Abstract
Aims
To describe and compare the functional and anatomical outcomes of untreated and treated diabetic macular edema (DME) in eyes with very good baseline visual acuity (VA) in a real-world setting.
Methods
A 12-month, retrospective, multicenter, observational cohort study, including DME patients with baseline visual acuity (VA) ≤ 0.1 logMAR (≥ 20/25 Snellen) and central subfield thickness (CST) > 250 µm with intra- and/or subretinal fluid seen on optical coherence tomography.
Results
A total of 249 eyes were included, of which 155 were treated and 94 were non-treated during follow-up. Most eyes maintained vision (VA gain or VA loss < 5 letters) at 12 months (treated: 58.1%; non-treated: 73.4%). In non-treated eyes with stable VA within the first 6 months, VA was maintained throughout the follow-up in most cases (86.3%). In non-treated eyes with VA loss ≥ 5 letters within 6 months (36.7%), further observation led to worse visual outcome than treatment (− 4.2 vs. − 7.8 letters, p = 0.013). In eyes in which treatment was initiated at baseline (n = 102), treatment with 8–12 anti-VEGF injections led to better visual outcome compared to treatment with less injections (− 0.3 ± 3.6 letters vs. − 3.8 ± 6.2 letters, p = 0.003).
Conclusion
In a real-world setting, the majority of DME patients with very good VA maintained vision at 12 months, regardless of whether the DME was treated or not. This study supports close observation of eyes with DME and very good VA with consideration of treatment when a one line drop in vision is observed.
Similar content being viewed by others
Introduction
Diabetic macular edema (DME) is the main cause of vision loss in diabetic patients affecting around 21 million people worldwide [1, 2]. Several treatment regimens, including macular laser, intravitreal anti-VEGF injections, intravitreal triamcinolone acetonide, and dexamethasone (DEX) intravitreal implant have been shown to be effective for DME in randomized controlled trials (RCT) [3,4,5,6,7,8,9,10,11,12]. However, RCTs excluded eyes with very good vision so far, and hence little is known about the visual prognosis of such eyes with or without treatment. Protocol V by DRCR.net is the first RCT on central-involved DME and good visual acuity comparing prompt focal/grid photocoagulation, observation and prompt anti-VEGF therapy [13]. This trial is currently ongoing and the first results are awaited.
The main purpose of this study was to assess the functional and anatomical outcome of patients with DME with very good baseline visual acuity in a real-world setting.
Methods
This is a retrospective, international, multicenter, observational cohort study comprising 16 study sites. Institutional review board (IRB) approval was obtained through the individual IRBs at the participating institutes for a retrospective consecutive chart review. This research adhered to the tenets of the Declaration of Helsinki.
Study participants
Medical records of patients from January 1st, 2010, to June 30th, 2017 with a diagnosis of DME were reviewed. The following were set as inclusion criteria, with all criteria being met: (1) age 18 years or older; (2) type 1 or 2 diabetes mellitus; study eye with (3) center-involving DME (DME defined by retinal thickness of > 250 µm in the central subfield thickness (CST)) and intra ± subretinal fluid on spectral-domain optical coherence tomography (SD-OCT). (4) Best corrected visual acuity ≤ 0.1 logMAR (≥ 0.8 decimal acuity, ≥ 20/25 or ≥ 80 EDTRS letters).
Exclusion criteria were (1) concomitant ocular disease that could cause macular edema (including choroidal neovascularization from any cause, retinal vein occlusion, uveitis and recent intraocular surgery); (2) any concomitant ocular or neurological condition that could affect vision except cataract; (3) laser panretinal photocoagulation (PRP) < 6 months prior to baseline; (4) intravitreal therapy < 3 months prior to study inclusion; and (5) intravitreal therapy during follow-up for proliferative diabetic retinopathy (PDR).
Data collection
For eligible patients, the following data were collected from their medical charts: demographic data (i.e., age, sex); duration of diabetes; stage of diabetic retinopathy [non-proliferative (NPDR) or PDR]; previous DME treatments (macular laser, intravitreal anti-VEGF injections, triamcinolone acetonide, DEX implant), previous laser PRP; lens status at baseline and 12 months; VA and CST at baseline, 3, 6, 9 and 12 months; and further treatment during follow-up (including macular laser, intravitreal anti-VEGF injections, triamcinolone acetonide, and DEX implant), laser PRP, and cataract surgery.
Outcome measures
Main outcome measures were the mean change in VA and CST from baseline to month 12. Secondary outcome measures included the mean change in VA and CST from baseline to month 6, the proportion of eyes which maintained vision (VA loss < 5 letters or VA gain), VA loss ≥ 5 letters, ≥ 10 letters, ≥ 15 letters, VA of ≥ 0.2 logMAR (≤ 75 letters, ≤ 20/32 Snellen equivalent) and VA of ≥ 0.3 logMAR (≤ 70 letters, ≤ 20/40 Snellen equivalent) at 12 months.
OCT analysis
All eyes were imaged with SD-OCT (Heidelberg Spectralis, Heidelberg, Germany; Optovue Avanti, Fremont, USA; Topcon 3D OCT-2000, Tokyo; Japan; or Cirrus, Zeiss, Oberkochen, Germany, Canon-OCT HS100, Tokyo, Japan). Quantitative assessment of DME-included CST calculated automatically by the instrument. Additionally, for all study participants the horizontal B-scans encompassing the fovea were exported. These images were graded for any disruption to the ellipsoid zone (EZ) by three independent and masked graders (CB, MI, MR).
Statistical analysis
Variables are expressed as mean ± standard deviation (SD). To control for the correlated nature of our data, we used a generalized estimating equations (GEE) procedure. Differences in VA and CST between baseline and month 6 or month 12 were analyzed by univariable linear regression. Difference in outcome measures between the subgroups were assessed by including the following confounding baseline variables: (1) age, (2) gender, (3) stage of diabetic retinopathy (NPDR vs. PDR), (4) duration of diabetes, (5) EZ disruption at baseline, (6) lens status at baseline and (7) after 12 months, (8) treatment naivety, (9) conduction of PRP during follow-up, and (10) baseline VA (for VA outcomes) and baseline CST (for CST outcomes). Variables with p ≤ 0.15 in the univariable analysis were included in the final GEE model. A backward selection procedure was applied that retained only those variables with p < 0.05. For continuous outcome variables, a linear regression model and for a binary outcome a logistic regression model was applied. Markov chain Monte Carlo multiple imputation procedure with 100 run imputations was used to impute missing data. Statistical analysis was performed with SPSS Statistics 22 (IBM, Armonk, NY, USA).
Results
The study included 249 eyes from 210 patients. Demographic and baseline characteristics are shown in Table 1. In the overall cohort, mean baseline VA was 0.06 ± 0.05 logMAR (82 letters, 20/25 Snellen equivalent) and mean baseline CST was 355.5 ± 77.3 µm (Table 2).
The majority of eyes were treatment naïve (186/249, 74.7%). One quarter (63 eyes) had received DME treatment prior to inclusion in the study; including macular laser in 38 eyes (15.3%), anti-VEGF therapy in 43 eyes (17.3%), intravitreal triamcinolone acetonide in 3 eyes (1.2%) and DEX Implant in 1 eye (0.4%).
Over the 12 months of follow-up, 94 eyes (37.7%) were non-treated (never treated), and 155 eyes (62.2%) received treatment. Types of DME treatment undertaken during the study period is shown in Table 3. The cohort receiving treatment during the study period showed signs of a more severe disease with increased proportion of PDR, were more likely to have been previously treated and more likely to have EZ disruption on OCT imaging at baseline (Table 1).
Functional and anatomical outcomes
Most eyes maintained vision (VA gain or VA loss < 5 letters) at 12 months (treated eyes: 58.1%; non-treated eyes: 73.4%; Table 4). Mean change in VA at 12 months in non-treated eyes was − 1.8 ± 5.6 letters and − 3.4 ± 5.8 letters in treated eyes (Table 2). A VA loss of ≥ 5 letters was seen in 26.6% (25/94 eyes) of the non-treated cohort, and in 41.9% (65/155 eyes) of the treated cohort.
There was no clinical relevant change in CST at 12 months compared to baseline in non-treated eyes (+ 11.3 ± 58.8 µm, p = 0.06). However, at 12 months CST was reduced in eyes that were treated (− 38.9 ± 97.7 µm, p < 0.001; Table 2).
Eyes non-treated at baseline
At the study baseline, treatment was commenced for 102 eyes (41.0%) whereas the other 147 eyes (59.0%) were initially non-treated.
In non-treated eyes with stable VA within the first 6 months, VA was maintained throughout the follow-up in most cases without any treatment (86.3%, Table 4). Only 1 eye dropped ≥ 10 letters (1.4%). In less than 10% (9.6% or 7/73 eyes) VA dropped to ≥ 0.2 logMAR (≤ 75 letters, ≤ 20/32 Snellen equivalent) at 12 months.
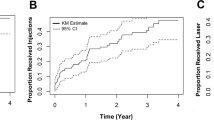
If a VA loss ≥ 5 letters within 6 months occured (36.7%), further observation led to worse visual outcome than treatment (− 4.2 vs. − 7.8 letters, p = 0.013). Despite this VA loss within the first 6 months, 21 eyes (38.9%) continued to be non-treated over the study period. Those eyes experienced on average a small, but worse functional and anatomical outcome at 12 months than the 33 eyes (61.1%) that were treated after experiencing reduction in VA (VA change at 12 months: − 7.8 ± 6.9 letters vs. − 4.1 ± 5.6 letters, p = 0.013, multivariable analysis; CST change at 12 months: +30.3 ± 58.0 µm vs. − 37.4 ± 70.3 µm, p < 0.001, multivariable analysis; Fig. 1). Furthermore, treated eyes tended to be less likely to present with persistent VA loss of ≥ 5 letters at month 12 compared to eyes that were further observed after experiencing VA loss (48.5% vs. 71.4%, p = 0.100, multivariable analysis; Table 4).
Eyes treated at baseline
Of the 102 eyes, in which treatment was initiated at baseline, 80 received anti-VEGF therapy with or without macular laser during the 12-month follow-up period.
The combination of anti-VEGF + macular laser was not superior to anti-VEGF therapy only (VA change at 12 months: p = 0.683, CST change at 12 months: p = 0.227, univariable analysis; Table 2). Macular laser alone tended to lead to worse outcomes compared to intravitreal therapy (Table 2). Eyes that received 8–12 anti-VEGF injections on average showed a significantly better visual outcome compared to those that received 1–7 injections (VA change at 12 months: − 0.3 ± 3.6 letters vs. − 3.8 ± 6.2 letters, p = 0.003, multivariable analysis; Table 2; Fig. 2). There was a corresponding greater reduction in CST at 12 months but this was not statistically significant (CST change at 12 months: − 85.9 ± 102.0 µm vs. − 41.4 ± 119.0 µm, p = 0.068, multivariable analysis; Table 2; Fig. 2).
Discussion
To our best knowledge, data on the real-world outcome of DME patients and very good baseline visual acuity have not been published. Previous RCTs and real-world studies did not include or report on DME eyes with baseline VA better than 78 letters [3,4,5,6,7,8,9,10,11, 14, 15]. Our study reveals that both non-treated and treated DME patients with very good visual acuity on average maintained very good vision after 12 months in a real-world setting. Untreated eyes without significant VA loss in the early observation phase maintained stable VA during the follow-up. However, in case of a significant VA loss under observation, treatment of those eyes led to better outcomes. In the treated cohort, intensive anti-VEGF treatment led to better functional and anatomical outcomes than less intense treatment. However, benefit reached by intensive treatment was small.
Kwon et al. reported on the natural course of DME by examining eyes with mild DME (CST 250–300 µm), but with worse VA [0.32 logMAR (20/50 Snellen equivalent) vs. 0.05 logMAR (20/25 Snellen equivalent)] than our cohort [16]. Similar to our cohort, VA acuity changes were small and a small but non-significant increase in CST in untreated DME eyes was observed [16]. Eyes that were never treated in our study on average maintained good visual acuity over the follow-up, raising the question if treatment should be considered in eyes with very good visual acuity. When eyes were non-treated and presented with a stable VA throughout the first 6 months, most eyes (86.3%) maintained VA over the whole study period. A relevant VA loss (≥ 5 letters) within the first 6 months was present in 36.7% of non-treated eyes. Our data indicate that in those eyes, treatment could be considered since VA outcomes were worse in eyes which continued to be non-treated compared to eyes which were treated.
Eyes that were treated intensively did not experience a VA gain in our study as reported before in RCTs [3, 4, 10,11,12]. This may be due to the ceiling effect when starting with good vision. In the whole cohort, an intensive anti-VEGF treatment on average led to better anatomical outcomes compared to no treatment, which may or may not lead to a better long-term vision. Randomized prospective studies are required and we eagerly await the results of the DRCR.net protocol V [13]. This RCT includes eyes with center-involving DME and good visual acuity (defined as a ≥ 20/25 Snellen equivalent, ≥ 79 letters) that receive (1) prompt focal/grid photocoagulation + deferred anti-VEGF, (2) observation + deferred anti-VEGF, or (3) prompt anti-VEGF therapy [13]. The primary outcome is set as VA loss of ≥ 5 letters after 2 years [13]. It is vital to know whether early treatment in DME patients with very good visual acuity leads to better long-term visual outcomes, since anti-VEGF treatment is not without ocular and systemic risk [17, 18], and cause high costs to healthcare system and patient [19].
Limitations of this study include its retrospective nature and the shortcomings of a real-world setting, especially the lack of defined treatment criteria among the study centers. Baseline characteristics between treated and untreated eyes were not well balanced, with unsurprisingly more severe cases in the treatment group. To account for this we included baseline characteristics as confounders in the statistical analyses. We were not able to report outcomes for untreated eyes with higher CST, since those patients tended to be treated in our real-world setting. Thus, our results might not be applicable for patients with CST > 400 µm. We did not have information on the course of DME in the individual eyes before inclusion of the study, which might have also influenced the outcome results. Furthermore, we conducted multiple testing, which could have led to false-positive results.
This study shows, in a real-world setting, that the majority of eyes with DME and very good visual acuity maintain very good vision at 12 months whether the DME is treated or not. In the treated cohort, many anti-VEGF treatments at high cost to the patient and healthcare system were required to obtain small and clinically not relevant gains in VA and reduction in CST. This study, therefore, supports a close observation of eyes with DME and very good visual acuity at least until a one line drop in vision is observed, however, longer, randomized prospective studies are required.
References
Arroba AI, Valverde AM (2017) Modulation of microglia in the retina: new insights into diabetic retinopathy. Acta Diabetol 54(6):527–533
Yau JW, Rogers SL, Kawasaki R et al (2012) Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 35(3):556–564
Nguyen QD, Brown DM, Marcus DM et al Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119(4):789–801
Diabetic Retinopathy Clinical Research N, Wells JA, Glassman AR et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med 2015;372(13):1193–1203
Gillies MC, Lim LL, Campain A et al (2014) A randomized clinical trial of intravitreal bevacizumab versus intravitreal dexamethasone for diabetic macular edema: the BEVORDEX study. Ophthalmology 121(12):2473–2481
Boyer DS, Yoon YH, Belfort R Jr et al (2014) Three-year, randomized, sham-controlled trial of dexamethasone intravitreal implant in patients with diabetic macular edema. Ophthalmology 121(10):1904–1914
Figueira J, Khan J, Nunes S et al (2009) Prospective randomised controlled trial comparing sub-threshold micropulse diode laser photocoagulation and conventional green laser for clinically significant diabetic macular oedema. Br J Ophthalmol 93(10):1341–1344
Ip MS, Bressler SB, Antoszyk AN et al (2008) A randomized trial comparing intravitreal triamcinolone and focal/grid photocoagulation for diabetic macular edema: baseline features. Retina 28(7):919–930
Diabetic Retinopathy Clinical Research N, Elman MJ, Aiello LP et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117(6):1064–1077 e35
Korobelnik JF, Do DV, Schmidt-Erfurth U et al (2014) Intravitreal aflibercept for diabetic macular edema. Ophthalmology 121(11):2247–2254
Brown DM, Schmidt-Erfurth U, Do DV et al (2015) Intravitreal aflibercept for diabetic macular edema: 100-week results from the VISTA and VIVID studies. Ophthalmology 122(10):2044–2052
Mitchell P, Bandello F, Schmidt-Erfurth U et al (2011) The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 118(4):615–625
ClinicalTrials.gov. Treatment for CI-DME in eyes with very good VA study (protocol V). https://clinicaltrials.gov/ct2/show/NCT01909791. Accessed 1 Oct 2018
Iglicki M, Busch C, Zur D et al (2019) Dexamethasone implant for diabetic macular edema in naive compared with refractory eyes: The International Retina Group Real-Life 24-Month Multicenter Study. The IRGREL-DEX Study. Retina 39(1):44–51
Patrao NV, Antao S, Egan C et al (2016) Real-world outcomes of ranibizumab treatment for diabetic macular edema in a united kingdom national health service setting. Am J Ophthalmol 172:51–57
Kwon SI, Baek SU, Park IW (2013) Comparison of natural course, intravitreal triamcinolone and macular laser photocoagulation for treatment of mild diabetic macular edema. Int J Med Sci 10(3):243–249
Falavarjani KG, Nguyen QD (2013) Adverse events and complications associated with intravitreal injection of anti-VEGF agents: a review of literature. Eye (Lond) 27(7):787–794
Bressler SB, Almukhtar T, Bhorade A et al (2015) Repeated intravitreous ranibizumab injections for diabetic macular edema and the risk of sustained elevation of intraocular pressure or the need for ocular hypotensive treatment. JAMA Ophthalmol 133(5):589–597
Kiss S, Chandwani HS, Cole AL et al (2016) Comorbidity and health care visit burden in working-age commercially insured patients with diabetic macular edema. Clin Ophthalmol 10:2443–2453
Author information
Authors and Affiliations
Consortia
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standard statement or human and animal rights disclosure
Institutional review board approval was obtained through the individual IRBs at the participating institutes for a retrospective consecutive chart review. The research adhered to the tenets of the Declaration of Helsinki.
Informed consent disclosure
None.
Additional information
Managed by Massimo Federici.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Busch, C., Fraser-Bell, S., Zur, D. et al. Real-world outcomes of observation and treatment in diabetic macular edema with very good visual acuity: the OBTAIN study. Acta Diabetol 56, 777–784 (2019). https://doi.org/10.1007/s00592-019-01310-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00592-019-01310-z