Abstract
Background
Proton pump inhibitors (PPIs) are affected by cytochrome P450 2C19 (CYP2C19) polymorphisms. This study compared the effect of two PPIs on early symptom relief in Japanese patients with reflux esophagitis, classified by the CYP2C19 phenotype.
Methods
Patients with reflux esophagitis were randomised to treatment with omeprazole 20 mg or rabeprazole 10 mg once daily. The CYP2C19 phenotype [homozygous extensive metaboliser (homoEM), heterozygous extensive metaboliser (heteroEM) or poor metaboliser (PM)] of each patient was determined. The primary efficacy endpoint was early, sufficient (Global Overall Symptom scale score 1 or 2), sustained (maintained for ≥7 days) reflux symptom relief.
Results
Of the 199 patients included in this analysis, the proportion achieving sufficient, sustained reflux symptom relief was higher with omeprazole than with rabeprazole on day 1 (35.6 vs. 22.4 %; p = 0.041) and day 2 (43.6 vs. 28.6 %; p = 0.028); there was no significant difference between the two groups on days 3–7. Among patients with the CYP2C19 PM phenotype, sufficient, sustained reflux symptom relief was higher with omeprazole than with rabeprazole on days 4–7 (62.5–66.9 vs 31.6 %; p ≤ 0.03); differences were not significant on days 1–3, or among those with the homoEM or heteroEM phenotypes on days 1–7.
Conclusions
In Japanese patients with reflux esophagitis, omeprazole 20 mg is more effective than rabeprazole 10 mg at achieving early, sufficient, sustained reflux symptom relief in individuals with the CYP2C19 PM phenotype, and is similarly effective to rabeprazole 10 mg in those with heteroEM or homoEM phenotypes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gastroesophageal reflux disease (GERD) is characterised by reflux of the stomach contents and/or bile into the esophagus [1], typically causing symptoms of heartburn and acid regurgitation [2]. Studies conducted in Japan have found that between 6.5 and 9.5 % of the population have reflux symptoms on at least 1 day per week, and the reported prevalence of reflux esophagitis ranges from 4.9 to 8.2 % [3]. Various lifestyle factors are reported to be associated with GERD [4], and reflux symptoms negatively affect health-related quality of life, work productivity, and health resource utilisation [5–8]. Moreover, reflux esophagitis is a risk factor for Barrett’s esophagus and esophageal adenocarcinoma [9]. Proton pump inhibitors (PPIs) are the most effective treatment for GERD, including endoscopically confirmed reflux esophagitis [1, 10]. Most individuals experience resolution of their reflux symptoms when taking a PPI [1, 10], with a concomitant overall improvement in health-related quality of life [11, 12].
PPIs are metabolised via the hepatic enzyme cytochrome P450 2C19 (CYP2C19). There are three genetic polymorphisms of CYP2C19, resulting in homozygous extensive metaboliser (homoEM), heterozygous extensive metaboliser (heteroEM) and poor metaboliser (PM) phenotypes [13]. These CYP2C19 phenotypes have different effects on the pharmacodynamic and pharmacokinetic profiles of PPIs. Gastric acid secretion is affected such that post-PPI intragastric pH values are highest in the PM group and lowest in the homoEM group following administration of omeprazole or rabeprazole [14]. The clinical relevance of these differences is especially important for patients in Japan, where the PM phenotype is much more common (prevalence 18.0–22.5 %) than in the USA or Europe (prevalence ≤3.7 %) [13].
Results from studies in healthy Japanese volunteers suggest that early effects on gastric acid inhibition in people with different CYP2C19 phenotypes may depend on the type of PPI used [15–17]. Compared with omeprazole 20 mg or lansoprazole 30 mg, rabeprazole 10 mg has been shown to exert a faster and more pronounced inhibition of gastric acid secretion in healthy Japanese volunteers with the homoEM or heteroEM phenotypes [15]; however, in another study also conducted in healthy Japanese volunteers with the homoEM or heteroEM phenotypes, lansoprazole 30 mg was shown to induce an earlier rise in blood PPI concentration and intragastric pH than rabeprazole 10 mg [16]. Furthermore, in healthy Japanese volunteers receiving omeprazole 20 mg or rabeprazole 10 mg, there was no significant difference between the two PPIs in early intragastric pH changes in individuals with the homoEM phenotype, but intragastric pH was significantly higher with omeprazole than with rabeprazole 6–8 h after PPI administration according to combined data from participants with the heteroEM and PM phenotypes [17]. Thus, whereas the pharmacodynamic and pharmacokinetic profiles of omeprazole and rabeprazole are clearly dependent on CYP2C19 phenotype, data from healthy volunteers on differences in early acid inhibitory effects between the two PPIs are inconsistent. In addition, there is a paucity of data on whether any differences in early acid inhibitory effects in healthy volunteers translate into early differences in clinical outcomes in patients with GERD.
We conducted this study to compare the efficacy of omeprazole 20 mg and rabeprazole 10 mg in achieving early symptom relief in Japanese patients with reflux esophagitis. Our analysis took into consideration outcomes based on CYP2C19 phenotype.
Methods
Study design
This was a 4-week, multicentre, randomised, open-label, parallel-group study conducted at 18 centres in Japan between January 2010 and March 2011. Eligible patients were randomly allocated by the study coordination centre (five patients per block per study centre) to receive omeprazole 20 mg or rabeprazole 10 mg orally once daily (in the morning) for 4 weeks. Participants were asked to complete the investigator-administered Global Overall Symptom (GOS) scale to determine symptom severity at baseline (screening visit) and at the end of 2 and 4 weeks of PPI therapy. In addition, participants used the GOS scale to record the severity of their reflux symptoms (heartburn and acid regurgitation) in a daily diary (before bedtime) during the first 2 weeks of the study. An overview of the study design is provided in Fig. 1.
Eligible participants were also asked to provide two 5 mL blood samples at screening to determine their Helicobacter pylori status (assessed using enzyme immunoassay) and CYP2C19 phenotype (homoEM, heteroEM or PM; assessed using gene analysis by fluorescence correlation spectroscopy).
The study protocol was reviewed and approved by the ethics review boards of all participating centres before the start of the investigation. The study was conducted in accordance with the principles of the Declaration of Helsinki and all patients provided informed consent as a condition of participation.
Patients
Patients of either sex aged 20 years and older were eligible for inclusion if they had diagnoses of reflux esophagitis (Los Angeles grades A–D) on endoscopy during the preceding 12 months. Individuals also had to have heartburn and/or acid regurgitation of at least moderate severity (GOS scale score ≥4) at baseline (screening visit).
Exclusion criteria were: ‘alarm’ features (e.g. vomiting, gastrointestinal haemorrhage and involuntary weight loss); peptic ulcer (other than those at the scarred stage); history of gastrointestinal resection or vagotomy; history of inflammatory bowel disease, irritable bowel syndrome, esophageal stenosis, esophageal achalasia, Zollinger–Ellison syndrome, malabsorption or cerebral disorders; serious hepatic, renal or cardiac disease; confirmed or suspected malignancies; or requirement for continued use of medication that might interact with the test drugs (e.g. atazanavir sulphate, diazepam, phenytoin, warfarin, tacrolimus hydrate, digoxin, methyldigoxin, itraconazole, gefitinib, voriconazole, acid suppressants containing aluminium hydroxide gel, or magnesium hydroxide). Women who were or might have been pregnant, or who were lactating, were also excluded from the study.
The following medications were discontinued at least 1 week before study entry and were not allowed during the study period: PPIs (other than the study PPIs), histamine-2 receptor antagonists, prokinetic agents, gastric mucosal protective agents, anticholinergic drugs, antidepressants, anxiolytics, antidiabetic agents, steroids (other than topical steroids), non-steroidal anti-inflammatory drugs [including acetylsalicylic acid (ASA) preparations and low-dose ASA], and bisphosphonates.
Efficacy assessments
The efficacy of omeprazole 20 mg and rabeprazole 10 mg was assessed on the basis of the GOS scale heartburn and acid regurgitation scores recorded by patients in their daily diary entries during the first 2 weeks, and from the GOS scale that was completed at the clinic after 2 and 4 weeks of PPI therapy.
The GOS scale has been validated for the assessment of upper gastrointestinal symptoms in the clinical trial setting [18], and has been used in clinical studies to assess symptoms of GERD (heartburn and acid regurgitation) and other upper gastrointestinal symptoms [19–21]. The GOS scale measures the severity of eight symptoms (heartburn, acid regurgitation, gastric pain, stomach feeling heavy, early satiety, feeling queasy, burping and feeling of fullness) on a 7-point scale, from 1 [‘no problem’ (no symptoms)] to 7 [‘very severe problem’ (cannot be ignored and markedly limits my daily activities and often requires rest)] [18]. The GOS scale was used in the current study to perform symptom-based evaluations, not to diagnose reflux esophagitis. Therefore, no cut-off value was implemented in this study.
Primary and secondary endpoints
The primary efficacy endpoint was the proportion of patients who had sufficient and sustained (for ≥7 consecutive days) relief of reflux symptoms, defined as the first day of PPI therapy on which the GOS scale score was 1 [‘no problem’ (no symptoms)] or 2 [‘minimal problem (can be easily ignored without effort)’]. Secondary efficacy endpoints included the proportion of patients who had: sufficient and sustained relief of reflux symptoms assessed by CYP2C19 phenotype; sufficient relief of reflux symptoms (GOS scale score of 1 or 2) after 2 and 4 weeks of PPI therapy (overall and by CYP2C19 phenotype); sufficient relief of upper gastrointestinal symptoms (GOS scale score of 1 or 2) after 2 and 4 weeks of PPI therapy (overall and by CYP2C19 phenotype); complete resolution of reflux symptoms (GOS scale score of 1) after 2 and 4 weeks of PPI therapy (overall and by CYP2C19 phenotype); and complete resolution of upper gastrointestinal symptoms (GOS scale score of 1) after 2 and 4 weeks of PPI therapy (overall and by CYP2C19 phenotype).
Safety assessments
Adverse events were recorded throughout the study period and were assessed according to whether or not they were serious, their relationship to the study drug, their time of onset, and the outcome. All adverse events were reported descriptively.
Statistical analyses
Sample size
The median time to reach heartburn control was estimated to be 2 days for the omeprazole 20 mg group and 3 days for the rabeprazole 10 mg group, based on data by Bytzer et al. [22], and taking into consideration the distribution of the different phenotypes and the treatment doses. Based on these median times, the proportion of patients with symptom improvement was estimated to be 90 % in the omeprazole 20 mg group and 77 % in the rabeprazole 10 mg group. Using these criteria, 97 patients were required to participate in the study to detect a 5 % (two-sided) inter-group difference in the primary variable using the log-rank test. A dropout rate of approximately 10 % was assumed; thus, the plan was to enrol approximately 220 patients (110 per group).
Efficacy and safety variables
The primary and secondary efficacy endpoints were analysed using data from all patients with at least one assessment of efficacy after the initiation of study treatment.
Sufficient symptom relief was defined as a score of 1 or 2 on the GOS scale, and complete symptom resolution was defined as a score of 1 on the GOS scale. Sufficient and sustained symptom relief was defined as maintenance of sufficient symptom relief for at least 7 consecutive days. Inter-group differences in the proportion of patients reaching the primary and secondary endpoints were analysed using the χ 2 test, with statistical significance defined as p < 0.05 (two-sided).
Results
Patient population
In total, 209 eligible patients with reflux esophagitis were randomised and received at least one dose of omeprazole 20 mg (n = 106) or rabeprazole 10 mg (n = 103). Evaluable data were available for 101 patients (95.3 %) in the omeprazole 20 mg group and 98 patients (95.1 %) in the rabeprazole 10 mg group. Ten patients (4.8 %) were excluded from the efficacy analysis because they did not provide daily diary records.
All patients included in this analysis had heartburn and/or acid regurgitation of at least moderate severity (GOS scale score ≥4) at baseline, in accordance with study inclusion criteria. Baseline demographics and clinical characteristics are listed in Table 1; there were no significant differences between the two treatment groups. Mean GOS scale scores at baseline were similar in the two treatment groups (Table 2).
Efficacy
Primary efficacy endpoint
On day 1 of PPI therapy, the proportion of patients achieving sufficient and sustained relief of their reflux symptoms for at least 7 consecutive days was 35.6 % with omeprazole 20 mg and 22.4 % with rabeprazole 10 mg (p = 0.041; Fig. 2). On day 2 of PPI therapy, it was 43.6 % with omeprazole 20 mg and 28.6 % with rabeprazole 10 mg (p = 0.028; Fig. 2). Sufficient and sustained relief of reflux symptoms continued to be observed in a greater proportion of patients in the omeprazole 20 mg group (46.5–61.4 %) than in the rabeprazole 10 mg group (39.8–52.0 %) on days 3–7, but the difference between the two groups was no longer statistically significant.
Proportion of patients achieving sustained and sufficient relief of their reflux symptoms on days 1–7 of therapy with omeprazole 20 mg or rabeprazole 10 mg. Sustained and sufficient symptom relief was defined as a GOS scale score of 1 or 2 that was maintained for at least 7 consecutive days. GOS Global Overall Symptom
Secondary efficacy endpoints
Among patients with the PM phenotype, a greater proportion in the omeprazole 20 mg than in the rabeprazole 10 mg group achieved sufficient and sustained relief of their reflux symptoms on days 1–7 of PPI therapy; between-group differences reached statistical significance on days 4–7 (omeprazole 62.5–66.9 % vs rabeprazole 31.6 %; p ≤ 0.03; Fig. 3a). Sufficient and sustained relief of reflux symptoms on days 1–7 of PPI therapy was not statistically different in the omeprazole 20 mg and the rabeprazole 10 mg groups in patients with either the homoEM or heteroEM phenotypes (Fig. 3b, c).
Proportion of patients achieving sustained and sufficient relief of their reflux symptoms on days 1–7 of therapy with omeprazole 20 mg or rabeprazole 10 mg in those with the CYP2C19 phenotype a PM, b heteroEM and c homoEM. Sustained and sufficient symptom relief was defined as a GOS scale score of 1 or 2 that was maintained for at least 7 consecutive days. CYP2C19 phenotype was unknown for two patients in the omeprazole group. CYP2C19 cytochrome P450 2C19, GOS Global Overall Symptom, heteroEM hetero extensive metaboliser, homoEM homo extensive metaboliser, PM poor metaboliser
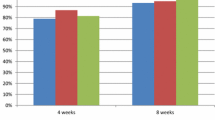
After 2 and 4 weeks of PPI therapy, the proportion of patients achieving sufficient relief of their reflux symptoms was similar in the omeprazole 20 mg and rabeprazole 10 mg groups overall (Fig. 4a), and in patients with the homoEM and the heteroEM phenotypes (Fig. 4b). In patients with the PM phenotype, however, a significantly greater proportion in the omeprazole 20 mg group than in the rabeprazole 10 mg group achieved sufficient relief of their reflux symptoms after 2 weeks (78.3 vs 42.1 %; p = 0.016) and 4 weeks (95.7 vs 68.4 %; p = 0.018) of PPI therapy (Fig. 4b).
Proportion of patients achieving sufficient relief of their reflux symptoms (GOS scale score of 1 or 2) after 2 and 4 weeks of therapy with omeprazole 20 mg or rabeprazole 10 mg a overall and b according to CYP2C19 phenotype. CYP2C19 phenotype was unknown for two patients in the omeprazole group. CYP2C19 cytochrome P450 2C19, GOS Global Overall Symptom, heteroEM hetero extensive metaboliser, homoEM homo extensive metaboliser, PM poor metaboliser
Complete resolution of reflux symptoms was achieved in a significantly greater proportion of patients in the omeprazole 20 mg than in the rabeprazole 10 mg group after both 2 weeks (44.0 vs 27.1 %; p = 0.013) and 4 weeks (55.0 vs 36.5 %; p = 0.009) of PPI therapy (Fig. 5a). When assessed by CYP2C19 phenotype, a significantly greater proportion of patients with the heteroEM phenotype in the omeprazole 20 mg than in the rabeprazole 10 mg group achieved complete reflux symptom resolution after 2 weeks of PPI therapy (43.6 vs 20.0 %; p = 0.024), but there was no significant difference after 4 weeks of therapy (Fig. 5b). No significant inter-group difference in this measure was noted at either time point for patients with the homoEM or PM phenotype (Fig. 5b).
Proportion of patients achieving complete resolution (GOS scale score of 1) of their reflux symptoms after 2 and 4 weeks of therapy with omeprazole 20 mg or rabeprazole 10 mg a overall and b according to CYP2C19 phenotype. CYP2C19 phenotype was unknown for two patients in the omeprazole group. CYP2C19 cytochrome P450 2C19, GOS Global Overall Symptom, heteroEM hetero extensive metaboliser, homoEM homo extensive metaboliser, PM poor metaboliser
For upper gastrointestinal symptoms, sufficient relief after 2 and 4 weeks of PPI therapy was similar in the omeprazole 20 mg and rabeprazole 10 mg groups overall, and in patients with the homoEM and the heteroEM phenotypes (Fig. 6). In patients with the PM phenotype, a significantly greater proportion in the omeprazole 20 mg group than in the rabeprazole 10 mg group achieved sufficient relief of their upper gastrointestinal symptoms after 2 weeks (73.9 vs 26.3 %; p = 0.002), but differences were not statistically significant after 4 weeks (Fig. 6b).
Proportion of patients achieving sufficient relief (GOS scale score of 1 or 2) of their upper gastrointestinal symptoms after 2 and 4 weeks of therapy with omeprazole 20 mg or rabeprazole 10 mg a overall and b according to CYP2C19 phenotype. CYP2C19 phenotype was unknown for two patients in the omeprazole group. CYP2C19 cytochrome P450 2C19, GOS Global Overall Symptom, heteroEM hetero extensive metaboliser, homoEM homo extensive metaboliser, PM poor metaboliser
Complete resolution of upper gastrointestinal symptoms was achieved in a significantly greater proportion of patients in the omeprazole 20 mg group than in the rabeprazole 10 mg group after 4 weeks of PPI therapy (37.0 vs 20.8 %; p = 0.013), but differences were not statistically significant at the earlier, 2-week time point (Fig. 7a). When assessed according to CYP2C19 polymorphism, a significantly greater proportion of patients with the PM phenotype in the omeprazole 20 mg group than in the rabeprazole 10 mg group achieved complete resolution of their upper gastrointestinal symptoms after 4 weeks of PPI therapy (34.8 vs 21.1 %; p = 0.033), but no significant difference was observed at the earlier, 2-week time point (Fig. 7b).
Proportion of patients achieving complete resolution (GOS scale score of 1) of their upper gastrointestinal symptoms after 2 and 4 weeks of therapy with omeprazole 20 mg or rabeprazole 10 mg a overall and b according to CYP2C19 phenotype was unknown for two patients in the omeprazole group. CYP2C19 cytochrome P450 2C19, GOS Global Overall Symptom, heteroEM hetero extensive metaboliser, homoEM homo extensive metaboliser, PM poor metaboliser
Safety
Four adverse events (one each of itching, abdominal fullness, thirst and rash/exanthema) were reported in four patients treated with omeprazole 20 mg, of whom three had the homoEM and one the PM phenotype. All adverse events were considered by the investigator to be related to the study drug but none were graded as being serious. No adverse events were recorded for patients treated with rabeprazole 10 mg.
Discussion
In this 4-week, randomised study in Japanese patients with endoscopically confirmed reflux esophagitis, omeprazole 20 mg once daily was significantly more effective than rabeprazole 10 mg once daily at achieving early, sufficient, and sustained reflux symptom relief. On both day 1 and day 2 of PPI therapy, the proportion of patients achieving sufficient and sustained reflux symptom relief was at least 50 % higher in the omeprazole 20 mg group than in the rabeprazole 10 mg group. The study thus met its primary efficacy endpoint of early onset of sufficient and sustained relief of reflux symptoms, defined as the first day of sufficient reflux symptom relief that was maintained for at least 7 consecutive days. Furthermore, a significantly greater proportion of patients achieved complete resolution of their reflux symptoms with omeprazole 20 mg than with rabeprazole 10 mg at both 2 and 4 weeks of PPI therapy, and a significantly greater proportion of patients treated with omeprazole 20 mg also experienced complete resolution of upper gastrointestinal symptoms after 4 weeks of PPI therapy.
The pharmacodynamics and pharmacokinetics of PPIs are affected by genetic polymorphisms of CYP2C19, such that intragastric pH and plasma PPI levels are highest in patients with the PM phenotype, lowest in those with the homoEM phenotype and intermediate in those with the heteroEM phenotype [13]. Given that the PM phenotype is particularly prevalent in the Japanese population [13], we also evaluated outcomes based on CYP2C19 phenotype. At baseline, 21 % of patients in our study were shown to have the PM phenotype, which is consistent with the prevalence for this trait in the general Japanese population (18.0–22.5 %) [13]. Our study showed that omeprazole 20 mg was also more effective than rabeprazole 10 mg at relieving and resolving reflux symptoms in patients with the PM phenotype; indeed, the proportion of patients achieving sufficient and sustained reflux symptom relief was at least 65 % higher in the omeprazole 20 mg group than in the rabeprazole 10 mg group in patients with the PM phenotype on days 1–7 of PPI therapy, and this difference reached statistical significance on days 4–7. Reflux symptom relief remained significantly more effective with omeprazole 20 mg than with rabeprazole 10 mg at 2 and 4 weeks of PPI therapy in patients with the PM phenotype.
These results are consistent with the finding that CYP2C19 phenotype status has a greater effect on the pharmacokinetics of omeprazole than on the pharmacokinetics of rabeprazole, with a greater difference in systemic exposure to omeprazole than to rabeprazole seen across CYP2C19 phenotype categories [14, 23]. Although, by inference, it might be expected that systemic exposure to omeprazole is reduced in heteroEM and particularly homoEM phenotype patients, and this might translate into a decline in efficacy, our study did not show any significant differences in reflux-related outcomes favouring rabeprazole in these subgroups.
In line with these findings, results from a study conducted in healthy Japanese volunteers showed that early acid inhibition (measured by gastric pH) was greater after omeprazole 20 mg than after rabeprazole 10 mg in a combined group of heteroEM and PM phenotypes, but not in homoEM phenotypes, although this significant difference did not persistent after 1 week of PPI therapy [17]. The results from our study suggest that these early differences in acid inhibition translate into improved, early reflux symptom relief with omeprazole 20 mg, compared with rabeprazole 10 mg, in patients with reflux esophagitis and the PM phenotype. However, early differences in acid inhibition seen with omeprazole 20 mg versus rabeprazole 10 mg in healthy volunteers with the homoEM or heteroEM phenotypes [15] may not translate into differences in early reflux symptom relief with the two PPIs. In our study, omeprazole 20 mg and rabeprazole 10 mg were broadly similarly effective at relieving and resolving reflux symptoms in patients with the homoEM and heteroEM phenotypes, with no significant differences observed at any of the time points analysed. Results from a multinational study conducted in Europe (where the prevalence of the PM phenotype is ≤3.7 % [13]) showed a median time to heartburn relief of 1.5 days for both omeprazole 20 mg and rabeprazole 20 mg in patients with reflux esophagitis, suggesting that the two PPIs are similarly effective at early symptom relief in a population with predominantly homoEM and heteroEM phenotypes, even when the higher dose of 20 mg rabeprazole is used [22]. Correspondingly, in another study conducted in Europe, decreases in 24-h esophageal acid exposure in response to PPI therapy in patients with GERD were similar with omeprazole 20 mg and rabeprazole 20 mg [24]. Taking into account the distribution of the different CYP2C19 phenotypes and the treatment doses, we consider it reasonable to expect that omeprazole 20 mg might be more effective than rabeprazole 10 mg at treating symptoms in Japanese patients with reflux esophagitis.
In addition to the typical symptoms of GERD (heartburn and acid regurgitation), patients with GERD commonly experience other upper gastrointestinal symptoms, including bloating, belching and abdominal pain [25]. Typical symptoms of GERD and dyspepsia-related symptoms often occur together, even in uninvestigated patients. Upper gastrointestinal symptoms have been shown to improve with PPI therapy [19]. The objective of our study was to evaluate improvement of symptoms in patients with reflux esophagitis, and the GOS scale, which measures upper gastrointestinal symptoms, was considered to be the appropriate questionnaire for this aim. When assessing a combination of eight upper gastrointestinal symptoms in this study, a significantly greater proportion of patients in the omeprazole 20 mg group than in the rabeprazole 10 mg group experienced complete symptom resolution at 4 weeks of PPI therapy. In patients with the PM phenotype, omeprazole 20 mg showed a significantly higher efficacy than rabeprazole 10 mg at relieving (at 2 weeks) and resolving (at 4 weeks) upper gastrointestinal symptoms. Furthermore, omeprazole 20 mg and rabeprazole 10 mg were broadly similarly effective at relieving and resolving these symptoms in patients with the homoEM and heteroEM phenotypes. Our results therefore suggest that improved early symptom relief with omeprazole 20 mg, compared with rabeprazole 10 mg, in patients with reflux esophagitis and the PM phenotype applies not only to reflux symptoms, but also to other upper gastrointestinal symptoms that are common in GERD.
Both omeprazole 20 mg and rabeprazole 10 mg were generally well-tolerated in this 4-week study. Only four adverse events were recorded (itching, abdominal fullness, thirst, rash/exanthema), all in patients receiving omeprazole 20 mg. Although the incidence of adverse events was low, there was no evidence of a relationship with the CYP2C19 phenotype.
Key strengths of our study include the use of a validated patient-reported outcome instrument to record symptoms, the inclusion of patients with endoscopically confirmed reflux esophagitis, the separate analyses according to all three CYP2C19 phenotypes, and the use of study drug doses relevant to clinical practice in Japan. A limitation of our study is the open-label design, which means that confounding of outcomes by treatment expectation cannot be excluded. In addition, the symptomatic improvements seen with omeprazole 20 mg over rabeprazole 10 mg were not corroborated by endoscopic evaluation of reflux esophagitis. Since there has been little published data regarding symptom-based evaluations in early-phase response to treatment in GERD [26–29], the primary endpoint of this study was symptom relief in the early phase of treatment rather than mucosal healing, which is why endoscopy was not included in the study protocol. Regarding 4–8-week initial therapies for reflux esophagitis, symptom relief was relevant to mucosal healing [30]. Although endoscopy should be performed to confirm cure of reflux esophagitis, the fact that many patients achieved sufficient relief of reflux symptoms at 4 weeks in this study indicates that many of them might be cured. Furthermore, although doses of omeprazole 20 mg and rabeprazole 10 mg were consistent with those approved for current clinical practice in Japan, evidence suggests that rabeprazole has a more potent effect on acid suppression than omeprazole on a mg for mg basis [31]. It is open to question whether the advantage for omeprazole 20 mg would have been sustained if rabeprazole had been administered at the same dose; however, doses were chosen based on current clinical practice in Japan.
In conclusion, the results of our study show that omeprazole 20 mg once-daily is significantly more effective than rabeprazole 10 mg once daily at achieving sufficient and sustained relief of reflux symptom in the first 2 days of PPI therapy in Japanese patients with reflux esophagitis. This significant advantage of omeprazole 20 mg therapy also extends to resolution of reflux and upper gastrointestinal symptoms. Omeprazole 20 mg is more effective than rabeprazole 10 mg at early relief and resolution of reflux symptoms and other upper gastrointestinal symptoms in patients with the PM phenotype, and has similar effectiveness to rabeprazole 10 mg in patients with the heteroEM and homoEM phenotypes. These findings can assist physicians with disease management in patients with reflux esophagitis in Japan, where the PM phenotype is much more common than in the USA or Europe [13].
References
Donnellan C, Sharma N, Preston C, Moayyedi P. Medical treatments for the maintenance therapy of reflux oesophagitis and endoscopic negative reflux disease. Cochrane Database Syst Rev. 2005;2:CD003245.
Vakil N, Veldhuyzen van Zanten S, Kahrilas P, Dent J, Jones R. The Montreal definition and classification of gastro-esophageal reflux disease (GERD)—a global evidence-based consensus. Am J Gastroenterol. 2006;101:1900–20.
Kinoshita Y, Adachi K, Hongo M, Haruma K. Systematic review of the epidemiology of gastroesophageal reflux disease in Japan. J Gastroenterol. 2011;46:1092–103.
Matsuki N, Fujita T, Watanabe N, et al. Lifestyle factors associated with gastroesophageal reflux disease in the Japanese population. J Gastroenterol. 2013;48:340–9.
Ronkainen J, Aro P, Storskrubb T, et al. Gastro-oesophageal reflux symptoms and health-related quality of life in the adult general population—the Kalixanda study. Aliment Pharmacol Ther. 2006;23:1725–33.
Wahlqvist P, Reilly M, Barkun AN. Systematic review: the impact of gastro-oesophageal reflux disease on work productivity. Aliment Pharmacol Ther. 2006;24:259–72.
Wahlqvist P, Karlsson M, Johnson D, Carlsson J, Bolge S, Wallander MA. Relationship between symptoms of gastroesophageal reflux disease and costs: a database study in a US cohort. Value Health. 2007;10:A149.
Wiklund I, Carlsson J, Vakil N. Gastroesophageal reflux symptoms and well-being in a random sample of the general population of a Swedish community. Am J Gastroenterol. 2006;101:18–28.
Ronkainen J, Talley NJ, Storskrubb T, et al. Erosive esophagitis is a risk factor for Barrett’s esophagus: a community-based endoscopic follow-up study. Am J Gastroenterol. 2011;106:1946–52.
van Pinxteren B, Numans ME, Bonis PA, Lau J. Short-term treatment with proton pump inhibitors, H2-receptor antagonists and prokinetics for gastro-oesophageal reflux disease-like symptoms and endoscopy negative reflux disease. Cochrane Database Syst Rev. 2004;4:CD002095.
Kulig M, Leodolter A, Vieth M, et al. Quality of life in relation to symptoms in patients with gastro-oesophageal reflux disease—an analysis based on the ProGERD initiative. Aliment Pharmacol Ther. 2003;18:767–76.
Pace F, Negrini C, Wiklund I, Rossi C, Savarino V. Quality of life in acute and maintenance treatment of non-erosive and mild erosive gastro-oesophageal reflux disease. Aliment Pharmacol Ther. 2005;22:349–56.
Furuta T, Sugimoto M, Shirai N, Ishizaki T. CYP2C19 pharmacogenomics associated with therapy of Helicobacter pylori infection and gastro-esophageal reflux diseases with a proton pump inhibitor. Pharmacogenomics. 2007;8:1199–210.
Shirai N, Furuta T, Moriyama Y, et al. Effects of CYP2C19 genotypic differences in the metabolism of omeprazole and rabeprazole on intragastric pH. Aliment Pharmacol Ther. 2001;15:1929–37.
Saitoh T, Fukushima Y, Otsuka H, et al. Effects of rabeprazole, lansoprazole and omeprazole on intragastric pH in CYP2C19 extensive metabolizers. Aliment Pharmacol Ther. 2002;16:1811–7.
Yamagishi H, Koike T, Ohara S, et al. Early effects of Lansoprazole orally disintegrating tablets on intragastric pH in CYP2C19 extensive metabolizers. World J Gastroenterol. 2008;14:2049–54.
Furuta K, Adachi K, Ohara S, et al. Relationship between the acid-inhibitory effects of two proton pump inhibitors and CYP2C19 genotype in Japanese subjects: a randomized two-way crossover study. J Int Med Res. 2010;38:1473–83.
Veldhuyzen Van Zanten SJ, Chiba N, Armstrong D, et al. Validation of a 7-point global overall symptom scale to measure the severity of dyspepsia symptoms in clinical trials. Aliment Pharmacol Ther. 2006;23:521–9.
Sakurai K, Nagahara A, Inoue K, et al. Efficacy of omeprazole, famotidine, mosapride and teprenone in patients with upper gastrointestinal symptoms: an omeprazole-controlled randomized study (J-FOCUS). BMC Gastroenterol. 2012;12:42.
Veldhuyzen van Zanten S, Armstrong D, Chiba N, et al. Esomeprazole 40 mg once a day in patients with functional dyspepsia: the randomized, placebo-controlled “ENTER” trial. Am J Gastroenterol. 2006;101:2096–106.
Dewan B, Philipose N. Lafutidine 10 mg versus rabeprazole 20 mg in the treatment of patients with heartburn-dominant uninvestigated dyspepsia: a randomized, multicentric trial. Gastroenterol Res Pract. 2011;2011:640685.
Bytzer P, Morocutti A, Kennerly P, Ravic M, Miller N. Effect of rabeprazole and omeprazole on the onset of gastro-oesophageal reflux disease symptom relief during the first seven days of treatment. Scand J Gastroenterol. 2006;41:1132–40.
Furuta T, Ohashi K, Kosuge K, et al. CYP2C19 genotype status and effect of omeprazole on intragastric pH in humans. Clin Pharmacol Ther. 1999;65:552–61.
Galmiche JP, Zerbib F, Ducrottè P, et al. Decreasing oesophageal acid exposure in patients with GERD: a comparison of rabeprazole and omeprazole. Aliment Pharmacol Ther. 2001;15:1343–50.
Vakil N, Halling K, Wernersson B, Ohlsson L. Rome III functional dyspepsia criteria show substantial symptom overlap with gastroesophageal reflux disease. Gut. 2011;60(Suppl 3):A154.
Fock KM, Teo EK, Ang TL, Chua TS, Ng TM, Tan YL. Rabeprazole vs esomeprazole in non-erosive gastro-esophageal reflux disease: a randomized, double-blind study in urban Asia. World J Gastroenterol. 2005;11:3091–8.
Archimandritis AJ, Nikolopoulou V, Kouklakis G, Paraskevas E, Avgerinos A, Tsianos E, Triantafillidis JK, Hellenic Rabeprazole Study Group. Effects of rabeprazole on early symptom relief in gastro-oesophageal reflux disease: the Hellenic Rabeprazole Study Group surveillance study. Curr Med Res Opin. 2005;21:603–10.
Mee AS, Rowley JL. Rapid symptom relief in reflux oesophagitis: a comparison of lansoprazole and omeprazole. Aliment Pharmacol Ther. 1996;10:757–63.
Tominaga K, Iwakiri R, Fujimoto K, GERD 4 Study Group, et al. Rikkunshito improves symptoms in PPI-refractory GERD patients: a prospective, randomized, multicenter trial in Japan. J Gastroenterol. 2012;47:284–92.
Khan M, Santana J, Donnellan C, Preston C, Moayyedi P. Medical treatments in the short term management of reflux oesophagitis. Cochrane Database Syst Rev. 2007;2:CD003244.
Stedman CA, Barclay ML. Review article: comparison of the pharmacokinetics, acid suppression and efficacy of proton pump inhibitors. Aliment Pharmacol Ther. 2000;14:963–78.
Acknowledgments
We would like to thank the following study investigators: Kiyoharu Ito (Ozaki Clinic), Tetsuya Murao (Ozaki Clinic), Satoshi Yamada (Yamada Clinic), Akihiko Kurosawa (Kurosawa Gastroenterological Clinic), Toshio Murai (Murai Clinic), Yukihiro Sakurai (Sakurai Digestive Disease Clinic), Shuhei Tazaki (Tazaki Clinic) and Ryuzo Murai (Onaka Clinic).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Nagahara, A., Suzuki, T., Nagata, N. et al. A multicentre randomised trial to compare the efficacy of omeprazole versus rabeprazole in early symptom relief in patients with reflux esophagitis. J Gastroenterol 49, 1536–1547 (2014). https://doi.org/10.1007/s00535-013-0925-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00535-013-0925-8