Abstract
Background
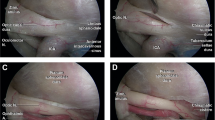
The interoptic triangle (IOT) offers a key access to the contralateral carotid artery’s ophthalmic segment (oICA) and its perforating branches (PB), the ophthalmic artery (OA), and the superior hypophyseal artery (SHA). It has been previously reported that the assessment of IOT’s size is relevant when attempting approaches to the contralateral oICA. However, previous studies have overseen that, since the oICA is a paramedian structure and a lateralized contralateral approach trajectory is then required, the real access to the oICA is further limited by the approach angle adopted by the surgeon with respect to the IOT’s plane. For this reason, we determined the surgical accessibility to the contralateral oICA and its branches though the IOT by characterizing the morphometry of this triangle relative to the optimal contralateral approach angle.
Methods
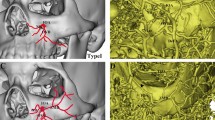
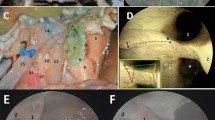
We defined the “relative interoptic triangle” (rIOT) as the two-dimensional projection of the IOT to the surgeon’s view, when the microscope has been positioned with a certain angle with respect to the midline to allow the maximal contralateral oICA visualization. We correlated the surface of the rIOT to the visualization of oICA, OA, SHA, and PBs on 8 cadavers and 10 clinical datasets, using for the last a 3D-virtual reality system.
Results
A larger rIOT correlated positively with the exposure of the contralateral oICA (R = 0.967, p < 0.001), OA (R = 0.92, p < 0.001), SHA (R = 0.917, p < 0.001), and the number of perforant vessels of the oICA visible (R = 0.862, p < 0.001). The exposed length of oICA, OA, SHA, and number PB observed increased as rIOT’s surface enlarged. The correlation patterns observed by virtual 3D-planning matched the anatomical findings closely.
Conclusions
The exposure of contralateral oICA, OA, SHA, and PB directly correlates to rIOT’s surface. Therefore, preoperative assessment of rIOT’s surface is helpful when considering contralateral approaches to the oICA. A virtual 3D planning tool greatly facilitates this assessment.






Similar content being viewed by others
References
Hassoun-Turkmani A, AL. D (2016) Microsurgery of paraclinoid aneurysms. In: Youmans W (ed) Neurological surgery, vol 4, 7th edn, pp 3298–3306
Akabane A, Saito K, Suzuki Y, Shibuya M, Sugita K (1995) Monitoring visual evoked potentials during retraction of the canine optic nerve: protective effect of unroofing the optic canal. J Neurosurg 82:284–287. https://doi.org/10.3171/jns.1995.82.2.0284
Boari N, Spina A, Giudice L, Gorgoni F, Bailo M, Mortini P (2017) Fronto-orbitozygomatic approach: functional and cosmetic outcomes in a series of 169 patients. J Neurosurg 1–9. https://doi.org/10.3171/2016.9.JNS16622
Chang HS, Joko M, Song JS, Ito K, Inoue T, Nakagawa H (2006) Ultrasonic bone curettage for optic canal unroofing and anterior clinoidectomy. Technical note. J Neurosurg 104:621–624. https://doi.org/10.3171/jns.2006.104.4.621
Chen S, Kato Y, Kumar A, Sinha R, Oguri D, Oda J, Watabe T, Imizu S, Sano H, Hirose Y (2013) Contralateral approach to unruptured superior hypophyseal artery aneurysms. J Neurol Surg A Cent Eur Neurosurg 74:18–24. https://doi.org/10.1055/s-0032-1326944
Clatterbuck RE, Tamargo RJ (2005) Contralateral approaches to multiple cerebral aneurysms. Neurosurgery 57:160–163 discussion 160-163
Day AL (1990) Aneurysms of the ophthalmic segment. A clinical and anatomical analysis. J Neurosurg 72:677–691. https://doi.org/10.3171/jns.1990.72.5.0677
de Oliveira E, Tedeschi H, Siqueira MG, Ono M, Fretes C, Rhoton AL Jr, Peace DA (1996) Anatomical and technical aspects of the contralateral approach for multiple aneurysms. Acta Neurochir 138:1–11 discussion 11
Fries G, Perneczky A, van Lindert E, Bahadori-Mortasawi F (1997) Contralateral and ipsilateral microsurgical approaches to carotid-ophthalmic aneurysms. Neurosurgery 41:333–342 discussion 342-333
Gelber BR, Sundt TM Jr (1980) Treatment of intracavernous and giant carotid aneurysms by combined internal carotid ligation and extra- to intracranial bypass. J Neurosurg 52:1–10. https://doi.org/10.3171/jns.1980.52.1.0001
Germanwala AV, Hofler R, Lagman C, Chung LK, Khalessi AA, Zada G, Smith ZA, Dahdaleh NS, Bohnen AM, Cho JM, Colen CB, Duckworth E, Kan P, Lam S, Kim CY, Li G, Lim M, Sherman JH, Wang VY, Yang I (2017) Neurosurgery concepts: key perspectives on endoscopic versus microscopic resection for pituitary adenomas, surgical decision-making in tuberculum sellae meningiomas, optic nerve mobilization during resection of craniopharyngiomas, and evaluation of headache and quality of life after endoscopic transphenoidal surgery for pituitary adenomas. Surg Neurol Int 8:52. https://doi.org/10.4103/sni.sni_17_17
Gibo H, Lenkey C, Rhoton AL Jr (1981) Microsurgical anatomy of the supraclinoid portion of the internal carotid artery. J Neurosurg 55:560–574. https://doi.org/10.3171/jns.1981.55.4.0560
Hongo K, Watanabe N, Matsushima N, Kobayashi S (2001) Contralateral pterional approach to a giant internal carotid-ophthalmic artery aneurysm: technical case report. Neurosurgery 48:955–959
Kakizawa Y, Tanaka Y, Orz Y, Iwashita T, Hongo K, Kobayashi S (2000) Parameters for contralateral approach to ophthalmic segment aneurysms of the internal carotid artery. Neurosurgery 47:1130–1137
Kang S, Yang Y, Kim T, Kim J (1997) Sudden unilateral blindness after intracranial aneurysm surgery. Acta Neurochir 139:221–226
Kobayashi S, Kyoshima K, Gibo H, Hegde SA, Takemae T, Sugita K (1989) Carotid cave aneurysms of the internal carotid artery. J Neurosurg 70:216–221. https://doi.org/10.3171/jns.1989.70.2.0216
Kockro RA, Killeen T, Ayyad A, Glaser M, Stadie A, Reisch R, Giese A, Schwandt E (2016) Aneurysm surgery with preoperative three-dimensional planning in a virtual reality environment: technique and outcome analysis. World Neurosurg 96:489–499. https://doi.org/10.1016/j.wneu.2016.08.124
McMahon JH, Morgan MK, Dexter MA (2001) The surgical management of contralateral anterior circulation intracranial aneurysms. J Clin Neurosci 8:319–324
Nacar OA, Rodriguez-Hernandez A, Ulu MO, Rodriguez-Mena R, Lawton MT (2014) Bilateral ophthalmic segment aneurysm clipping with one craniotomy: operative technique and results. Turk Neurosurg 24:937–945. https://doi.org/10.5137/1019-5149.JTN.12586-14.1
Nakao S, Kikuchi H, Takahashi N (1981) Successful clipping of carotid-ophthalmic aneurysms through a contralateral pterional approach: report of two cases. J Neurosurg 54:532–536
Nishio S, Matsushima T, Fukui M, Sawada K, Kitamura K (1985) Microsurgical anatomy around the origin of the ophthalmic artery with reference to contralateral pterional surgical approach to the carotid-ophthalmic aneurysm. Acta Neurochir 76:82–89
Oikawa S, Kyoshima K, Kobayashi S (1998) Surgical anatomy of the juxta-dural ring area. J Neurosurg 89:250–254. https://doi.org/10.3171/jns.1998.89.2.0250
Oshiro EM, Rini DA, Tamargo RJ (1997) Contralateral approaches to bilateral cerebral aneurysms: a microsurgical anatomical study. J Neurosurg 87:163–169. https://doi.org/10.3171/jns.1997.87.2.0163
Renn WH, Rhoton AL Jr (1975) Microsurgical anatomy of the sellar region. J Neurosurg 43:288–298. https://doi.org/10.3171/jns.1975.43.3.0288
Rhoton AI Jr (2002) Cranial anatomy and surgical approaches. Neurosurgery 53:1–746
Rizzo JF 3rd (1995) Visual loss after neurosurgical repair of paraclinoid aneurysms. Ophthalmology 102:905–910
Rozen WM, Chubb D, Stella DL, Taylor GI, Ashton MW (2009) Evaluating anatomical research in surgery: a prospective comparison of cadaveric and living anatomical studies of the abdominal wall. ANZ J Surg 79:913–917. https://doi.org/10.1111/j.1445-2197.2009.05143.x
Serrano LE, Ayyad A, Archavlis E, Schwandt E, Nimer A, Ringel F, Kantelhardt SR (2018) A literature review concerning contralateral approaches to paraclinoid internal carotid artery aneurysms. Neurosurg Rev. https://doi.org/10.1007/s10143-018-01063-3
Shiokawa Y, Aoki N, Saito I, Mizutani H (1988) Combined contralateral pterional and interhemispheric approach to a subchiasmal carotid-ophthalmic aneurysm. Acta Neurochir 93:154–158
Vajda J, Juhasz J, Pasztor E, Nyary I (1988) Contralateral approach to bilateral and ophthalmic aneurysms. Neurosurgery 22:662–668
van Lindert E, Perneczky A, Fries G, Pierangeli E (1998) The supraorbital keyhole approach to supratentorial aneurysms: concept and technique. Surg Neurol 49:481–489 discussion 489-490
Yamada K, Hayakawa T, Oku Y, Ushio Y, Yoshimine T, Kawai R (1984) Contralateral pterional approach for carotid-ophthalmic aneurysm: usefulness of high resolution metrizamide or blood computed tomographic cisternography. Neurosurgery 15:5–8
Yaşargil MG (1984) Clinical considerations, surgery of the intracranial aneurysms and results, vol 2. Thieme
Zimmerman CF, Van Patten PD, Golnik KC, Kopitnik TA Jr, Anand R (1995) Orbital infarction syndrome after surgery for intracranial aneurysms. Ophthalmology 102:594–598
Acknowledgements
We want to thank very much Mr. Thomas Bauer for assistance with drawings for the Fig. 1, which greatly improved the intended message of our manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. The cadaveric study followed all ethical and hygienic procedures ruled by the Hygiene and Health Department of our institution and current national and international standards. Ethical committee approval was not required as presented data correspond to cadaveric specimens and anonymized patient’s datasets, so that there is no risk of identification.
Informed consent
Consent was not obtained given that presented data corresponding to patient’s datasets are anonymized and there is no risk of identification.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Comments
The authors are to be congratulated for their meticulous work in studying the exposures that can be achieved through the interoptic triangle from the surgeons’ perspective. The study is performed on 8 cadaveric specimens as well as 10 clinical datasets using a 3D virtual reality system. The exposure of the contralateral ophthalmic segment of the carotid, the ophthalmic artery, as well as the superior hypophyseal artery is studied, evaluating the relative area exposed with a variety of surgical manipulations like ipsilateral clinoidectomy, drilling of the tuberculum or planum, and contralateral optic nerve mobilization. The study does not necessarily offer something radical, but there are subtle novelties that may make it a worthwhile addition to the literature.
Georgios A. Zenonos
Jacques J. Morcos
FL,USA
In this article, the authors describe the “relative interoptic triangle” (rIOT) and how size of the rIOT influences access to the contralateral ICA, OphA, SHA, and perforators. Eight cadavers and 10 clinical datasets using a 3D-VR system were used. The authors found that a larger rIOT correlated with greater exposure of the contralateral ICA, OphA, SHA, and perforators, which makes logical sense. This concept is demonstrated in Fig. 1, which shows how the surgical approaches alters rIOT size and, therefore, influences visualization of contralateral vascular structures. Figure 4 also demonstrates that significant anterior skull base removal and optic nerve mobilization greatly increase rIOT.
The ideal approach to proximal ICA pathology (i.e., aneurysm) is rarely, if ever, a contralateral approach. If a patient has bilateral ICA aneurysms, the side of approach is that of the more concerning aneurysm, and the look contralaterally is only to assess clip feasibility of the aneurysm to spare the patient a second craniotomy. While the rIOT is influenced by the craniotomy of choice, it also changes with each move of the microscope. Therefore, surgeons can increase or decrease visibility of contralateral lesions by changing their working angle. It is not clear how pre-operative assessment and determination of rIOT from a specific working angle would influence intra-operative decisions to look contralaterally or not. In addition, surgeons will only clip contralateral ICA aneurysms if they can safely do so and this is something that can only be determined at surgery.
Fady Charbel
Illinois, USA
This article is part of the Topical Collection on Neurosurgical Anatomy
Rights and permissions
About this article
Cite this article
Serrano, L.E., Archavlis, E., Ayyad, A. et al. The approach angle to the interoptic triangle limits surgical workspace when targeting the contralateral internal carotid artery. Acta Neurochir 161, 1535–1543 (2019). https://doi.org/10.1007/s00701-019-03911-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-019-03911-7