Abstract
Two Pseudomonas putida aminotransferases (ArAT I and ArAT II) that exhibit activity toward l-tryptophan were purified 104- and 395-fold using a six-stage purification procedure involving ammonium sulfate fractionation and chromatographic separation on phenyl-Sepharose, Sephadex G-100 superfine, DEAE-cellulose and Protein-Pack Q8 HR columns. Mass spectrometry analysis resulted in the identification of 27 and 20 % of the total ArAT I and ArAT II amino acid sequences. In addition, N-terminal sequence fragments of ArAT I and ArAT II were determined using the Edman degradation method. Based on the analyses performed, the studied proteins were identified as products of the tyrB-2 and phhC genes, and the presence of these genes in the investigated bacterial strain was confirmed using molecular biology methods. Extensive analysis of the substrate specificities of ArAT I and ArAT II revealed that both enzymes most efficiently catalyzed reactions involving aromatic amino acids and 2-oxoacids followed by dicarboxylic compounds. The best substrates for ArAT I and ArAT II were l-phenylalanine and phenylpyruvate. Based on these results, the studied proteins were classified as aromatic amino acid aminotransferase isozymes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
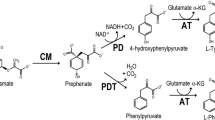
Pseudomonas putida is an aerobic, rod-shaped, gram-negative bacterium that lives in water and soil, particularly in association with plant roots, and along with P. fluorescens is considered the most important species within the Pseudomonas genus in the aspect of plant growth stimulation (Botelho and Mendonça-Hagler 2006; Khakipour et al. 2008). Of the various mechanisms of plant growth promotion by P. putida and other bacteria, production of indole-3-acetic acid through the indole-3-pyruvate (IPyA) pathway is thought to play a central role in this process (Lambrecht et al. 2000; Patten and Glick 2002; Spaepen et al. 2007). The first reaction of the IPyA pathway, where l-tryptophan is converted to IPyA, is catalyzed by an aromatic amino acid aminotransferase (EC 2.6.1.57, ArAT), which is a pirydoxal phosphate-dependent enzyme that reversibly transfers the amino group of aromatic amino acids (l-phenylalanine, l-tryptophan and l-tyrosine) onto 2-oxoglutarate. The proteins with ArAT activity, particularly toward l-tryptophan, and the genes encoding ArATs were therefore recently studied in various bacteria species, including Azospirillum brasilense, Enterobacter cloacae and Pantoea agglomerans (Koga et al. 1994; Sergeeva et al. 2007; Ge et al. 2009; Castro-Guerrero et al. 2012) as well as in some plants (Simpson et al. 1997; Phillips et al. 2011; Won et al. 2011). In our previous methodological paper, we reported the detection of two aminotransferases that were capable of transaminating l-tryptophan also in P. putida (Szkop et al. 2012). However, until now, there were no experimental data concerning the primary structure of the proteins exhibiting ArAT activity or the identity of the genes encoding them in P. putida. The only data available resulted from computational analyses of the recently sequenced genomes of several P. putida strains (e.g., KT2440, Nelson et al. 2002; Bird-1, Matilla et al. 2011; ND6, Li et al. 2012). Based on gene sequence similarity, the putative functions of the two genes, which were designated as tyrB (NCBI Gene ID: 1043062) and tyrB-2 (NCBI Gene ID: 1045962), were predicted to encode ArAT proteins. Considering that a variety of bacterial aminotransferases exist in a single microorganism and that these enzymes usually possess broad and overlapping substrate specificities, the biochemical approach for their identification is required (Xing and Whitman 1992). Particularly that on the basis of primary structure homology ArAT has been classified along with alanine, aspartate and histydynol-phosphate aminotransferases in one subgroup whose representatives exhibit similar enzymatic properties (Metha et al. 1993). Moreover, it was shown in diverse bacterial species that activity toward aromatic amino acids and 2-oxoacids exhibits to some extent also branched-chain amino acid aminotransferase (Whitaker et al. 1982; Xing and Whitman 1992; Yvon et al. 2000). Therefore, the objective of the present study was to purify, identify and properly classify aminotransferases exhibiting activity toward l-tryptophan in the P. putida bacterium.
Materials and methods
Pseudomonas putida strain and culture conditions
The Pseudomonas putida strain A used in this study was isolated from wheat rhizosphere and was identified using the ribotyping method by Blirt S.A. DNA-Gdańsk, Poland. The 16S rRNA gene sequence of this strain is deposited in the DNA Data Bank of Japan under accession number AB667903. The bacteria were cultured in 1 l of King B liquid medium (King et al. 1954) while shaking (150 rpm) at room temperature for 48 h. The bacterial culture was centrifuged (20,000×g; 12 min), and the bacterial pellet was washed two times and resuspended in 50 mM phosphate buffer (pH 8.1) containing 5 μM pirydoxal phosphate (PLP) and 5 mM 2-mercaptoethanol (2-ME) (extraction buffer) before being broken with ultrasonication. The crude bacterial extract was centrifuged (20,000×g; 12 min), and the obtained supernatant (homogenate) was used as the starting material for enzyme purification.
Purification of enzymes
All purification steps were performed at 4 °C. The proteins in the homogenate were fractionated with ammonium sulfate (40–60 % saturation). The collected protein precipitate was dissolved in the extraction buffer, and after centrifugation (20,000×g; 12 min), it was loaded onto a phenyl-Sepharose column (2 × 15 cm) equilibrated with 50 mM phosphate (pH 8.1) and 0.3 M (NH4)2SO4. The bound proteins were eluted with a linear gradient (0–100 %) of 50 mM phosphate (pH 8.1) supplemented with 2 μM PLP and 1 mM 2-ME. The fractions exhibiting l-tryptophan:2-oxoglutarate activity were pooled and concentrated to a volume of approximately 12 ml using an Amicon concentrator (Millipore Corporation; Bedford, MA, USA) supplied with a PM 10 membrane. The concentrated preparation was separated by gel filtration on a Sephadex G-100 superfine column (2.6 × 85 cm) equilibrated with 10 mM phosphate (pH 8.1) containing 2 μM PLP and 1 mM 2-ME (buffer A). The fractions with l-tryptophan:2-oxoglutarate activity were pooled, concentrated to a volume of approximately 10 ml and applied to a DEAE-cellulose column (1 × 10 cm) equilibrated with buffer A. Proteins bound to the resin were eluted with a linear gradient (0–100 %) of 0.1 M phosphate buffer (pH 6.8) containing 2 μM PLP and 1 mM 2-ME. The fractions corresponding to the two peaks with l-tryptophan:2-oxoglutarate activity (the first peak corresponded to ArAT II activity, and the second peak corresponded to ArAT I activity) were separately pooled, concentrated to a volume of approximately 4 ml and rechromatographed on the Sephadex G-100 superfine column under the same conditions. Finally, each preparation was subjected to anion exchange chromatography on a Protein-Pack Q8 HR column attached to an HPLC system (Waters Corporation; Milford, MA, USA). For the preparation of ArAT I, the column was equilibrated with 40 mM phosphate buffer (pH 8.1) supplemented with 2.5 % (w/v) sucrose, 2 μM PLP and 1 mM 2-ME. For the preparation of ArAT II, the column was equilibrated with 10 mM phosphate buffer (pH 8.1) supplemented with 2.5 % (w/v) sucrose, 2 μM PLP and 1 mM 2-ME. In both cases, the bound proteins were eluted with a linear gradient (0–100 %) of 0.15 M phosphate buffer (pH 6.8) containing 2.5 % (w/v) sucrose, 2 μM PLP and 1 mM 2-ME. The active fractions were pooled, enriched with sucrose to a final concentration of 20 % (w/v) and stored at −80 °C.
Aminotransferase activity assays
l-Tryptophan:2-oxoglutarate activity during purification was determined using a method previously developed in our laboratory (Szkop et al. 2012). One unit (U) of enzyme activity was defined as the release of 1 μmol of IPyA per minute. Specific activity was expressed in U per mg of protein. The protein concentration was determined using the method of Bradford (1976). Enzyme specificity analysis towards 2-oxoacids was performed using the above-mentioned method except that 2-oxoglutarate was replaced with various 2-oxoacids at 0.25 or 3 mM concentration in the reaction mixture. Controls omitting one of the substrates were run with each assay. Specificity of the enzymes towards amino acids was determined using the RP-HPLC-fluorescence method based on l-glutamate (the amino acid product of 2-oxoglutarate transamination) quantification. Here also, controls omitting one of the substrates were run with each assay. The reaction mixture consisted of 0.1 M phosphate buffer (pH 8.0), 2.5 or 10 mM amino acid, 3 mM 2-oxoglutarate, 10 μM pyridoxal phosphate and enzyme preparation. The reactions were performed at 35 °C in a total volume of 0.5 ml and were terminated by the addition of 125 μl of 2 M borate buffer (pH 12.5) containing 0.7 M 2-ME. The samples were centrifuged and filtered (Amicon Ultra 0.5 ml centrifugal filters Z677094, Sigma–Aldrich, Inc.; St. Louis, MO, USA), and the filtrate was derivatized with orthophthalic aldehyde (Van Eijk et al. 1993) and analyzed using HPLC (binary HPLC 1525 pump, Waters). Separations were performed on C18 columns (Symmetry 4.6 × 150 mm, 5 μm, Waters) fitted with a C18 guard column (Symmetry 3.9 × 20 mm, 5 μm, Waters) and fluorimetric detection (λex = 338/λem = 425 nm, Model 474, Waters) was used. The samples were automatically derivatized through the autosampler (Model 717plus, Waters) in the following manner. First, 100 μl of orthophthalic aldehyde solution (15 mg ml−1 (v/v) in 50 % methanol) was added into 450 μl of the filtered reaction mixture. After two cycles of mixing and a 3-min delay, 100 μl of the sample was injected on the column. Chromatographic separations were performed with 60 mM phosphate buffer (pH 6.8; eluent A) and 80 % acetonitrile (eluent B) using two different gradient programs. A shorter program (the mobile phase started with eluent A:eluent B at 100:0 % and varied to 0:100 % and 100:0 % in 12 and 16 min, respectively) was used when the transamination reaction was performed with the participation of all of the analyzed amino acids except l-aspartate. In the latter case, a longer program was applied (the mobile phase started with eluent A:eluent B at 100:0 %, and it was varied to 70:30, 0:100 and 100:0 % in 18, 25 and 31 min, respectively). The flow rate of the mobile phase was 1 ml min−1. The concentration of l-glutamate produced in the course of the transamination reactions was quantified based on the prepared calibration curve.
Electrophoretic separation and enzyme activity staining
Electrophoresis under native and denaturing conditions was performed in a discontinuous system (4 and 10 % stacking and resolving gels were used, respectively) according to the method of Laemmli (1970). The protein bands in the gels after SDS-PAGE were stained with Colloidal Coomassie Brilliant Blue (Roth; Karlsruhe, Germany) according to the manufacturer’s protocol. The gels were calibrated with Unstained Protein Molecular Weight Marker (Fermentas; Vilnius, Lithuania). The aromatic amino acid:2-oksoglutarate activity bands after native PAGE were visualized according to the method described by Pedraza et al. (2004) with modifications. Gels after electrophoresis were incubated in a mixture containing 0.1 M phosphate buffer (pH 8.0), 10 mM l-phenylalanine, 10 mM l-tryptophan or 2.5 mM l-tyrosine, 3 mM 2-oxoglutarate, 2 μM PLP, 0.6 mM NBT and 0.1 mM PMS until activity bands appeared. Gels were washed with distilled water and photographed. When the gels were stained for l-aspartate:2-oxoglutarate activity, 8 mM l-cysteine sulfinic acid was used as the amino acid substrate (Stejskal 1994).
Protein identification
Proteins were identified by mass spectrometry (Laboratory of Mass Spectrometry, Institute of Biochemistry and Biophysics, Polish Academy of Sciences, Warsaw, Poland) and by N-terminal sequencing using the Edman degradation method (Biocentrum; Kraków, Poland). For mass spectrometry analysis, the protein bands obtained in SDS-PAGE were excised and digested with trypsin. The resulting peptides were separated on a nanoAcquity UPLC (Ultra Performance LC) and analyzed with an Orbitrap-based mass spectrometer. N-terminal sequencing of the proteins was performed from PVDF membranes using an automated sequencer Procise 491 system (Applied Biosystems, Foster City, CA, USA).
Obtaining a partial sequence of the genes encoding the studied enzymes
Bacterial genomic DNA was isolated using the Genomic DNA Purification Kit (Fermentas). The following primers were used for PCR: FArAT I (5′-GTGTTCAAACATGTCGATGC-3′), RArAT I (5′-TTCGAGAACGAGTTGCTGAC-3′), FArAT II (5′-ATGCACTTCGGAGCCATC-3′) and RArAT II (5′-GACCATGTACACGCTGTGCT-3′). These primers were designed based on the nucleotide sequence of putative ArAT from P. putida KT2440 (NCBI Gene ID: 1045962; tyrB-2) and from P. entomophila L48 (NCBI Gene ID: 4086633; phhC). The PCR amplifications were performed using Pfu polymerase (Fermentas) using the following program: 10 min at 95 °C, 30 cycles of 1 min at 95 °C, 1.5 min at 56 °C and 4 min at 70 °C and a final extension step for 10 min at 72 °C. The resulting PCR products were ligated into a pJET1.2/blunt cloning vector (Fermentas), amplified in Escherichia coli TOP10 cells and sequenced in the Laboratory of DNA Sequencing and Oligonucleotide Synthesis (Institute of Biochemistry and Biophysics, Polish Academy of Sciences). The obtained nucleotide sequences were analyzed using bioinformatics tools.
Results and discussion
Zymographic analysis of the proteins from the crude bacterial extract revealed the presence of two aminotransferases capable of catalyzing the reaction involving aromatic amino acids and l-aspartate as substrates. According to their electrophoretic mobility, the enzymes were designated as ArAT I and ArAT II (Fig. 1). ArAT I and ArAT II were subjected to a six-step purification procedure that consisted of ammonium sulfate fractionation (40–60 %), hydrophobic interaction chromatography on phenyl-Sepharose, gel filtration chromatography on a Sephadex G-100 superfine and anion exchange chromatography on DEAE-cellulose as well as gel filtration rechromatography and anion exchange chromatography on a Protein-Pack Q8 HR (HPLC). The most effective step was the anion exchange chromatography on a DEAE-cellulose column, which permitted the separation of the studied proteins and resulted in an approximate 8-fold purification of ArAT II and a 2.5-fold purification of ArAT I. The purification procedure resulted in a final 104-fold purification of ArAT I and 395-fold purification of ArAT II with a yield of 0.95 and 1.71 % and specific activities of 14.7 and 55.7 U mg−1 protein, respectively (Table 1). Highly purified ArAT I and ArAT II were used for the determination of their substrate specificity, which is the main criterion for enzyme classification. For this purpose, we used thirteen l-amino acids and nine 2-oxoacids with various side chain structures. Among the amino acids tested, both enzymes most efficiently catalyzed the reactions involving aromatic amino acids and l-aspartate (Table 2). The best substrate for ArAT I and ArAT II was l-phenylalanine, and the activities of both isozymes toward l-tryptophan, l-aspartate and l-tyrosine ranged from 56 to 90 % of the maximum activity at 10 mM amino acid concentration. Activity of ArAT I toward the other tested amino acids did not exceed 10 % of the maximum activity. ArAT II was characterized by a broader substrate specificity and catalyzed relatively efficiently also reactions involving l-histidine (38 % of the maximum activity at 10 mM concentration), l-isoleucine and l-leucine (17 and 14 % of the maximum activity at 10 mM concentration, respectively). Among the studied 2-oxoacid substrates, ArAT I and ArAT II exhibited the highest activities toward aromatic and dicarboxylic 2-oxoacids; however, different results were obtained depending on the 2-oxoacid concentration. At 0.25 mM 2-oxoacid concentration, both isozymes converted phenylpyruvate and p-hydroxyphenylpyruvate with the highest rate. When 3 mM 2-oxoacid was used, ArAT I and ArAT II catalyzed the reactions involving oxaloacetate most efficiently. The observed phenomenon was associated with the occurrence of progressive substrate inhibition when aromatic 2-oxoacids were used at concentrations above 0.25 mM, which indicated a high affinity of the aminotransferases towards these substrates (Paris and Magasanik 1981). For dicarboxylic 2-oxoacids, progressive substrate inhibition was observed when the concentration of these compounds exceeded 3 mM (data not shown). Activity of ArAT I toward other tested 2-oxoacids did not exceed 5 % of the maximum activity, whereas ArAT II catalyzed with a relatively high rate reaction involving imidazole-pyruvate (31 % of the maximum activity at 3 mM concentration). These results clearly show that the best substrates for ArAT I and ArAT II were aromatic amino acids and 2-oxoacids followed by dicarboxylic compounds, which allowed for the classification of the studied aminotransferases as ArAT isozymes. High activities toward dicarboxylic substrates characterized also ArATs isolated from other sources. The activity of ArAT from Escherichia coli toward l-aspartate reached 65 % of the maximum activity determined with l-tyrosine (Powell and Morrison 1978), and activity of ArAT from Brevibacterium linens toward l-aspartate reached 50 % of the maximum activity determined with l-phenylalanine (Lee and Desmazeaud 1985). For comparison, aspartate aminotransferases studied in parallel with ArATs by Powell and Morrison (1978) and Lee and Desmazeaud (1985) were characterized by considerably different relative activities toward amino acid substrates. Aspartate aminotransferase from E. coli catalyzed the reaction involving aromatic amino acids at a rate of approximately 20 % of the maximum activity reached with l-aspartate, and the same enzyme from B. linens catalyzed the transamination reactions with the participation of various aromatic amino acids six to ten times less efficiently than reactions involving l-aspartate. Similar kinetic properties characterized also aspartate aminotransferase from Pyrococcus furiosus (Ward et al. 2002).
The final preparations of ArAT I and ArAT II were subjected to SDS-PAGE. Such an analysis of the ArAT II preparation revealed a single protein band with a molecular mass of approximately 44.2 kDa (Fig. 2b). The identity of this protein band was determined by mass spectrometry. As a result, five peptides with 100 % identity and one peptide with 92 % identity with sequence fragments of putative ArAT from P. entomophila L48 (NCBI RefSeq protein accession no: YP_609388.1; NCBI Gene ID: 4086633; phhC) were obtained (Fig. 3b). The identified peptides constituted 20 % of the total enzyme sequence. For ArAT I preparation, SDS-PAGE analysis showed the presence of five visible protein bands (Fig. 2a). All of these bands were separately excised from the gel and analyzed using mass spectrometry. Examination of the protein band with a molecular mass of approximately 43.8 kDa resulted in the identification of seven peptides with 100 % identity to sequence fragments of a putative ArAT from P. putida KT2440 (NCBI RefSeq protein accession no: NP_745726.1; NCBI Gene ID: 1045962; tyrB-2) (Fig. 3a). The identified peptides constituted 27 % of the total enzyme sequence. Analysis of other four protein bands did not result in the identification of any peptides similar to any putative ArAT sequence (data not shown). Densitometric analysis of the gel showed that the protein band corresponding to ArAT I constituted approximately 24 % of the total protein content in the final enzyme preparation. To further establish the identity of the studied isozymes, N-terminal fragments of ArAT I and ArAT II sequences were determined using the Edman degradation method. For ArAT II, an unambiguous 20 amino-acid sequence (MHFGAIARVPGDPILGLMEA) was obtained. This sequence showed a 90 % identity with the N-terminal sequence of YP_ 609388.1 protein (18 amino acids identical, Gly in place of Ala at position 4 and Ala in place of Gly at position 7). For ArAT I, a five amino acid sequence with an uncertain amino acid at position three: MF(K+G)HV was obtained. With the adoption of the presence of Lys (K) at that position, this sequence showed 100 % identity with the N-terminal sequence of NP_745726.1 protein. The consistent results obtained by mass spectrometry and Edman degradation analysis of the ArAT I and ArAT II amino acid sequences allowed the selection of appropriate nucleotide sequences from the NCBI database, which were used as a template to design the primers for PCR. For ArAT I, the primers were designed on the basis of the nucleotide sequence of the tyrB-2 gene encoding a putative ArAT from P. putida KT2440; for ArAT II, the primers were designed based on the nucleotide sequence of the phhC gene, which encodes a putative ArAT from P. entomophila. As a result, PCR products of 740 and 1,110 bp were obtained and sequenced. These sequences, which represented 62 % (tyrB-2) and 93 % (phhC) of the total gene sequences, have been deposited in the GeneBank under accession numbers KC415773 and KC415774, respectively. It should be noted that for both enzymes, we observed 100 % identity between all amino acid-sequence fragments obtained by biochemical analysis of the purified proteins and amino acid sequences deduced from the obtained nucleotide sequences. These results demonstrate that the tyrB-2 and phhC genes of the studied P. putida strain, taxonomy of which was confirmed using the precise ribotyping method, encode aromatic amino acid aminotransferase isozymes. The function of the tyrB-2 gene in bacteria of the Pseudomonas genus was predicted on the basis of its sequence homology, and the function of the phhC gene has been described only in P. aeruginosa (Gu et al. 1998). Moreover, to the best of our knowledge, this is the first report demonstrating the presence of the phhC gene in the genome of P. putida. In other strains of these bacteria, which have been previously sequenced, the only genes encoding proteins with predicted ArAT function were tyrB and tyrB-2. The fact that only two ArAT isozymes were detected in the studied bacterial strain may indicate a lack of the tyrB gene in this strain or that the authentic function of this gene is different.
SDS-PAGE of the final ArAT I (a) and ArAT II (b) preparations. Approximately 3 μg (ArAT I) and 0.5 μg (ArAT II) of protein were loaded. WM, molecular weight standards: β-galactosidase (116.0 kDa), albumin (66.2 kDa), ovalbumin (45.0 kDa), lactate dehydrogenase (35.0 kDa), REase Bsp98 l (25.0 kDa) and β-lactoglobulin (18.4 kDa)
The alignment of the deduced amino acid sequences of ArAT I (a) and ArAT II (b) with putative ArAT proteins and peptides obtained by mass spectrometry analysis. NP_745726.1 and YP_609388.1, deduced amino acid sequences of putative ArAT from P. putida KT2440 and P. entomophila L48, respectively. MS, peptides derived from mass spectrometry analysis. KC415773 and KC415774, deduced amino acid sequences of ArAT I and ArAT II (studied enzymes), respectively. N-terminal fragments of ArAT I and ArAT II determined using the Edman degradation method are shown in bold font. Identical amino acids within aligned sequences are presented on a black background
Concluding remarks
In this paper, we demonstrate evidence based on biochemical approaches for the determination of previously predicted function of the proteins and genes that two P. putida aminotransferases, whose substrate specificity allowed for their classification as ArAT isozymes, are encoded by the tyrB-2 and phhC genes. The physiological importance of the existence of two ArAT isozymes, especially in the aspect of their contribution to IAA biosynthesis by the P. putida species, remains to be shown and is a subject of our current study.
References
Botelho GR, Mendonça-Hagler LC (2006) Fluorescent Pseudomonads associated with the rhizosphere of crops—an overview. Braz J Microbiol 37:401–416
Bradford MM (1976) A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Castro-Guerrero J, Romero A, Aguilar JJ, Xiqui ML, Sandoval JO, Baca BE (2012) The hisC1 gene, encoding aromatic amino acid aminotransferase-1 in Azospirillum brasilense Sp7, expressed in wheat. Plant Soil 356:139–150
Ge SM, Tao L, Chen SF (2009) Expression and functional analysis of aminotransferase involved in indole-3-acetic acid biosynthesis in Azospirillum brasilense Yu62. Biochemistry (Mosc) 74(1):81–84
Gu W, Song J, Bonner CA, Xie G, Jensen RA (1998) PhhC is an essential aminotransferase for aromatic amino acid catabolism in Pseudornonas aeruginosa. Microbiology 144(11):3127–3134
Khakipour N, Khavazi K, Mojallali H, Pazira E, Asadirahmani H (2008) Production of auxin hormone by fluorescent pseudomonads. Am-Eurasian J Agric Environ Sci 4(6):687–692
King EO, Ward MK, Raney DE (1954) Two simple media for the demonstration of pyocyanin and fuorescein. J Lab Clin Med 44:301–302
Koga J, Syono K, Ichikawa T, Adachi T (1994) Involvement of l-tryptophan aminotransferase in indole-3-acetic acid biosynthesis in Enterobacter cloacae. Biochim Biophys Acta 1209(2):241–247
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lambrecht M, Okon Y, Vande Broek A, Vanderleyden J (2000) Indole-3-actic acid: a reciprocal signaling molecule in bacteria-plant interaction. Trends Microbiol 8(7):298–300
Lee CW, Desmazeaud MJ (1985) Partial purification and some properties of an aromatic-amino-acid and an aspartate aminotransferase in brevibacterium linens 47. Microbiology 131(3):459–467
Li S, Zhao H, Li Y, Niu S, Cai B (2012) Complete genome sequence of the naphthalene-degrading Pseudomonas putida strain ND6. J Bacteriol 194(18):5154–5155
Matilla MA et al (2011) Complete genome of the plant growth-promoting rhizobacterium Pseudomonas putida BIRD-1. J Bacteriol 193(5):1290
Metha PK, Halle TI, Christen P (1993) Aminotransferases: demonstration of homology and division into evolutionary subgroups. Eur J Biochem 214:549–561
Nelson KE et al (2002) Complete genome sequence and comparative analysis of the metabolically versatile Pseudomonas putida KT2440. Environ Microbiol 4(12):799–808
Paris CG, Magasanik B (1981) Purification and properties of aromatic amino acid aminotransferase from Klebsiella aerogenes. J Bacteriol 145(1):266–271
Patten CL, Glick BR (2002) Role of Pseudomonas putida indoleacetic acid in development of the host plant root system. Appl Environ Microbiol 68(8):3795–3801
Pedraza RO, Ramirez-Mata A, Xiqui ML, Baca BE (2004) Aromatic amino acid aminotransferase activity and indole-3-acetic acid production by associative nitrogen-fixing bacteria. FEMS Microbiol Lett 233:15–21
Phillips KA et al (2011) Vanishing tassel2 encodes a grass-specific tryptophan aminotransferase required for vegetative and reproductive development in maize. Plant Cell 23(2):550–566
Powell JT, Morrison JF (1978) The purification and properties of the aspartate aminotransferase and aromatic-amino-acid aminotransferase from Escherichia coli. Eur J Biochem 87(2):391–400
Sergeeva E, Hirkala DLM, Nelson LM (2007) Production of indole-3-acetic acid, aromatic amino acid aminotransferase activities and plant growth promotion by Pantoea agglomerans rhizosphere isolates. Plant Soil 297(1–2):1–13
Simpson RM, Nonhebel HM, Christie DL (1997) Partial purification and characterisation of an aromatic amino acid aminotransferase from mung bean (Vigna radiata L. Wilczek). Planta 201:71–77
Spaepen S, Vanderleyden J, Remans R (2007) Indole-3-acetic acid in microbal and microorganism-plant signaling. FEMS Microbiol Rev 31:425–448
Stejskal J (1994) Aspartate aminotransferase isozymes in plants: comparison of two staining methods in polyacrylamide gels. Biol Plant 36:359–364
Szkop M, Sikora P, Orzechowski S (2012) A novel, simple, and sensitive colorimetric method to determine aromatic amino acid aminotransferase activity using the Salkowski reagent. Folia Microbiol 57(1):1–4
Van Eijk HMH, Rooyakkers DR, Deutz NEP (1993) Rapid routine determination of amino acids in plasma by high-performance liquid chromatography with a 2–3 μm Spherisorb ODS II column. J Chromatogr 620:143–148
Ward DE, De Vos WM, Van der Oost J (2002) Molecular analysis of the role of two aromatic aminotransferases and a broad-specificity aspartate aminotransferase in the aromatic amino acid metabolism of Pyrococcus furiosus. Archaea 1:133–141
Whitaker RJ, Gaines CG, Jensen RA (1982) A multispecific quintet of aromatic aminotransferases that overlap different biochemical pathways in Pseudomonas aeruginosa. J Biol Chem 257(22):13550–13556
Won C, Shen X, Mashiguchi K, Zheng Z, Dai X, Cheng Y, Kasahara H, Kamiya Y, Chory J, Zhao Y (2011) Conversion of tryptophan to indole-3-acetic acid by TRYPTOPHAN AMINOTRANSFERASES OF ARABIDOPSIS and YUCCAs in Arabidopsis. PNAS 108(45):18518–18523
Xing RY, Whitman WB (1992) Characterization of amino acid aminotransferases of Methanococcus aeolicus. J Bacteriol 174(2):541–548
Yvon M, Chambellon E, Bolotin A, Roudot-Algaron F (2000) Characterization and role of the branched-chain aminotransferase (BcaT) isolated from Lactococcus lactis subsp. cremoris NCDO 763. Appl Environ Microbiol 66(2):571–577
Acknowledgments
This work was supported by the Polish Ministry of Science and Higher Education (Grant No. NN310 304639).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Szkop, M., Bielawski, W. tyrB-2 and phhC genes of Pseudomonas putida encode aromatic amino acid aminotransferase isozymes: evidence at the protein level. Amino Acids 45, 351–358 (2013). https://doi.org/10.1007/s00726-013-1508-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00726-013-1508-y