Abstract
Phototrophic primary producers are regarded as valuable indicators in aquatic habitats, since they are spread in a wide range of environmental conditions. However, the communities harbored by extreme ecosystems such as the saline lakes have been scarcely investigated with respect to their diversity and roles in the ecosystem functioning. The present study investigates the structure, diversity, and distribution of autotrophs from 19 lakes located in the Transylvanian Basin (Romania). Overall, 83 aquatic taxa were identified, belonging to Bacillariophyta (67), Cyanobacteria (10), Chlorophyta (4), Euglenophyta (1), and Xanthophyta (1). Standardized limnological methods were employed for sampling and analyses: measurements of morphometric, physical and chemical parameters, sampling techniques, optical microscopy identification of taxa and multivariate processing of data. Because saline lakes typically sustain undiversified biotic communities, usually well adapted to extreme conditions, we hypothesized that: (1) The main environmental driver of taxonomic diversity (expressed as species richness) would be the salinity; and (2) the same halophilic dominant taxa would occur in all hypersaline lakes (TDS > 50 g L−1). However, our results disproved both statements. Firstly, salinity was found to be an important driver of taxa diversity, but only subsequent to high habitat heterogeneity, lake area and nutrient concentrations. Secondly, high percentages of halotolerant, and not halophilic species were found, together with increased numbers of rare species (appearing in only one lake). Thus, the present study helps improve the current knowledge on the diversity of primary producers from saline lakes, opening new perspectives in understanding the functioning of such extreme environments.

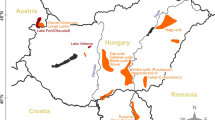
source: Google Earth Pro—7.3.2.5491)




Similar content being viewed by others
References
Abid O, Sellami-Kammoun A, Ayadi H, Drira Z, Bouain A, Aleya L (2008) Biochemical adaptation of phytoplankton to salinity and nutrient gradients in a coastal solar saltern, Tunisia. Estuarine Coastal Shelf Sci 80:391–400. https://doi.org/10.1016/j.ecss.2008.09.007
Afonina EY, Tashlykova NA (2018) Plankton community and the relationship with the environment in saline lakes of Onon-Torey plain, Northeastern Mongolia. Saudi J Biol Sci 25:399–408. https://doi.org/10.1016/j.sjbs.2017.01.003
Alcocer J, Hammer UT (1998) Saline lake ecosystems of Mexico. Aquat Ecosyst Health Managem 1:291–315. https://doi.org/10.1016/S1463-4988(98)00011-6
Alexe M (2010) Study of salt lakes in the Transylvanian Basin. Cluj University Press, Cluj-Napoca (in Romanian)
Alexe M, Șerban G, Baricz A, Andrei A-Ș, Cristea A, Battes KP, Cîmpean M, Momeu L, Muntean V, Porav SA, Banciu HL (2018) Limnology and plankton diversity of salt lakes from Transylvanian Basin (Romania): a review. J Limnol 77:17–34. https://doi.org/10.4081/jlimnol.2017.1657
Anderson GC (1958) Seasonal characteristics of two saline lakes in Washington. Limnol Oceanogr 3:51–68
Andrei A-Ş, Baricz A, Păușan M, Muntean V, Sicora C, Alexe M, Rakosy-Tican E, Banciu HL (2017) Spatial distribution and molecular diversity of archaeal communities in the extreme hypersaline meromictic Brâncoveanu Lake (Transylvanian Basin, Romania). Geomicrobiol J 34:130–138. https://doi.org/10.1080/01490451.2016.1149527
Andrei A-Ş, Robeson MS, Baricz A, Coman C, Muntean V, Ionescu A, Etiope G, Alexe M, Sicora CI, Podar M, Banciu HL (2015) Contrasting taxonomic stratification of microbial communities in two hypersaline meromictic lakes. ISME J 9:2642–2656. https://doi.org/10.1038/ismej.2015.60
Baricz A, Coman C, Andrei A-Ş, Muntean V, Keresztes ZG, Păușan M, Alexe M, Banciu HL (2014) Spatial and temporal distribution of archaeal diversity in meromictic, hypersaline Ocnei Lake (Transylvanian Basin, Romania). Extremophiles 18:399–413. https://doi.org/10.1007/s00792-013-0625-6
Baricz A, Cristea A, Muntean V, Teodosiu G, Andrei A-Ş, Molnár I, Alexe M, Rakosy-Tican E, Banciu HL (2015) Culturable diversity of aerobic halophilic archaea (Fam. Halobacteriaceae) from hypersaline, meromictic Transylvanian lakes. Extremophiles 19:525–537. https://doi.org/10.1007/s00792-015-0738-1
Baricz A, Chiriac CM, Andrei A-Ș, Bulzu P-A, Levei EA, Cadar O, Battes KP, Cîmpean M, Șenilă M, Cristea A, Muntean V, Alexe M, Coman C, Szekeres EK, Sicora CI, Ionescu A, Blain D, O’Neill WK, Edwards J, Hallsworth JE, Banciu HL (2020) Spatio-temporal insights into microbiology of the freshwater-to-hypersaline, oxic-hypoxic-euxinic waters of Ursu Lake. Environm Microbiol (Early View). https://doi.org/10.1111/1462-2920.14909
Bolgovics Á, Ács É, Várbíró G, Görgényi J, Borics G (2016) Species area relationship (SAR) for benthic diatoms: a study on aquatic islands. Hydrobiologia 764:91–102. https://doi.org/10.1007/s10750-015-2278-1
Bulgǎreanu VAC (1996) Protection and management of anthroposaline lakes in Romania. Lakes Reservoirs 2:211–229
Cărăuș I (2017) Algae of Romania. A distributional checklist of actual algae. Version 2.4. Stud Cercet Biol 7:1–1002
Chavent M, Kuentz V, Labenne A, Liquet B, Saracco J (2014) Multivariate analysis of mixed data: the PCAmixdata R package. Available at: http://arxiv.org/abs/1411.4911
Demeter L, Stoicescu A (2008) A review of the distribution of large branchiopods (Branchiopoda: Anostraca, Notostraca, Spinicaudata, Laevicaudata) in Romania. North-West J Zool 4:203–223
Dodds W (2002) Freshwater ecology: concepts and environmental applications. Academic Press Elsevier, San Diego
Ettl H (1983) Chlorophyta 1. Phytomonadina. Gustav Fischer Verlag, Stuttgart
Gao YN, Dong J, Fu QQ, Wang YP, Chen C, Li JH, Li R, Zhou CJ (2017) Allelopathic effects of submerged macrophytes on phytoplankton. Allelopathy J 40:1–22
Gheorghievici LM, Gheorghievici G, Tănase I (2015) The phytoplankton composition features of five Romanian pelogenous ecosystems. Environm Engin Managem J 14:975–984
Gheorghievici LM, Pompei I, Gheorghievici G, Tănase I (2012) The influence of abiotic factors on suppliers of organic matter in the peloidogenesis process from Lake Techirghiol, Romania. AACL Bioflux 5:69–78
Google Earth Pro Version 7.3.2.5491 (2018) Transylvania, Romania. 46°17′07.50″N, 24°50′23.26″E; eye altitude 424.68 km. Available at: http://www.earth.google.com. Accessed 25 Oct 2018
Gross EM, Hilt S, Lombardo P, Mulderij G (2007) Searching for allelopathic effects of submerged macrophytes on phytoplankton: state of the art and open questions. In: Gulati RD, Lammens E, De Pauw N, Van Donk E (eds) Shallow Lakes in a Changing World Developments in Hydrobiology, vol. 196. Springer, Dordrecht, pp 77–88. https://doi.org/10.1007/978-1-4020-6399-2_8
Hammer UT (1986) Saline lake ecosystems of the World. Kluwer Academic, Dordrecht
Hodgson DA, Vyverman W, Sabbe K (2001) Limnology and biology of saline lakes in the Rauer Islands, eastern Antarctica. Antarct Sci 13:255–270
Horne AJ, Goldman CR (1994) Limnology, 2nd edn. McGraw-Hill, New York
Huber-Pestalozzi G (1955) Das Phytoplankton des Süsswassers, Band XVI, 4. Teil: Euglenophyceen. Schweizerbart Science Publishers, Stuttgart
Ionescu V, Năstăsescu M, Spiridon L, Bulgăreanu VAC (1998) The biota of Romanian saline lakes on rock salt bodies: a review. Int J Salt Lake Res 7:45–80
Jolliffe IT (2002) Principal component analysis, 2nd edn. Springer, New York
Kelly MG, Cazaubon A, Coring E, Dell’Uomo A, Ector L, Goldsmith B, Guasch H, Hürlimann J, Jarlman A, Kawecka B, Kwandrans J, Laugaste R, Lindstrøm E-A, Leitao M, Marvan P, Padisák J, Pipp E, Prygiel J, Rott E, Sabater S, van Dam H, Vizinet J (1998) Recommendations for the routine sampling of diatoms for water quality assessments in Europe. J Appl Phycol 10:215–224. https://doi.org/10.1023/A:1008033201227
Keresztes ZG, Felföldi T, Somogyi B, Székely G, Dragoş N, Márialigeti K, Bartha C, Vörös L (2012) First record of picophytoplankton diversity in Central European hypersaline lakes. Extremophiles 16:759–769. https://doi.org/10.1007/s00792-012-0472-x
Klymiuk V, Barinova S (2016) Phytoplankton cell size in saline lakes. Res J Pharm Biol Chem Sci 7:1077–1085
Komárek J, Anagnostidis K (1998) Cyanoprokariota, 1.Teil: Chroococcales. In: Ettl H, Gärtner G, Heynig H, Mollenhauer D (eds) Süβwasserflora von Mitteleuropa. Spektrum Akademischer Verlag, Heidelberg, pp 1–759
Krammer K, Lange-Bertalot H (1986) Bacillariophyceae, 1. Teil: Naviculaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süßwasserflora von Mitteleuropa 2/1. Gustav Fischer Verlag, Stuttgart, pp 1–876
Krammer K, Lange-Bertalot H (1988) Bacillariophyceae, 2. Teil: Bacillariaceae, Epithemiaceae, Surirellaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süßwasserflora von Mitteleuropa 2/2. Gustav Fischer Verlag, Stuttgart, pp 1–596
Krammer K, Lange-Bertalot H (1991a) Bacillariophyceae, 3. Teil: Centrales, Fragilariaceae, Eunotiaceae. In: Ettl H, Gerloff J, Heynig H, Mollenhauer D (eds) Süßwasserflora von Mitteleuropa 2/3. Gustav Fischer Verlag, Stuttgart, pp 1–598
Krammer K, Lange-Bertalot H (1991b) Bacillariophyceae, 4. Teil: Achnanthaceae. Kritische Ergänzungen zu Navicula (Lineolatae) und Gomphonema. In: Ettl H, Gärtner G, Gerloff J, Heynig H, Mollenhauer D (eds) Süßwasserflora von Mitteleuropa 2/4. Gustav Fischer Verlag, Stuttgart, pp 1–437
Liu X, Hou W, Dong H, Wang S, Jiang H, Wu G, Yang J, Li G (2016) Distribution and diversity of cyanobacteria and eukaryotic algae in Qinghai-Tibetan lakes. Geomicrobiol J 33:860–869. https://doi.org/10.6084/m9.figshare.1626687.V2
Lv T, He Q, Hong Y, Liu C, Yu D (2019) Effects of water quality adjusted by submerged macrophytes on the richness of the epiphytic algal community. Frontiers Pl Sci 9:1980. https://doi.org/10.3389/fpls.2018.01980
Mulderij G, Van Nes EH, Van Donk E (2007) Macrophyte-phytoplankton interactions: the relative importance of allelopathy versus other factors. Ecol Model 204:85–92. https://doi.org/10.1016/j.ecolmodel.2006.12.020
Muntean V, Crișan R, Paşca D, Kiss S, Drăgan-Bularda M (1996) Enzymological classification of salt lakes in Romania. Int J Salt Lake Res 5:35–44
Nagy L (2012) Comunități de diatomee din unele ape stătătoare cu grade diferite de salinitate de la Turda. PhD Thesis, Babeș-Bolyai University, Cluj-Napoca (in Romanian)
Oprean L (2008) Biodinamica lacurilor de la Ocna Sibiului. Editura Universităţii „Lucian Blaga”, Sibiu (in Romanian)
Padisák J (2004) Phytoplankton. In: O’Sullivan P, Reynolds CS (eds) The lakes handbook, vol. 1. Limnology and limnetic ecology. Blackwell Publishing, Malden, pp 251–308
Padisák J, Vasas G, Borics G (2016) Phycogeography of freshwater phytoplankton: traditional knowledge and new molecular tools. Hydrobiologia 764:3–27. https://doi.org/10.1007/s10750-015-2259-4
Panigrahi S, Wikner J, Panigrahy RC, Satapathy KK, Acharya BC (2009) Variability of nutrients and phytoplankton biomass in a shallow brackish water ecosystem (Chilika Lagoon, India). Limnology 10:73–85. https://doi.org/10.1007/s10201-009-0262-z
Shiklomanov IA (1990) Global water resources. Nat Resources 26:34–43
Smith VH, Foster BL, Grover JP, Holt RD, Leibold MA, deNoyelles F (2005) Phytoplankton species richness scales consistently from laboratory microcosms to the world’s oceans. Proc Natl Acad Sci USA 102:4393–4396. https://doi.org/10.1073/pnas.0500094102
Somogyi B, Vörös L, Pálffy K, Székely G, Bartha C, Keresztes ZG (2014) Picophytoplankton predominance in hypersaline lakes (Transylvanian Basin, Romania). Extremophiles 18:1075–1084. https://doi.org/10.1007/s00792-014-0685-2
Tenenhaus M, Pagès J, Ambroisine L, Guinot C (2005) PLS methodology for studying relationships between hedonic judgements and product characteristics. Food Qual Preference 16:315–325. https://doi.org/10.1016/j.foodqual.2004.05.013
Várbíró G, Görgényi J, Tóthmérész B, Padisák J, Hajnal É, Borics G (2017) Functional redundancy modifies species-area relationship for freshwater phytoplankton. Ecol Evol 7:9905–9913. https://doi.org/10.1002/ece3.3512
Wang Z, Qi Y, Chen J, Xu N, Yang Y (2006) Phytoplankton abundance, community structure and nutrients in cultural areas of Daya Bay, South China Sea. J Marine Syst 62:85–94. https://doi.org/10.1016/j.jmarsys.2006.04.008
Washington HG (1984) Diversity, biotic and similarity indices: a review with special relevance to aquatic ecosystems. Water Resources 18:653–694
Watson SB, McCauley E, Downing JA (1997) Patterns in phytoplankton taxonomic composition across temperate lakes of differing nutrient status. Limnol Oceanogr 42:487–495. https://doi.org/10.4319/lo.1997.42.3.0487
Welch PS (1948) Limnological methods. McGraw-Hill Book Company, New York
Wetzel RG (2001) Limnology: lake and river ecosystems, 3rd edn. Academic Press Elsevier, San Diego
Wetzel RG, Likens G (2000) Limnological analyses, 3rd edn. Springer, New York
Williams WD (1978) Limnology of Victorian Salt Lakes, Australia. Verh Int Vereinigung Theor Limnol 20:1165–1174. https://doi.org/10.1080/03680770.1977.11896667
Williams WD (1998) Management of inland saline waters. Guidelines of lake management, vol. 6. ILEC/UNEP, Kusatsu
Wurtsbaugh WA, Miller C, Null SE, DeRose RJ, Wilcock P, Hahnenberger M, Howe F, Moore J (2017) Decline of the world’s saline lakes. Nat Geosci 10:816. https://doi.org/10.1038/ngeo3052
Acknowledgements
This work was financially supported through grants of the Romanian National Authority for Scientific Research, CNCS – UEFISCDI, project numbers PN-II-ID-PCE-2011-3-0546 and PN-III-P4-ID-PCE-2016-0303. We are grateful to Dr. O. Mera, D. Buta, J. Nagy-Fülöp, and I. Costea for granting the permission to enter the study areas. We thank Dr. E.A. Levei, A.M. Incze, and Dr. M. Șenilă at INCDO-INOE 2000—Research Institute for Analytical Instrumentation (Cluj-Napoca, Romania) for performing the chemical analyses of sampled water.
Funding
This study was funded by the Romanian National Authority for Scientific Research, CNCS—UEFISCDI (grant numbers PN-II-ID-PCE-2011–3-0546 and PN-III-P4-ID-PCE-2016–0303).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Not applicable.
Additional information
Handling Editor: Bogdan-Iuliu Hurdu.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Information on Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Information on Electronic Supplementary Material
Information on Electronic Supplementary Material
Online Resource 1. List of the main physico-chemical and morphometric parameters measured for the 19 saline lakes from the Transylvanian Basin (central Romania), together with habitat heterogeneity classes.
Online Resource 2. Segregation of taxa groups along the two major Principal Component Analysis (PCA) axes, based on their presence in the 19 saline lakes considered for the present study (axes F1 and F2: 52.87%).
Online Resource 3. List of the parameters considered in the Partial Least Square Regression (PLS-R) analyses.
Rights and permissions
About this article
Cite this article
Şuteu, A.M., Momeu, L., Battes, K.P. et al. Diversity and distribution of phototrophic primary producers in saline lakes from Transylvania, Romania. Plant Syst Evol 307, 12 (2021). https://doi.org/10.1007/s00606-020-01733-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00606-020-01733-0