Abstract
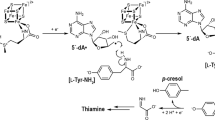
We have designed, synthesized, and evaluated tyrosine homologues and their O-methyl derivatives as potential inhibitors for tyrosine phenol lyase (TPL, E.C. 4.1.99.2). Recently, we reported that homologues of tryptophan are potent inhibitors of tryptophan indole-lyase (tryptophanase, TIL, E.C. 4.1.99.1), with K i values in the low µM range (Do et al. Arch Biochem Biophys 560:20–26, 2014). As the structure and mechanism for TPL is very similar to that of TIL, we postulated that tyrosine homologues could also be potent inhibitors of TPL. However, we have found that homotyrosine, bishomotyrosine, and their corresponding O-methyl derivatives are competitive inhibitors of TPL, which exhibit K i values in the range of 0.8–1.5 mM. Thus, these compounds are not potent inhibitors, but instead bind with affinities similar to common amino acids, such as phenylalanine or methionine. Pre-steady-state kinetic data were very similar for all compounds tested and demonstrated the formation of an equilibrating mixture of aldimine and quinonoid intermediates upon binding. Interestingly, we also observed a blue-shift for the absorbance peak of external aldimine complexes of all tyrosine homologues, suggesting possible strain at the active site due to accommodating the elongated side chains.





Similar content being viewed by others
Abbreviations
- PLP:
-
Pyridoxal-5′-phosphate
- TPL:
-
Tyrosine phenol-lyase [EC 4.1.99.2]
- TIL:
-
Tryptophan indole-lyase [EC 4.1.99.1]
- SOPC:
-
S-(o-Nitrophenyl)-l-cysteine
References
Antson AA, Demidkina TV, Gollnick P, Dauter Z, Von Tersch RL, Long J, Berezhnoy SN, Phillips RS, Harutyunyan EH, Wilson KS (1993) Three-dimensional structure of tyrosine phenol-lyase. Biochemistry 32:4195–4206
Chen HY, Demidkina TV, Phillips RS (1995a) Site-directed mutagenesis of tyrosine-71 to phenylalanine in Citrobacter freundii tyrosine phenol-lyase: evidence for dual roles of tyrosine-71 as a general acid catalyst in the reaction mechanism and in cofactor binding. Biochemistry 34:12276–12283
Chen H, Gollnick P, Phillips RS (1995b) Site-directed mutagenesis of His343-Ala in Citrobacter freundii tyrosine phenol-lyase. Effects on the kinetic mechanism and rate-determining step. Eur J Biochem 229:540–549
Chenault HK, Dahmer J, Whitesides GM (1989) Kinetic resolution of unnatural and rarely occurring amino acids: enantioselective hydrolysis of N-acyl amino acids catalyzed by acylase I. J Am Chem Soc 111:6354–6364
Cleland WW (1979) Statistical analysis of enzyme kinetic data. Meth Enzymol 63:103–138
Di Martino P, Merieau A, Phillips R, Orange N, Hulen C (2002) Isolation of an Escherichia coli strain mutant unable to form biofilm on polystyrene and to adhere to human pneumocyte cells: involvement of tryptophanase. Can J Microbiol 48:132–137
Do QT, Nguyen GT, Celis V, Phillips RS (2014) Inhibition of escherichia coli tryptophan indole-lyase by homologues of tryptophan. Arch Biochem Biophys 560:20–26
Hayashi H, Mizuguchi H, Miyahara I, Islam MM, Ikushiro H, Nakajima Y, Hirotsu K, Kagamiyama H (2003) Strain and catalysis in aspartate aminotransferase. Biochim Biophys Acta 1647:103–109
Isupov MN, Antson AA, Dodson EJ, Dodson GG, Dementieva IS, Zakomirdina LN, Wilson KS, Dauter Z, Lebedev AA, Harutyunyan EH (1998) Crystal structure of tryptophanase. J Mol Biol 276:603–623
Kumagai H, Matsui H, Ohgishi K, Ogata K, Yamada H, Ueno T, Fukami H (1969) Synthesis of 3,4-dihydroxyphenyl-l-alanine from l-tyrosine and pyrocatechol by crystalline β-tyrosinase. Biochem Biophys Res Comm 34:266–270
Kumagai H, Yamada H, Matsui H, Ohgishi H, Ogata K (1970a) Tyrosine phenol lyase. I. Purification, crystallization, and properties. J Biol Chem 245:1767–1772
Kumagai H, Yamada H, Matsui H, Ohgishi H, Ogata K (1970b) Tyrosine phenol lyase. II. Cofactor requirements. J Biol Chem 245:1773–1777
Matheson IBC (1990) A critical comparison of least absolute deviation fitting (robust) and least-squares fitting: the importance of error distributions. Comput Chem 14:49–57
Milić D, Demidkina TV, Faleev NG, Matkovic-Calogovic D, Antson AA (2008) Insights into the catalytic mechanism of tyrosine phenol-lyase from X-ray structures of quinonoid intermediates. J Biol Chem 283:29206–29214
Milić D, Demidkina TV, Faleev NG, Phillips RS, Matkovic-Calogovic D, Antson AA (2011) Crystallographic snapshots of tyrosine phenol-lyase show that substrate strain plays a role in C-C bond cleavage. J Am Chem Soc 133:16468–16476
Murashige R, Hayashi Y, Ohmori S, Torii A, Aizu Y, Muto Y, Murai Y, Oda Y, Hashimoto M (2011) Comparisons of O-acylation and Friedel-Crafts acylation of phenols and acyl chlorides and Fries rearrangement of phenyl esters in trifluoromethanesulfonic acid: effective synthesis of optically active homotyrosines. Tetrahedron 67:641–649
Muro T, Nakatani H, Hiromi K, Kumagai H, Yamada H (1978) Elementary processes in the interaction of tyrosine phenol-lyase with inhibitors and substrate. J Biochem (Tokyo) 84:633–640
Phillips RS (1987) Reactions of O-acyl-l-serines with tryptophanase, tyrosine phenol-lyase, and tryptophan synthase. Arch Biochem Biophys 256:302–310
Phillips RS (1991) Reaction of indole and analogues with amino acid complexes of Escherichia coli tryptophan indole-lyase: detection of a new reaction intermediate by rapid-scanning stopped-flow spectrophotometry. Biochemistry 30:5927–5934
Phillips RS (2000) Proton transfer and carbon-carbon bond cleavage in the elimination of indole catalyzed by Escherichia coli tryptophan indole-lyase. J Am Chem Soc 122:1008–1014
Phillips RS, Ravichandran K, Tersch RV (1989) Synthesis of l-tyrosine from phenol and S-(o-nitrophenyl)-l-cysteine catalyzed by tyrosine phenol-lyase. Enz Microb Technol 11:80–83
Phillips RS, Demidkina TV, Zakomirdina LN, Bruno S, Ronda L, Mozzarelli A (2002) Crystals of tryptophan indole-lyase and tyrosine phenol-lyase form stable quinonoid complexes. J Biol Chem 277:21592–21597
Phillips RS, Chen HY, Faleev NG (2006) Aminoacrylate intermediates in the reaction of Citrobacter freundii tyrosine phenol-lyase. Biochemistry 45:9575–9583
Shimohigashi Y, Lee S, Izumiya N (1976) Resolution of amino acids. XII. Preparation of L-2-amino-5-arylpentanoic acids, constituent amino acids in AM-toxins. Bull Chem Soc Jpn 49:3280–3284
Snell EE (1975) Tryptophanase: structure, catalytic activities, and mechanism of action. Adv Enzymol Relat Areas Mol Biol 42:287–333
Sundararaju B, Antson AA, Phillips RS, Demidkina TV, Barbolina MV, Gollnick P, Dodson GG, Wilson KS (1997) The crystal structure of Citrobacter freundii tyrosine phenol-lyase complexed with 3-(4′-hydroxyphenyl)propionic acid, together with site-directed mutagenesis and kinetic analysis, demonstrates that arginine-381 is required for substrate specificity. Biochemistry 36:6502–6510
Ueno T, Nakashima T, Hayashi Y, Fukami H (1975) Structures of AM-toxin I and II, host specific phytotoxic metabolites produced by Alternaria mali. Agri Biol Chem 39:1115–1122
Venkatesh M, Mukherjee S, Wang H, Li H, Sun K, Benechet AP, Qiu Z, Maher L, Redinbo MR, Phillips RS, Fleet JC, Kortagere S, Mukherjee P, Fasano A, Le Ven J, Nicholson JK, Dumas ME, Khanna KM, Mani S (2014) Symbiotic bacterial metabolites regulate gastrointestinal barrier function via the xenobiotic sensor PXR and toll-like receptor 4. Immunity 41:296–310
Watanabe T, Snell EE (1972) Reversibility of the tryptophanase reaction: synthesis of tryptophan from indole, pyruvate, and ammonia. Proc Nat Acad Sci USA 69:1086–1090
Watkins EB, Phillips RS (2001) Enzymatic synthesis of aza-l-tyrosines. Bioorg Med Chem Lett 11:2099–2100
Yamada H, Kumagai H, Matsui H, Ohgishi H, Ogata K (1968) Crystallization and properties of β-tyrosinase from Esherichia intermedia. Biochem Biophys Res Comm 33:10–14
Yamada M, Nagashima N, Hasegawa J, Takahashi S (1998) A highly efficient asymmetric synthesis of methoxyhomophenylalanine using Michael addition of phenethylamine. Tet. Lett. 39:9019–9022
Acknowledgments
Financial support to Q. D. during his PhD work was provided by the University of Georgia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: C. Schiene-Fischer.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Do, Q., Nguyen, G.T. & Phillips, R.S. Inhibition of tyrosine phenol-lyase by tyrosine homologues. Amino Acids 48, 2243–2251 (2016). https://doi.org/10.1007/s00726-016-2263-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-016-2263-7