will have already been taken at their previous hospital. If cancer is diagnosed based on this biopsy, we will not conduct a biopsy from the lesion again. As a negative biopsy, only a surrounding biopsy is taken, and endoscopic treatment is performed. Lesions found at our hospital underwent biopsy and were diagnosed by a pathologist who specializes in the digestive tract. Therefore, the condition of the preoperative pathology diagnosis differs according to the case. The cases aggregated this time are all cases who presented at our hospital during the study period. Because the preoperative biopsy diagnosis is not uniform, a strong bias is likely. Therefore, the inclusion of the preoperative biopsy results was considered inappropriate and was not considered for analysis.
In conclusion, advanced age and mixed PDA were predictors of noncurative resection. Compared to mixed PDA, the rate of curative resection for pure SRC was found to be as high as that of differentiated-type early gastric cancer.
References
Gotoda T, Yanagisawa A, Sasako M, Ono H, Nakanishi Y, Shimoda T, et al. Incidence of lymph node metastasis from early gastric cancer: estimation with a large number of cases at two large centers. Gastric Cancer. 2000;3:219–25.
Hirasawa T, Gotoda T, Miyata S, Kato Y, Shimoda T, Taniguchi H, et al. Incidence of lymph node metastasis and the feasibility of endoscopic resection for undifferentiated-type early gastric cancer. Gastric Cancer. 2009;12:148–52.
Japanese Gastric Cancer Association. Japanese gastric cancer treatment guidelines, ver. 4. Gastric Cancer. 2014;2017(20):1–19.
Takizawa K, Shimoda T, Nakanishi Y, Taniguchi H, Oda I, Gotoda T. Expanded indication for endoscopic resection from pathological viewpoint: the possibility of sm invasion by undifferentiated-type early gastric cancer. Stomach Intest. 2006;41:9–17 (in Japanese).
Lee IS, Lee S, Park YS, Gong CS, Yook JH, Kim BS. Applicability of endoscopic submucosal dissection for undifferentiated early gastric cancer: mixed histology of poorly differentiated adenocarcinoma and signet ring cell carcinoma is a worse predictive factor of nodal metastasis. Surg Oncol. 2017;26:8–12.
Imamura T, Komatsu S, Ichikawa D, Kawaguchi T, Kosuga T, Okamoto K, et al. Early signet ring cell carcinoma of the stomach is related to favorable prognosis and low incidence of lymph node metastasis. J Surg Oncol. 2016;114:607–12.
Okada K, Fujisaki J, Yoshida T, Ishikawa H, Suganuma T, Kasuga A, et al. Long-term outcomes of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Endoscopy. 2012;44:122–7.
Abe S, Oda I, Suzuki H, Nonaka S, Yoshinaga S, Odagaki T, et al. Short- and long-term outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Endoscopy. 2013;45:703–7.
Inokuchi Y, Kobayashi M, Kudo K, Yamada H, Inoue S, Nishimura K, et al. Outcomes and precautions of endoscopic submucosal dissection for undifferentiated-type early gastric cancer. Therap Adv Gastroenterol. 2015;8:255–62.
Kamada K, Tomatsuri N, Yoshida N. Endoscopic submucosal dissection for undifferentiated early gastric cancer as the expanded indication lesion. Digestion. 2012;85:111–5.
Yamamoto Y, Fujisaki J, Hirasawa T, Ishiyama A, Yoshimoto K, Ueki N, et al. Therapeutic outcomes of endoscopic submucosal dissection of undifferentiated-type intramucosal gastric cancer without ulceration and preoperatively diagnosed as 20 millimetres or less in diameter. Dig Endosc. 2010;22:112–8.
Kim YY, Jeon SW, Kim J, Park JC, Cho KB, Park KS, et al. Endoscopic submucosal dissection for early gastric cancer with undifferentiated histology: could we extend the criteria beyond? Surg Endosc. 2013;12:4656–62.
Kang HY, Kim SG, Kim JS, Jung HC, Song IS. Clinical outcomes of endoscopic submucosal dissection for undifferentiated early gastric cancer. Surg Endosc. 2010;24:509–16.
Kim JH, Lee YC, Kim H, Song KH, Lee SK, Cheon JH, et al. Endoscopic resection for undifferentiated early gastric cancer. Gastrointest Endosc. 2009;69:e1–9.
Park YD, Chung YJ, Chung HY, Yu W, Bae HI, Jeon SW, et al. Factors related to lymph node metastasis and the feasibility of endoscopic mucosal resection for treating poorly differentiated adenocarcinoma of the stomach. Endoscopy. 2008;40:7–10.
Centers for Disease Control and Prevention. Public health and aging: trends in aging: United States and Worldwide. JAMA. 2003;289:1371–3.
Fried L, Barron J. Older adults. In: Handbook of urban health: population, methods, and practice. Springer: New York; 2005
Kusano C, Iwasaki M, Kaltenbach T, Conlin A, Oda I, Gotoda T. Should elderly patients undergo additional surgery after non-curative endoscopic resection for early gastric cancer? Long-term comparative outcomes. Am J Gastroenterol. 2011;106:1064–9.
Chiu CT, Kuo CJ, Yeh TS, Hsu JT, Liu KH, Yeh CN, et al. Early signet ring cell gastric cancer. Dig Dis Sci. 2011;56:1749–56.
Huh CW, da Jung H, Kim JH, Lee YC, Kim H, Kim H, et al. Signet ring cell mixed histology may show more aggressive behavior than other histologies in early gastric cancer. J Surg Oncol. 2013;107:124–9.
Hyung WJ, Noh SH, Lee JH, Huh JJ, Lah KH, Choi SH, et al. Early gastric carcinoma with signet ring cell histology. Cancer (Phila). 2002;94:78–83.
Kunisaki C, Shimada H, Nomura M, Matsuda G, Otsuka Y, Akiyama H. Therapeutic strategy for signet ring cell carcinoma of the stomach. Br J Surg. 2004;91:1319–24.
Gronnier C, Messager M, Robb WB, Thiebot T, Louis D, Luc G, et al. Is the negative prognostic impact of signet ring cell histology maintained in early gastric adenocarcinoma? Surgery (St. Louis). 2013;154:1093–9.
Nakamura K, Sugano H. Microcarcinoma of the stomach measuring less than 5 mm in the largest diameter and its histogenesis. Prog Clin Biol Res. 1983;132:107–16.
Bang CS, Park JM, Baik GH, Park JJ, Joo MK, Jang JY, et al. Therapeutic outcomes of endoscopic resection of early gastric cancer with undifferentiated-type histology: a Korean ESD registry database analysis. Clin Endosc. 2017;50:569–77.
Kim JH, Kim YH, da Jung H, Jeon HH, Lee YC, Lee H, et al. Follow-up outcomes of endoscopic resection for early gastric cancer with undifferentiated histology. Surg Endosc. 2014;28:2627–33.
Yamaguchi N, Isomoto H, Fukuda E, Ikeda K, Nishiyama H, Akiyama M, et al. Clinical outcomes of endoscopic submucosal dissection for early gastric cancer by indication criteria. Digestion. 2009;80:173–81.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
There is no conflict of interest in this study.
Human rights statement and informed consent
This study was conducted in compliance with the principle of the “Declaration of Helsinki” issued in 1964 and revised thereafter. Before registration, all personal identifiers were deleted, and informed consent was obtained from all patients to allow the use of pathological specimens and imaging data for this study. The study has been approved by the institutional review board at the Cancer Institute Hospital (IRB no.2017-1033).
Rights and permissions
About this article
Cite this article
Horiuchi, Y., Fujisaki, J., Yamamoto, N. et al. Mixed poorly differentiated adenocarcinoma in undifferentiated-type early gastric cancer predicts endoscopic noncurative resection. Gastric Cancer 21, 689–695 (2018). https://doi.org/10.1007/s10120-017-0788-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10120-017-0788-4