Abstract
Background
Acute subdural hematoma (ASDH) is a serious traumatic disease, and predictive methods for hematoma growth are necessary to decide whether emergent operation is necessary. This study aimed to evaluate the incidence of “leakage” using computed tomography angiography (CTA) in patients with ASDH and to identify its prognostic value.
Methods
Sixty-seven patients with ASDH were examined using CTA (mean age 64.1 ± 20.6 years; 24 men) by analyzing two serial scans (CTA phase and delayed phase). We defined a positive leakage sign as a > 10% increase in Hounsfield units (HU) in the region of interest. Hematoma expansion was determined using plain CT after 24 h in patients who did not undergo emergent surgery.
Results
Of the 67 patients, conservative therapy was administered to 35 patients; of these patients, 9 showed hematoma expansion, and 8 of these 9 patients (88.9%) showed positive leakage signs. The sensitivity and specificity of leakage signs to hematoma expansion in the no-surgery group were 88.8% and 76.1%, respectively. All positive leakage signs were found within 4.5 h of injury; patients showing negative leakage signs showed a decreased tendency towards hematoma 24 h after injury. Patients presenting with positive leakage signs had poor outcomes.
Conclusions
The results indicated that the leakage sign is a sensitive predictor of hematoma expansion and poor outcomes in ASDH. If the hematoma is small but leakage sign-positive, strict observation is necessary and aggressive surgery may improve outcomes.
Similar content being viewed by others
Introduction
Acute subdural hematoma (ASDH) is a serious disease with high morbidity and mortality. Many cases require emergent operation on admission to prevent brain herniation. Contrarily, some patients with a small hematoma and faint disturbance of consciousness on admission show a delayed, sudden increase in hematoma size, whereas other cases show no increase in hematoma size, resulting in good outcomes. Thus, the timing and decision of surgical intervention is an important issue for ASDH patients [5, 6, 14]. Correctly predicting the expansion of the hematoma is crucial. This prediction helps in choosing aggressive surgery and avoids unnecessary surgical operations.
We have previously reported a sensitive predictive method named “leakage sign” for contusional hematoma cases, with high sensitivity, specificity, and predictive value for hematoma expansion [9]. The purpose of this study was to establish a sensitive predictive method for ASDH expansion using this leakage sign. We expected that the leakage sign would be valuable in the selection of optimal operative strategy.
Materials and methods
Patient selection
All patients with traumatic head injury that were transferred to our institute between April 2012 and August 2015 were initially included in this prospective study (n = 152). We performed computed tomography angiography (CTA) on all patients with ASDH to determine whether any vascular lesions were present. If for any reason CTA could not be performed, the patient was excluded. Patients with chronic subdural hematoma, patients with Glasgow Coma Scale (GCS) score of 3 points with bilateral dilated pupils, patients allergic to the contrast medium, patients with kidney dysfunction, and patients with only diffuse axonal injury or traumatic subarachnoid hemorrhage were excluded. CTA was not performed for patients with rapidly progressive symptoms, and they were also excluded. A total of 67 cases of ASDH were included in this prospective study. This study was approved by the review board and Ethics Committee of our institution. Informed consent was obtained from all patients.
Clinical data
The following patient clinical data were recorded at admission: age, sex, arterial blood pressure, and the time from onset to admission. In addition, coagulation status at admission was evaluated using the international normalized ratio, prothrombin time, partial thromboplastin time, and use of modifying treatments such as antiplatelet therapy, anticoagulation therapy, administration of fresh frozen plasma, vitamin K therapy, and platelet transfusion. The onset time was determined by emergency records. When onset time was unclear, the case was excluded.
Detection of leakage sign by image acquisition
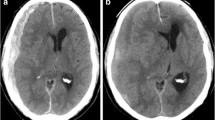
The leakage sign method has been previously documented [9, 10]. CT acquisitions were performed according to standard departmental protocols using eight-section General Electric helical CT scanners (BrightSpeed Edge, GE Healthcare, Wisconsin, USA). The first CT scan was performed for CTA (CTA phase), and the second scan (delayed phase CT) was performed 5 min after the CTA (Fig. 1). Plain CT was performed 24 h after the first CT to evaluate the hematoma size and other intracranial findings; a detailed method has been described previously [9].
The definition of leakage sign and the clinical examination process used in this study was based on computed tomography angiography (CTA) and delayed phase CT images. The region of interest (ROI; 10-mm diameter) was placed on the delayed phase images to identify leakage of the contrast medium into the hematoma. Hounsfield unit (HU) values in the ROI were determined in each section of the CTA and delayed phase images, and a > 10% increase in HU was considered as a positive leakage sign
For the CTA, 70 mL ioversol (Optiray; Fuji Pharma Co., Ltd., Tokyo, Japan; 320 mg I/mL) was intravenously injected at a rate of 3–3.5 mL/s via a power injector through an intravenous line. All plain CT scans were reviewed by two neuroradiologists blinded to the clinical data. The initial and follow-up plain CT studies were evaluated during separate sessions; the images were anonymous and randomized so that the reviewer was blinded to the patient’s identity and the timing of the images (admission or follow-up). The images were evaluated for hematoma size, which was based on the section with the largest hemorrhage size (mm2) of all the serial sections.
The following criteria for detection of leakage signs were used: based on the arterial and delayed phase CT images, a region of interest (ROI) of 10-mm diameter was set on the delayed phase images for the leakage of the contrast medium into the hematoma; the HU values in the ROI were determined in each section of the arterial and delayed phase images; a > 10% increase in HU was considered as a positive leakage sign (Fig. 1).
Measurement of changes in hemorrhage size
A region was set in the selected section that included the region of the hemorrhage, and the area was automatically measured with hemorrhage-HU (60–80) using INFINITT PACS (Infinitt Japan Co.; Japan). The leakage sign is usually present in extra-axial hematomas. To determine if the hematoma size had increased, we compared the measurements of the hemorrhage at initial presentation and at follow-up (24 h later).
Statistical analysis
Baseline demographics, hematoma volumes, and medication/medical history were compared between leakage sign-positive and leakage sign-negative groups using Fisher exact tests, t tests, analysis of variance, or McNemar tests, as appropriate. The relationship between hematoma expansion and leakage sign was analyzed in patients who did not undergo surgery. Statistical analyses were performed using the JMP version 13 software package (SAS Institute Inc., Cary, NC, USA).
Results
Sixty-seven patients with ASDH (39 men and 28 women) were included in this prospective study. The mean patient age was 72.1 (range, 27–95) years, and the median GCS score at admission was 9 (range, 3–15) points. The leakage sign-positive group had significantly lower GCS scores on admission (P < 0.05). There were no significant differences in the distributions of age, sex, platelet count, and international normalized ratio between the leakage sign-positive and leakage sign-negative patients. Contrastingly, the leakage sign-negative group had many patients with a history of hypertension (Table 1).
The leakage sign was positive in 44 patients (65.6%) (Table 1). The clinical course of all ASDH patients is shown in Fig. 2.
Emergent hematoma evacuation at admission was performed in 32 patients (47.7%); 26 of these 32 patients (81.2%) were leakage sign-positive. Of the remaining 35 patients, 5 were treated by delayed hematoma evacuation because they showed a decrease in consciousness level or late hematoma expansion; all 5 cases were leakage sign-positive. In the no-surgery group (n = 35), 17 patients were leakage sign-negative; one patient with subacute subdural hematoma experienced hematoma expansion. The other 18 patients in the no-surgery group were leakage sign-positive; 8 leakage sign-positive patients (44.4%) experienced hematoma expansion, of which 5 patients died within 24 h. Among all patients, 38 (56.7%) experienced poor outcomes (severe disability or death), including 22 patients (32.8%) who died during hospitalization.
Relationship between hematoma expansion and predictive value of the leakage sign
The relationship between hematoma expansion and leakage sign was analyzed in 35 patients who did not undergo emergent surgery at admission. Of these, 18 patients were leakage sign-positive; the 5 patients who died within 24 h were excluded from the analysis. Nine of the remaining 30 patients experienced hematoma expansion, and 8 of these 9 patients (88.8%) were leakage sign-positive (Fig. 2). The leakage sign showed high specificity (88.8%) and sensitivity (76.1%) for hematoma expansion (Table 2). Patients with a positive leakage sign showed a significantly greater increase in maximum hematoma size than patients with negative leakage signs (182.1 ± 263.9 mm2 vs − 198.1 ± 268.9 mm2; P < 0.05) (Fig. 3). Patients with negative leakage signs showed a decrease in hematoma size 24 h after imaging.
We analyzed the relationship between the interval from onset to first CT scan and change in hematoma size after 24 h (Fig. 4). According to our data, positive leakage signs were found until 4.5 h after injury. No cases with positive leakage signs were found after longer time intervals. Most cases (8/11) with negative leakage signs showed a decrease in hematoma size.
All patients who were transferred to our institute more than 5 h after injury were leakage sign-negative and the size of their hematoma had decreased (Fig. 4).
Leakage sign and clinical outcomes
We analyzed the relationship between outcomes measured by GCS score and the presence of leakage sign. The favorite outcomes (good recovery and moderately disabled on the Glasgow Outcome Scale) were significantly lower in cases with positive leakage signs than in cases with negative leakage signs (34.0% vs 60.8%; positive vs negative; P < 0.05). In the surgical group, the favorite outcomes were significantly lower when the leakage sign was positive than when it was negative (34.6% vs 66.6%; P < 0.05).
Discussion
Our prospective study of ASDH showed that the presence of leakage signs is closely related to hematoma growth and poor outcomes. The leakage sign-positive group was ranked as severe according to the GCS score on admission (Table 1). Previous reports have shown that in leakage sign-positive cases, hematoma expansion occurs in intracerebral hemorrhage [9] and contusional hematoma [10]. Many previous studies have attempted to develop methods for the prediction of hematoma expansion in patients with intracerebral hemorrhage. Specific signs such as the blend sign and black hole sign have been used to predict the expansion of hematomas in a cerebral hemorrhage without using contrast media [7, 8, 15, 16]. However, there have been few reports that focused on traumatic hemorrhagic diseases. Furthermore, among all methods that use predictive signs observed in brain scans, detection of leakage signs has the highest sensitivity and specificity. Contrast media is frequently used in trauma cases for whole body scans to detect other possible hemorrhagic lesions, and the leakage sign could be an important predictor in traumatic patients.
The detection of spot signs is capable of revealing the extravasation of contrast media on CTA and predicting patient prognosis [1,2,3,4, 12, 13], but few studies have examined predictive factors in patients with acute subdural hematomas.
Our results indicated that the presence or absence of leakage signs can predict hematoma expansion within 24 h of scanning with high sensitivity (88.8%) and specificity (76.1%) (Table 2). Furthermore, our study showed that in leakage sign-negative cases, acute subdural hematomas tend to decrease in size (Fig. 3), and that these decreases are more pronounced with longer time intervals between injury and CT scanning. This phenomenon was not observed in leakage sign-positive cases. We think that the hematoma may be washed away by cerebrospinal fluid, once the bleeding stops. In stark contrast, the hematoma size generally increased in cases with positive leakage signs (Fig. 4). Thus, with passing time, hematomas may be more likely to decrease in size in the absence of a leakage sign.
The leakage sign cannot predict clinical outcomes in patients with contusional hematomas directly [10]. However, the presence of a leakage sign on CT of patients with ASDH was found to be significantly associated with poor outcomes. Patients who received emergent evacuation of hematoma on admission showed the same trend (Fig. 5). This finding indicated that ASDH affects the prognosis more strongly than brain contusion. Therefore, early identification of this sign and aggressive management with rapid surgical evacuation could be very important, even if the patient’s neurological condition does not appear serious.
It may be critical to even wait for 5 min to perform a CT scan. In the present study, we excluded patients exhibiting anisocoria, unstable vital signs, or sudden deterioration in consciousness level, although no serious complications were observed during CT examination. However, measurement of the vital signs and observations that are in a state are necessary when I consider the possibility that a state turns worse. I may exceed a risk when I think about the possibility that leakage sign can predict the increase of the hematoma. We suggest that 5 min is an appropriate and possibly, a safe time period to delay the second CT and that the clinical data might be more important than the risk.
Thus, detection of leakage signs may be a very useful method in predicting the increase in hematoma size in ASDH as well the patient’s outcome. Selective aggressive treatments for leakage sign-positive patients, such as earlier surgical operation, treatment to decrease excessive blood pressure, and specific hemostat medication [11] may improve outcomes in ASDH patients.
Conclusions
Leakage signs can be reliably identified and are associated with hematoma expansion and poor outcomes. We expect that this method will be helpful in understanding the dynamics of ASDH in clinical medicine.
References
Almandoz JE, Kelly HR, Schaefer PW, Brouwers HB, Yoo AJ, Stone MJ, Goldstein JN, Rosand J, Lev MH, Gonzalez RG, Romero JM (2012) CT angiography spot sign predicts in-hospital mortality in patients with secondary intracerebral hemorrhage. J Neurointervent Surg 4:442–447
Brouwers HB, Goldstein JN, Romero JM, Rosand J (2012) Clinical applications of the computed tomography angiography spot sign in acute intracerebral hemorrhage. Stroke 43:3427–3432
Dowlatshahi D, Wasserman JK, Momoli F, Petrcich W, Stotts G, Hogan M, Sharma M, Aviv RI, Demchuk AM, Chakraborty S (2014) Evolution of computed tomography angiography spot sign is consistent with a site of active hemorrhage in acute intracerebral hemorrhage. Stroke 45:277–280
Du FZ, Jianq R, Gu M, He C, Guan J (2014) The accuracy of spot sign in predicting hematoma expansion after intracerebral hemorrhage: a systematic review and meta-analysis. PLoS One 9:e115777
Karibe H, Hayashi T, Hirano T, Kameyama M, Nakagawa A, Tominaga T (2014) Surgical management of traumatic acute subdural hematoma in adults: a review. Neurol Med Chir 54:887–894
Leitgeb J, Mauritz W, Brazinova A, Janciak I, Majdan M, Wilbacher I, Rusnak M (2012) Outcome after severe brain trauma due to acute subdural hematoma. J Neurosurg 117:324–333
Li R, Yang M (2017) A comparative study of the blend sign and the black hole sign on CT as a predictor of hematoma expansion in spontaneous intracerebral hemorrhage. Biosci Trends 11:682–687
Li Q, Zhang G, Xiong X, Wang XC, Yang WS, Li KW, Wei X, Xie P (2016) Black hole sign: novel imaging marker that predicts hematoma growth in patients with intracerebral hemorrhage. Stroke 47:1777–1781
Orito K, Hirohata M, Nakamura Y, Takeshige N, Aoki T, Hattori G, Sakata K, Abe T, Uchiyama Y, Sakamoto T, Morioka M (2016) Leakage sign for primary intracerebral hemorrhage: a novel predictor of hematoma growth. Stroke 47:958–963
Orito K, Hirohata M, Nakamura Y, Yamamoto M, Takeshige N, Aoki T, Hattori G, Sakata K, Takeuchi Y, Uzu H, Takasu O (2018) Predictive value of leakage signs for pure brain contusional hematoma expansion. J Neurotrauma 35:760–766
Rehmani R, Han A, Hassan J, Farkas J (2017) Role of prothrombin complex concentrate (PCC) in acute intracerebral hemorrhage with positive CTA spot sign: an institutional experience at a regional and state designated stroke center. Emerg Radiol 24:241–247
Rosa Junior M, da Rocha AJ, Maia Junior AC, Saade N, Gagliardi RJ (2015) The active extravasation of contrast (spot sign) depicted on multidetector computed tomography angiography might predict structural vascular etiology and mortality in secondary intracranial hemorrhage. J Comput Assist Tomogr 39:217–221
Thompson AL, Kosior JC, Gladstone DJ, Hopyan JJ, Symons SP, Romero F, Dzialowski I, Roy J, Demchuk AM, Aviv RI, PREDICT/Sunnybrook ICH CTA Study Group (2009) Defining the CT angiography ‘spot sign’ in primary intracerebral hemorrhage. Can J Neurol Sci 36:456–461
Wilberger JE Jr, Harris M, Diamond DL (1991) Acute subdural hematoma: morbidity, mortality, and operative timing. J Neurosurg 74:212–218
Yu Z, Zheng J, Guo R, Ma L, Li M, Wang X, Lin S, Li H, You C (2017) Performance of blend sign in predicting hematoma expansion in intracerebral hemorrhage: a meta-analysis. Clin Neurol Neurosurg 163:84–89
Xiong X, Li Q, Yang WS, Wei X, Hu X, Wang XC, Zhu D, Li R, Cao D, Xie P (2018) Comparison of swirl sign and black hole sign in predicting early hematoma growth in patients with spontaneous intracerebral hemorrhage. Med Sci Monit 24:567–573
Acknowledgements
The authors thank Mr. Naoki Yamamoto, the radiological technician, for assistance.
Funding
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Sports, Science and Culture of Japan.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Research involving human participants and/or animals
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This study was approved by the review board and Ethics Committee of our institution.
Informed consent
Informed consent was obtained from all patients.
Additional information
This article is part of the Topical Collection on Brain Trauma
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Yamamoto, M., Orito, K., Nakamura, Y. et al. Leakage sign for acute subdural hematoma in clinical treatment. Acta Neurochir 161, 233–238 (2019). https://doi.org/10.1007/s00701-018-3755-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-018-3755-x