Abstract
Objectives
This in vitro study aimed to analyze the anti-inflammatory and wound healing potential of green tea extract (GTE) in human gingival epithelial keratinocytes (HGEK) treated with lipopolysaccharides (LPS).
Materials and methods
A cell viability assay was conducted using MTT to determine nontoxic levels of GTE on immortalized HGEK. Cells were concomitantly treated with LPS (1 μg/ml) and GTE (1 mg/ml, 2.5 mg/ml, 5 mg/ml, and 10 mg/ml) to assess inflammation. Gene expression levels of inflammatory markers IL-β1, IL-6, and TNFα were measured by RT-PCR and their protein production was assessed by ELISA. The scratch wound healing assay was used to investigate the effects of different concentrations of GTE on cell migration. We also explored the effect of GTE on the induction of the Nrf2/HO-1 pathway in the cells with or without LPS.
Results
GTE at concentrations of 2.5 mg/ml, 5 mg/ml, and 10 mg/ml significantly enhanced cell viability (p < 0.05). And IL-β1, IL-6, and TNFα gene expression presented up to 10-fold decrease compared with LPS-treated cells, which was also similarly found on the protein levels. At the same concentrations, cell migration increased.
Conclusions
The mechanism results showed that GTE produced the anti-inflammatory response by activating the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway and increasing the level of anti-oxidant protein heme oxygenase-1 (HO-1).
Clinical relevance
GTE may be potentially used as oral rinse anti-inflammatory drug for treatment and prevention of oral inflammatory diseases, which is shown here by the ability to reduce the inflammation and increase in cell migration in a dose-dependent manner.
Similar content being viewed by others
Introduction
Growing interest in natural remedies has brought about an increase in scientific reports on substances such as GTE (Camellia sinensis). Green tea is a worldwide popular drink that has many compounds containing large amounts of important antimutagenic and anticarcinogenic polyphenol nutrients used medicinally in Asia for 5000 years [1]. Green tea consumption has been associated with health promotion as it is a natural product without side effects [2]. Components of GTE are basically known as polyphenols, such as epicatechin (EC), epicatechin gallate (ECG), epigallocatechin (EGC), and epigallocatechin-3-gallate (EGCG) [3,4,5]. Among the green tea polyphenol components, EGCG was found to be the most abundant constituent with over 30% of total catechins [6], and it was shown to reduce dentin surface loss under erosive/abrasive conditions in oral mouth rinsing [7,8,9]. EGCG has also been suggested to influence the cell anti-inflammatory activity by suppressing the synthesis and inhibiting action of many pro-inflammatory mediators, nitric oxide synthase, peroxynitrite, reactive oxygen/nitrogen species, and cyclooxygenase-2 [10,11,12,13,14,15,16]. Oral tissue inflammation is primarily initiated by epithelial gingival cells and macrophages when exposed to pro-inflammatory cytokines, interferon-γ, or oral bacterial LPS (lipopolysaccharides) [17]. Activation of macrophage may stimulate the release of inflammatory mediators also known as pro-inflammatory cytokines (interleukin-β1, interleukin-6, tumor necrosis factor α) [17, 18]. The production of inflammatory mediators and cytokines in prolonged oral tissue inflammation (i.e., periodontitis) can cause extensive cellular and tissue destruction.
To better prevent or avoid prolonged side effect of oral tissue inflammation, development of more anti-inflammatory agents is still needed as to be employed in treatment of oral diseases. In recent years, the inflammatory-inhibitory potential of naturally derived GTE substances has gained increasing attention. In comparison with steroidal or non-steroidal chemical drugs for treating inflammation, naturally derived substances for preventing prolonged inflammation have limited side effects and fewer intolerance issues, and could be available at lower costs than synthetic drugs [10]. Previous studies have shown green tea polyphenols, such as EGCG, to inhibit matrix-metalloproteinase-2 and matrix-metalloproteinase-9 key proteases involved in destructive periodontal diseases [19]. However, the exact role of these EGCGs in mediating inflammation on oral tissues is still unknown since most of previous research have been focusing on the use of green tea EGCC on reducing oral hard tissue erosion-abrasion [7, 9]. As previous studies have focused on the use of green tea extract polyphenols on inflammation in other cell line types and in animal models, it seems expedient to study the application of green tea phenols for their potential to block cytokine-involved inflammatory cascades in oral cells. Further, more knowledge is needed on how these EGCG polyphenols would improve oral tissue wound healing. In addition, other pathways were pointed to assist on progression of inflammation and one of these pathways is the nuclear factor erythroid 2-related factor 2 (Nrf2) pathway [20]. The activation of Nrf2 triggered by medication has increasingly gained attention on its possible use in therapies for inflammatory disorders [21, 22] and proved that Nrf2 pathway indeed may play a critical role in inflammation. However, there have been only few Nrf2 activators found to be employed to support oral therapies. Thus, the development of new activating clinical drugs of Nrf2 pathway should be an important goal in the pharmaceutical industry.
Accordingly, the purpose of the present study was to analyze the distinct anti-inflammatory activity and wound healing potential, given the ease of application, of green tea extract topically on the oral epithelium via oral mouth rinse hygiene products. To this end, we have demonstrated the anti-inflammatory potential of green tea extract in human gingival epithelial keratinocytes treated with periodontopathogenic bacterial endotoxin (LPS) by assessing the positive effects on cell viability, wound healing, and downregulation of important inflammatory markers. Also, our findings suggest that GTE could be a strong potential Nrf2 activator for the treatment and prevention of oral inflammatory diseases.
Materials and methods
Green tea extract solutions
The stock solution of the green tea extract (OM24®, 100% Camellia sinensis leaf extract) contained a mixture of catechin concentrations of EGCG (epigallocatechin-3-gallate, 70%), EGC (epigallocatechin, 4%), ECG (epicatechin gallate, 0.6%), and EC (epicatechin, 3%) (Omnimedica, Zürich, Switzerland). The green tea working solutions of 1 mg/ml, 2.5 mg/ml, 5 mg/ml, and 10 mg/ml were prepared prior to use by mixing GTE powder with 1× phosphate-buffered saline (PBS, Sigma-Aldrich). PBS 1× was used as negative control in the cell treatment experiments. This concentration was chosen based on previous studies of dentin erosion using oral mouth rinses containing GTE [7, 9, 19].
Cell culture and LPS induction
Immortalized human gingival epithelial keratinocytes (HGEK-16) were donated by the Oral Microbiology Institute, Center of Dental Medicine, University of Zurich. The cell line was previously established by transducing E6/E7 oncoproteins from human papillomavirus type 16 to primary cells [23]. Gingival epithelial keratinocyte cells were cultured in an incubator (5% CO2, 95% humidity at 37 °C) and passaged at regular intervals depending on their growth characteristics using 0.25% trypsin (Seromond Biochrom, Berlin, Germany) and maintained in a complete epithelial medium consisting of a defined keratinocyte serum-free medium (Gibco, 10744-019), supplemented with 100 U/ml penicillin (Sigma-Aldrich, 15140-122), 100 mg/ml streptomycin (Sigma-Aldrich, 15140-122), 2 mM l-glutamine (Sigma-Aldrich, G7513), and 0.25 mg/ml fungizone (Sigma-Aldrich, 15290-018). Medium was changed every 3 to 4 days and cells were passaged once a week. The cells used in this study were between the fifth and fifteenth passage. There were six treatments used for this study where inflammation was induced in the gingival epithelial keratinocytes: (1) negative control (PBX 1×); (2) positive control (1 μg/ml LPS from Porphyromonas gingivalis, Sigma-Aldrich); (3) HGEK treated with 1 μg/ml LPS and 1 mg/ml GTE; (4) HGEK treated with 1 μg/ml LPS and 2.5 mg/ml GTE; (5) HGEK treated with 1 μg/ml LPS and 5 mg/ml GTE; and (6) HGEK treated with 1 μg/ml LPS and 10 mg/ml GTE. The cells were incubated for 24 h and cell-free supernatant was used for the IL-β1, IL-6, and TNFα gene expression assay.
Cell viability assay
HEGK cell viability was determined by 3-(4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT, Sigma-Aldrich) dye reduction assay (5 mg/ml in PBS 1×). Gingival epithelial keratinocytes (0.1 × 106 cells/ml) were re-cultured in 12-well plates supplemented with fresh medium containing different concentrations of GTE (1 mg/ml, 2.5 mg/ml, 5 mg/ml, and 10 mg/ml). After an exposure time of 24 h, the solutions were aspirated and the cells washed with PBS 1× before culture medium was newly added. At 24 h after exposure to the respective GTE solution, 500 ml of MTT was added to each well and incubated for 4 h at 37 °C in the dark. In the next step, MTT was removed by aspiration from the wells and isopropanol was added (200 ml, 1 N HCl) to solubilize the MTT-formazan crystals formed. The test absorbance at 570 nm and reference absorbance at 630 nm were measured using a spectrophotometer plate reader (Corning Costar, Corning, NY, USA). Experiments were performed in triplicate.
Real-time quantitative polymerase chain reaction analysis
Total ribonucleic acid was isolated using Trizol reagent and RNAeasy Mini kit (QIAGEN), 24 h after GTE exposure in different concentrations (1 mg/ml, 2.5 mg/ml, 5 mg/ml, and 10 mg/ml) and LPS (1 μg/ml). Primer sequences for genes encoding IL-β1, IL-6, and TNFα were designed from Primer3 (version 0.4.0) as follows: IL-β1 (forward primer: 5′-TAGAGCTGCTGGCCTTGTTA-3′, reverse primer: 5′-ACCTGTAAAGGCTTCTCGGA-3′); TNFα (forward primer: 5′-TGCCTATGTCTCAGCCTCTT-3′, reverse primer: 5′-GAGGCCATTTGGGAACTTCT-3′); IL-6 (forward primer: 5′-ATGAACTCCTTCTCCACAAGC-3′, reverse primer: 5′-GTTTTCTGCCAGTGC CTCTTTG-3′); and GAPDH (forward primer: 5′-GCTCTCTGCTCCTCCCTGTT -3′, reverse primer: 5′-CACACCGACCTTCACCATCT -3′). Following Trizol extraction, real-time quantitative polymerase chain reaction (RT-PCR) analysis was performed using 15 ml final reaction volume of TaqMan’s One-Step Master Mix kit (Applied Biosystems). Total ribonucleic acid (40 ng) was used per sample well. Each sample contained pooled messenger ribonucleic acid from Trizol extractions collected from the cell cultures exposed with and without LPS + GTE at different concentrations at 24 h. All samples were tested in triplicate and 3 independent experiments were performed. The 2-∆∆ct method was used to calculate gene expression levels relative to GAPDH and normalized to negative control cells.
Enzyme-linked immunosorbent assay–protein expression
Protein levels of inflammatory markers were determined from cell culture supernatant using human IL-1β (RAB0273), IL-6 (RAB0306), and TNFα (RAB0476) enzyme-linked immunosorbent assay (ELISA) Kits (Sigma-Aldrich, St. Louis, MO, USA) following the manufacturer’s protocol. Briefly, gingival epithelial keratinocytes (0.1 × 106 cells/ml) were re-cultured in 12-well plates supplemented with fresh medium containing different concentrations of GTE (1 mg/ml, 2.5 mg/ml, 5 mg/ml, and 10 mg/ml) and LPS (1 μg/ml) and incubated overnight at 37 °C. After incubation time, the cell culture supernatant was collected in preparation for the ELISA.
Scratch wound healing migration assay
To determine the effect of GTE concentrations on wound healing, a scratch-wounded monolayer model was used. The cells were seeded at a density of 0.1 × 106 cells/ml and cultured into each well of a 12-well plate and incubated for 24 h at 37 °C until confluent. Prior to the scratch assay, the cells were exposed to 10 μg/ml of mitomycin C (Sigma-Aldrich) in serum-free media for 2 h, which inhibited mitosis of the cells. The wound was produced by scratching with a 10-μl pipette tip (700–900 μm in diameter). Following PBS 1× washes to remove cell debris, the remaining adherent cells were divided into 6 treatment groups: (1) negative control (PBX 1×); (2) positive control (1 μg/ml LPS from P. gingivalis, Sigma-Aldrich); (3) HGEK treated with 1 μg/ml LPS and 1 mg/ml GTE; (4) HGEK treated with 1 μg/ml LPS and 2.5 mg/ml GTE; (5) HGEK treated with 1 μg/ml LPS and 5 mg/ml GTE; (6) HGEK treated with 1 μg/ml LPS and 10 mg/ml GTE. Digital images were captured using a camera-equipped, inverted microscope (Carl Zeiss, Inc., Thorwood, NY, USA) and wound width measurements were subtracted from wound width at time 0 to obtain the net wound closure. The distance between edges of injured monolayers was measured using the ImageJ software (National Institutes of Health, USA) in pixels and wound closure was expressed as the difference in widths at 0 h and 24 h after wound simulation.
Western blot analysis
Western blotting was performed to determine the protein expression of Nrf2 and HO-1 proteins after cell treatment with LPS and GTE. The cells (0.1 × 106 cells/ml) were seeded in a 12-well plate and incubated for 24 h at 37 °C. After incubation, the cells were treated with various concentrations of GTE (1 mg/ml, 2.5 mg/ml, 5 mg/ml, and 10 mg/ml) and exposed to LPS (1 μg/ml) for 24 h. Protein was extracted with radio-immunoprecipitation assay (RIPA) buffer containing a protease inhibitor cocktail and centrifuged at 12,000×g for 15 min at 4 °C. The supernatant protein was quantified using a bicinchoninic acid assay (BCA, Thermo Fisher Scientific, Rockford, USA) and stored at − 80 °C. Total lysates were resolved in SDS-PAGE. Proteins were blotted onto a nitrocellulose membrane and incubated with primary antibodies and the corresponding secondary antibodies. Immune complexes were visualized by the use of an enhanced chemiluminescence western blotting system (BioRad, Richmond, CA). Antibodies used for immunoblotting were as follows: antibody against Nrf2 (ab137550), HO-1 (ab90492), and GAPDH (ab9484) (Abcam).
Statistical analysis
The mean values and standard deviations were computed for the cell viability test and multiple comparisons were conducted by analysis of variance (ANOVA), followed when appropriate by Bonferroni post hoc tests with a global significance level of 5% to assess the statistical significance of the differences between the experimental groups (SPSS version 22.0, Munich, Germany). All the in vitro experiments were performed in triplicate and from three independent experiments unless otherwise mentioned. Differences were considered significant at p < 0.05.
Results
Effect of GTE on HGEK cell viability
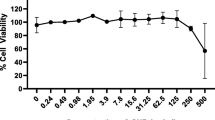
The cell viability, as a preliminary study, was shown to be significantly enhanced after GTE exposure at concentrations of 2.5 mg/ml, 5 mg/ml, and 10 mg/ml compared with the cells cultured without treatment (p < 0.05 at 24 h) and with LPS treatment by performing the cell viability assay. Based on these results and previous studies, the green tea extract concentrations of 1 mg/ml, 2.5 mg/ml, 5 mg/ml, and 10 mg/ml were selected for further experiments. GTE at concentrations of 2.5 mg/ml, 5 mg/ml, and 10 mg/ml showed significant increase a maximum of 1.5-fold compared with untreated control, while 1 mg/ml did not increase viability (Fig. 1).
Increase in cellular activity of keratinocyte cells 24 h after exposure. A significant increase in keratinocyte cellular activity was detected for GTE at 2.5 mg/ml, 5 mg/ml, and 10 mg/ml compared with non-treated cells (control). The circle in the figure indicates the outliners. Data is shown of 3 samples (3 wells each). *p < 0.001
Green tea extract downregulation of markers for inflammation
To investigate whether GTE could inhibit LPS-induced inflammatory gene expression of IL-β1, IL-6, and TNFα, cells were simultaneously pretreated with GTE (1 mg/ml, 2.5 mg/ml, 5 mg/ml, and 10 mg/ml) and LPS (1 μg/ml) for 24 h. The results showed that LPS (1 μg/ml) treatment remarkedly increased by almost 10-fold the expression of IL-β1, IL-6, and TNFα compared with untreated cells (Fig. 2a). Thus, LPS-treated keratinocytes showed the highest level of all pro-inflammatory cytokines and modulators tested in this study. Further, the positive control LPS (1 μg/ml) was used as a standard to obtain GTE inflammatory inhibition activity toward IL-β1, IL-6, and TNFα expression. GTE at concentrations of 2.5 mg/ml, 5 mg/ml, and 10 mg/ml decreased all pro-inflammatory cytokines tested in this study compared with each positive control (8-fold change decrease) (LPS 1 μg/ml) (Fig. 2a). The protein levels of IL-β1, IL-6, and TNFα by ELISA analysis have also shown significant decrease on GTE test cell groups (GTE at 2.5 mg/ml, 5 mg/ml, and 10 mg/ml and LPS at 1 μg/ml) compared with the positive control (LPS 1 μg/ml) (p < 0.05), confirming the RT-PCR results (Fig. 2b).
GTE-stimulated downregulation of inflammatory markers and protein production of same markers on gingival keratinocytes exposed to LPS. a RT-PCR analysis shows gene expression decrease of IL-1β, IL-6, and TNFα under simultaneous exposure to GTE (1 mg/ml, 2.5 mg/ml, 5 mg/ml, and 10 mg/ml) and LPS (1 μg/ml) compared with control (white bars). b ELISA results showed decrease in translational production of inflammatory proteins IL-1β, IL-6, and TNFα in the cells treated with GTE and LPS compared with control (white bars). *p < 0.05. Mean ± standard deviation
Scratch wound healing assay (cell migration)
HEGK cells exposed to GTE at concentrations of 2.5 mg/ml, 5 mg/ml, and 10 mg/ml similarly elicited increase in cell migration at 24 h compared with untreated negative control and positive control (LPS 1 μg/ml) (up to 40%). Wound closure was observed to be almost complete after 24 h in the presence of GTE (2.5 mg/ml, 5 mg/ml, and 10 mg/ml). Positive and negative controls showed around 80% incomplete healing patterns at 24 h (Fig. 3).
Induction of keratinocyte cell migration on in vitro scratch wound healing assay after GTE (1 mg/ml, 2.5 mg/ml, 5 mg/ml, and 10 mg/ml) and LPS (1 μg/ml) exposure. Representative images are shown from 3 independent experiments and light gray areas define the areas lacking cells (scale bar 300 μm). Images were analyzed using the ImageJ software to calculate wound area. Data are expressed as the mean values of percentage wound closure relative to the corresponding 0 h time point and represent the mean percentage closure ± standard deviation (n = 3): *p < 0.05 vs. time-matched treated control for 12 h and 24 h
Mechanism analysis via Nrf2 signaling pathway
In order to understand whether GTE could exert its anti-inflammatory effect through activating the Nrf2 pathway, the downstream protein HO-1 of the Nrf2 pathway was investigated in this study. Compared with LPS-stimulated HEGK cells, GTE (10 mg/ml) increased Nrf2 and HO-1 gene expression and protein production (p < 0.05, Fig. 4a, b).
The effect of GTE on the activation of Nrf2 and the expressions of HO-1 in LPS-stimulated oral keratinocytes. The total proteins of the cells were prepared and the expressions of Nrf2 (a) and HO-1 (b) were analyzed using western blot. Results are expressed as mean ± standard deviation of three independent experiments (n = 3). *p < 0.05
Discussion
The purpose of this study was to ascertain the anti-inflammatory and wound healing stimulating effects of green tea extract on human gingival epithelial keratinocyte cells challenged with LPS. Our findings sustain the hypothesis that GTE is a powerful anti-inflammatory agent and in a dose-dependent condition would elicit wound healing cell migration—with the majority of these properties attributed to the green tea plant’s polyphenolic compounds, i.e., catechins in the leaves. Consistent with our results, other studies reported biological response in other cell types, such as antioxidant, antimicrobial, and anti-inflammation effects of EGCG which is one of green tea’s compounds [10,11,12,13,14, 24,25,26]. Green tea also has shown antimicrobial activity against most oral bacteria. Additionally, it may enhance oral peroxidase activity and prevent the establishment and progression of periodontitis [27,28,29]. Based on its anti-inflammatory and antioxidant effects, green tea EGCG could be considered for treatment of innumerous diseases, including neurological diseases, diabetes, and hypertension [30,31,32]. GTE compounds can suppress nuclear factor “kappa-light-chain-enhancer” of activated B cells and downstream signaling inflammatory mediators (nitric oxide, inducible nitric oxide synthase, cyclooxygenase-2, prostaglandine-E2) in vitro and could be responsible for its significant anti-cancer or chemopreventive activity [33]. Our results (Fig. 1) suggested that GTE at concentrations of 2.5 mg/ml, 5 mg/ml, and 10 mg/ml has a positive effect on oral gingival keratinocyte cell viability by almost duplicating viability compared with the non-treated control. However, it was shown that green tea EGCG could provoke increased cytotoxicity in cells when applied at higher concentrations. An EGCG concentration higher than 100 μM was reported to cause production increase of H2O2 and oxidative DNA damage, while concentrations of more than 200 μM EGCG could even affect cell cycle progression [34, 35]. Consequently, the concentration of EGCG in the test green tea solution should be carefully controlled for in in vitro assay applications. In this study, green tea solutions containing a maximum of ≈ 50 μM EGCG concentration were observed to be safe based on the cell viability test (Fig. 1). GTE solutions at 2.5 mg/ml, 5 mg/ml, and 10 mg/ml concentrations downregulated the expression of inflammation markers IL-β1, IL-6, and TNFα at 24 h (Fig. 2a) and reduced protein production of the same analyzed genes (Fig. 2b). IL-β1, IL-6, and TNFα decreased in gene expression compared with those in the control, and the combination with GTE (at 2.5 mg/ml, 5 mg/ml, and 10 mg/ml) and LPS (1 μg/ml) still showed the positive effects of GTE by reducing inflammatory status. LPS is an important component of the outer membrane of Gram-negative bacteria, which promotes cellular signal transduction through toll-like receptors and secretion of pro-inflammatory interleukins, eicosanoids, and nitric oxide [36]. As seen in cells treated only with LPS (Fig. 2a, b, darker columns), the gingival keratinocytes treated with lipopolysaccharide here resulted in a significant increase of IL-β1, IL-6, and TNFα gene expression and protein production compared with the negative control. This is a predicted biological reaction to LPS bacterial endotoxin, especially from the highly oral pathogenic bacteria P. gingivalis. A decrease in inflammation was also observed in previous in vitro studies using different cell lines, which showed GTE’s potential for use as anti-inflammatory drugs, providing its ability to reduce the production of nitric oxide, cyclooxygenase-2, interleukin-6, interleukin-1β, and tumor necrosis factor α in active macrophages [37,38,39]. Tumor necrosis factor α is responsible for increase in levels of inflammation by upregulating other pro-inflammatory cytokines (e.g., interleukin-6, interleukin-1) and consequently inducing angiogenesis and nuclear factor “kappa-light-chain-enhancer” of activated B cell transcription, and stimulating nitric oxide production [40]. Tumor necrosis factor α has been targeted by anti-inflammatory screening agents due to its multiple roles in inflammation [41]. Interleukin-β1 induces secretion of interleukin-6 and interleukin-8 which also play a role as pro-inflammatory cytokines and are important for the initiation and increase of the inflammatory response to microbial infection [42]. In addition, GTE anti-inflammatory effect was confirmed in animal studies on LPS-induced retinal inflammation [43, 44].
Regarding the possible wound healing effect of green tea via our in vitro scratch wound healing assay, increase in difference with regard to the control was found with the GTE at concentrations of 2.5 mg/ml, 5 mg/ml, and 10 mg/ml at 24 h, while 1 mg/ml stimulation was non-significant. Despite GTE component’s EGCG association with wound healing in pharmacological animal studies [45, 46], in vitro studies with cancer cells showed that EGCG significantly decreased or inhibited cell migration in a dose-dependent manner [47, 48]. Based on our experimental observations, normal gingival keratinocytes showed an ≈ 50% increase in cell migration or change in migrating behavior after exposure to GTE (Fig. 3). In contrast to its effect on normal keratinocytes, GTE component’s EGCG was found to be specially antiproliferative and apoptotic on cancer cells [48,49,50,51,52]. However, it has been suggested that these effects of EGCG are cancer specific and EGCG shows pronounced growth inhibitory effect on cancerous cells but not on normal cells [53]. Another study demonstrated that, in cultured skin keratinocytes, EGCG acts to enhance differentiation without triggering apoptosis [54]. Topical application of EGCG on human skin which has been identified and reported earlier results in increased cell proliferation and reduced keratinocyte apoptosis [55]; however, to the best of our knowledge, there is no report of green tea on oral cells. Our results suggest that oral gingival epithelial keratinocytes presented significant decrease in cell mobility/migration activity challenged with P. gingivalis LPS (1 μg/ml) at 24 h (Fig. 3). Moreover, GTE at concentrations of 2.5 mg/ml, 5 gm/ml, and 10 mg/ml could successfully stimulate cells in an inflammatory environment when exposed to LPS. The unchanged migration found after 1 mg/ml GTE exposure could be explained by its lower concentration and EGCG content, which reduces green tea stimulation potency. And it indicates that there is possibly a threshold for gingival keratinocytes’ responsiveness to green tea. We also have to consider that any difference in green tea effects on migration likely reflects relative sensitivity of specific cell types to LPS. But further examinations of molecular basis are still needed to support this hypothesis. Regarding the signaling pathway analysis, our results showed that GTE may activate the Nrf2 pathway, bringing into play its anti-inflammatory effect on cells exposed to LPS (Fig. 4a). Also, the Nrf2-dependent anti-oxidant gene HO-1 was also increased (Fig. 4b). Some reports previously confirmed that the activation of the Nrf2 pathway prevents LPS-induced upregulation of pro-inflammatory cytokines, such as IL−β1, IL-6, and TNFα analyzed here [20, 56]. Drugs used to activate Nrf2 pathway could be considered for use as potential oral therapies for oral inflammatory diseases, such as gingivitis or periodontitis. Until today, there are few Nrf2 activating medicaments used in the dental clinic. Therefore, GTE could be used to develop new and safer Nrf2 activators for clinical use in dentistry.
This study has taken a step in the direction of defining the effect of green tea extract on inflammation suppression associated with wounding. However, the results are neither viewed nor are they presented as conclusive. In addition, it is important to emphasize that problems in methodological research design of in vitro assays place limitations on interpretations. Further, the potential for green tea catechins to aid in epithelial formation bears further study. Although this study did not support the finding of earlier animal studies [57], other research designs may yet show a benefit in its use. In addition, in vivo angiogenesis and granulation tissue augmentation by GTE have been demonstrated [30]. To our knowledge, there is a lack on studies evaluating GTE’s effect on oral epithelial healing. Therefore, further animal model research is needed.
Conclusions
In summary, our results sustained the hypothesis that GTE attenuates the inflammation in gingival epithelial keratinocytes treated with LPS by downregulating inflammatory cytokines in a dose-dependent manner. The results from our experiment support that GTE can be considered a potent anti-inflammatory agent with a potential as an oral therapeutic against inflammation.
References
Kaegi E (1998) Unconventional therapies for cancer: 2. Green tea. Can Med Assoc J 158:1033–1035
Chacko SM, Thambi PT, Kuttan R, Nishigaki I (2010) Beneficial effects of green tea: a literature review. Chin Med J 5:13
Seeram NP, Henning SM, Niu Y, Lee R, Scheuller HS, Heber D (2006) Catechin and caffeine content of green tea dietary supplements and correlation with antioxidant capacity. J Agric Food Chem 54:1599–1603
Pekal A, Drozdz P, Biesaga M, Pyrzynska K (2012) Screening of the antioxidant properties and polyphenol composition of aromatised green tea infusions. J Sci Food Agric 92:2244–2249
Chan EW, Soh EY, Tie PP, Law YP (2011) Antioxidant and antibacterial properties of green, black, and herbal teas of Camellia sinensis. Pharmacognosy Res 3:266–272
Cabrera C, Artacho R, Gimenez R (2006) Beneficial effects of green tea–a review. J Am Coll Cardiol 25:79–99
Barbosa CS, Kato MT, Buzalaf MA (2011) Effect of supplementation of soft drinks with green tea extract on their erosive potential against dentine. Aust Dent J 56:317–321
Kato MT, Magalhães AC, Rios D, Hannas AR, Attin T, Buzalaf MA (2009) Protective effect of green tea on dentin erosion and abrasion. J Appl Oral Sci 17:560–564
Magalhães AC, Wiegand A, Rios D, Hannas A, Attin T, Buzalaf MA (2009) Chlorhexidine and green tea extract reduce dentin erosion and abrasion in situ. J Dent 37:994–998
Zhong Y, Chiou YS, Pan MH, Shahidi F (2012) Anti-inflammatory activity of lipophilic epigallocatechin gallate (EGCG) derivatives in LPS-stimulated murine macrophages. Food Chem 134:742–748
Paquay JBG, Haenen GRMM, Stender G, Wiseman SA, Tijburg LBM, Bast A (2000) Protection against nitric oxide toxicity by tea. J Agric Food Chem 48:5768–5772
Nagai K, Jiang MH, Hada J, Nagata Y, Yajima Y, Yamamoto S, Nishizaki T (2002) (−)-Epigallocatehin gallate protects against NO stress-induced neuronal damage after ischemia by acting as an antioxidant. Brain Res 956:319–322
Tedeschi E, Menegazzi M, Yao Y, Suzuki H, Forstermann U, Kleinert H (2004) Green tea inhibits human inducible nitric-oxide synthase expression by down-regulating signal transducer and activator of transcription-1α activation. Mol Pharm 65:111–120
Tachibana H, Koga K, Fujimura Y, Yamada K (2004) A receptor for green tea polyphenol EGCG. Nat Struct Mol Biol 11:380–381
Tachibana H (2008) Green tea polyphenol EGCG signaling pathway through the 67-kDa laminin receptor. Nihon Yakurigaku Zasshi 132:145–149
Fujimura Y, Sumida M, Sugihara K, Tsukamoto S, Yamada K, Tachibana H (2012) Green tea polyphenol EGCG sensing motif on the 67-kDa laminin receptor. PLoS One 7:e37942
Duque GA, Descoteaux A (2014) Macrophage cytokines: involvement in immunity and infectious diseases. Front Immunol 5:491
Albandar JM (2011) Underestimation of periodontitis in NHANES surveys. J Periodontol 82:337–341
Demeule M, Brossard M, Pagé M, Gingras D, Béliveau R (2000) Matrix metalloproteinase inhibition by green tea catechins. Biochim Biophys Acta 1478:51–60
Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H (2003) Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest 112:1821–1830
Crunkhorn S (2012) Deal watch: Abbott boosts investment in NRF2 activators for reducing oxidative stress. Nat Rev Drug Discov 11:96
Kim J, Cha YN, Surh YJ (2010) A protective role of nuclear factor-erythroid 2-related factor-2 (Nrf2) in inflammatory disorders. Mutat Res 690:12–23
Bao K, Akguel B, Bostanci N (2014) Establishment and characterization of immortalized gingival epithelial and fibroblastic cell lines for the development of organotypic cultures. Cells Tissues Organs 199:228–237
Saffari Y, Sadrzadeh SM (2004) Green tea metabolite EGCG protects membranes against oxidative damage in vitro. Life Sci 74:1513–1518
Divya S, Naveen Krishna K, Ramachandran S, Dhanaraju MD (2011) Wound healing and in vitro antioxidant activities of Croton bonplandianum leaf extract in rats. G J Pharmacol 5:159–163
Khalaji N, Neyestani T (2007) The inhibitory effects of black and green teas (Camellia sinensis) on growth of pathogenic Escherichia coli, in vitro. I J Nutri Sci 1:33–38
Araghizadeh A, Kohanteb J, Fani MM (2013) Inhibitory activity of green tea (Camellia sinensis) extract on some clinically isolated cariogenic and periodontopathic bacteria. Med Prin Pract 22:368–372
Kushiyama M, Shimazaki Y, Murakami M, Yamashita Y (2009) Relationship between intake of green tea and periodontal disease. J Periodontol 80:372–377
Narotzki B, Levy Y, Aizenbud D, Reznick AZ (2013) Green tea and its major polyphenol EGCG increase the activity of oral peroxidases. Adv Exp Med Biol 756:99–104
Kim HH, Kawazoe T, Han DW, Matsumara K, Suzuki S, Tsutsumi S, Hyon SH (2008) Enhanced wound healing by an epigallocatechin gallate-incorporated collagen sponge in diabetic mice. Wound Repair Regen 16:714–720
Bogdanski P, Suliburska J, Szulinska M, Stepien M, Pupek-Musialik D, Jablecka A (2012) Green tea extract reduces blood pressure, inflammatory biomarkers, and oxidative stress and improves parameters associated with insulin resistance in obese, hypertensive patients. Nutrition Research 32:421–427
Lee H, Bae JH, Lee SR (2004) Protective effect of green tea polyphenol EGCG against neuronal damage and brain edema after unilateral cerebral ischemia in gerbils. J Neurosci Res 77:892–900
Hou CC, Chen YP, Wu JH, Huang CC, Wang SY, Yang NS, Shyur LF (2007) A galactolipid possesses novel cancer chemopreventive effects by suppressing inflammatory mediators and mouse B16 melanoma. Cancer Res 67:6907–6915
Sugisawa A, Umegaki K (2002) Physiological concentrations of (−)-epigal- locatechin-3-O-gallate (EGCg) prevent chromosomal damage induced by reactive oxygen species in WIL2-NS cells. J Nutri 132:1836–1839
Hou Z, Sang S, You H, Lee MJ, Hong J, Chin KV, Yang CS (2005) Mechanism of action of (-)-epigallocatechin-3-gallate: auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res 65:8049–8056
Wang X, Quinn PJ (2010) Endotoxins: lipopolysaccharides of gram-negative bacteria. Subcell Biochem 53:3–25
Novilla A, Djamhuri DS, Nurhayati B, Rihibiha DD, Afifah E, Widowati W (2017) Anti-inflammatory properties of oolong tea (Camellia sinensis) ethanol extract and epigallocatechin gallate in LPS-induced RAW 264.7 cells. Asian Pac J Trop Biomed 7:1005–1009
Wu YR, Choi HJ, Kang YG, Kim JK, Shin JW (2017) In vitro study on anti-inflammatory effects of epigallocatechin-3-gallate-loaded nano- and microscale particles. Int J Nanomedicine 12:7007–7013
Hou DX, Masuzaki S, Tanigawa S, Hashimoto F, Chen J, Sogo T, Fujii M (2010) Oolong tea theasinensins attenuate cyclooxygenase-2 expression in lipopolysaccharide (LPS)-activated mouse macrophages: structure-activity relationship and molecular mechanisms. J Agric Food Chem 58:12735–12743
Damte D, Reza MA, Lee SJ, Jo WS, Park SC (2011) Anti-inflammatory activity of dichloromethane extract of Auricularia auricula-judae in RAW264.7 cells. Toxicol Res 27:11–14
Henriques BO, Corrêa O, Azevedo EPC, Pádua RM, de Oliveira VLS, Oliveira THC, Boff D, Dias ACF, de Souza DG, Amaral FA, Teixeira MM, Castilho RO, Braga FC (2016) In vitro TNF-inhibitory activity of Brazilian plants and anti-inflammatory effect of Stryphnodendron adstringens in an acute arthritis model. Evid Based Complement. Alternat Med 6:9872598
Turner MD, Nedjai B, Hurst T, Pennington DJ (2014) Cytokines and chemokines: at the crossroads of cell signalling and inflammatory disease. Biochim Biophys Acta 1843:2563–2582
Ren JL, Yu QX, Liang WC, Leung PY, Ng TK, Chu WK, Pang CP, Chan SO (2018) Green tea extract attenuates LPS-induced retinal inflammation in rats. Sci Rep 8:429
Chu KO, Chan KP, Wang CC, Chu CY, Li WY, Choy KW, Rogers MS, Pang CP (2010) Green tea catechins and their oxidative protection in the rat eye. J Agric Food Chem 58:1523–1534
Asadi SY, Parsaei P, Karimi M, Ezzati S, Zamiri A, Mohammadizadeh F, Rafieian-Kopaei M (2013) Effect of green tea (Camellia sinensis) extract on healing process of surgical wounds in rat. Int J Surg 11:332–337
Kapoor M, Howard R, Hall I, Appleton I (2004) Effects of epicatechin gallate on wound healing and scar formation in a full thickness incisional wound healing model in rats. Am J Pathol 165:299e307
Chen X, Chang L, Qu Y, Liang J, Jin W, Xia X (2018) Tea polyphenols inhibit the proliferation, migration, and invasion of melanoma cells through the down-regulation of TLR4. Int J Immunopathol Pharmacol 32:394632017739531
Seo EJ, Wu CF, Ali Z, Wang YH, Khan SI, Walker LA, Khan IA, Efferth T (2016) Both phenolic and non-phenolic green tea fractions inhibit migration of cancer cells. Front Pharm 7:398
Valcic S, Timmermann BN, Alberts DS, Wachter GA, Krutzsch M, Wymer J, Guillen JM (1996) Inhibitory effect of six green tea catechins and caffeine on the growth of four selected human tumor cell lines. Anticancer Drugs 7:461–468
Yang GY, Liao J, Kim K, Yurkow EJ, Yang CS (1998) Inhibition of growth and induction of apoptosis in human cancer cell lines by tea polyphenols. Carcinogenesis 19:611–616
Liang YC, Chen YC, Lin YL, Lin-Shiau SY, Ho CT, Lin JK (1999) Suppression of extracellular signals and cell proliferation by the black tea polyphenol, theaflavin-3,3-digallate. Carcinogenesis 20:733–736
Gupta S, Ahmad N, Nieminen AL, Mukhtar H (2000) Growth inhibition, cellcycle dysregulation, and induction of apoptosis by green tea constituent (-)-epigallocatechin3-gallate in androgen-sensitive and androgen-insensitive human prostate carcinoma cells. Toxicol Appl Pharmacol 164:82–90
Chen ZP, Schell JB, Ho CT, Chen KY (1998) Green tea epigallocatechin gallate shows a pronounced growth inhibitory effect on cancerous cells but not on their normal counterparts. Cancer Lett 129:173–179
Balasubramanian S, Sturniolo MT, Dubyak GR, Eckert RL (2005) Human epidermal keratinocytes undergo (-)-epigallocatechin-3-gallate-dependent differentiation but not apoptosis. Carcinogenesis 26:1100–1108
Chung JH, Han JH, Hwang EJ, Seo JY, Cho KH, Kim KH, Youn JI, Eun HC (2003) Dual mechanisms of green tea extract (EGCG)-induced cell survival in human epidermal keratinocytes. FASEB J 17:1913–1915
Yang HL, Lin SW, Lee CC, Lin KY, Liao CH, Yang TY, Wang HM, Huang HC, Wu CR, Hseu YC (2015) Induction of Nrf2-mediated genes by Antrodia salmonea inhibits ROS generation and inflammatory effects in lipopolysaccharide-stimulated RAW264.7 macrophages. Food Funct 6:230–241
Neves ALA, Komesu MC, Matteo MASD (2010) Effects of green tea use on wound healing. Int J Morphol 28:905–910
Acknowledgments
We thank the Oral Microbiology Institute, Center of Dental Medicine, University of Zurich, for the donation of the human gingival epithelial keratinocyte cell line and Omnimedica AG for providing the green tea extract.
Funding information
This study was further supported by Clinic of Conservative and Preventive Dentistry, Center of Dental Medicine, University of Zurich, Zurich, Switzerland.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hagiu, A., Attin, T., Schmidlin, P.R. et al. Dose-dependent green tea effect on decrease of inflammation in human oral gingival epithelial keratinocytes: in vitro study. Clin Oral Invest 24, 2375–2383 (2020). https://doi.org/10.1007/s00784-019-03096-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00784-019-03096-4