Abstract
Objective
This overview of systematic reviews aims to critically appraise and consolidate evidence from current systematic reviews (SRs)/meta-analyses on the effects of exercise interventions on cancer-related fatigue (CRF) in breast cancer patients.
Methods
SRs/meta-analyses that explored the effects of exercise interventions on CRF in breast cancer patients compared with the routine methods of treatment and care were retrieved from nine databases. The methodological quality of the included SRs was appraised using A MeaSurement Tool to Assess systematic Reviews II (AMSTAR II). The Grading of Recommendations Assessment, Development and Evaluation (GRADE) was used to calculate the grading of outcomes in the included SRs. The exercise type, frequency, duration, and inclusion/absence of supervision were further evaluated with subgroup analyses. The Stata 16.0 software was utilized for data analysis.
Results
Twenty-nine reviews were included. The overall methodological quality and level of evidence of the included reviews were unsatisfactory, with only three reviews rated as high methodological quality and no review identified as high-quality evidence. Moderate certainty evidence indicated that exercise could improve fatigue in breast cancer patients (SMD = − 0.40 [95%CI − 0.58, − 0.22]; P = 0.0001). Subgroup analysis based on the types of exercise showed that yoga (SMD = − 0.30 [95%CI − 0.56, − 0.05]; I2 = 28.7%) and aerobic exercise (SMD = − 0.29 [95%CI − 0.56, − 0.02]; I2 = 16%) had a significantly better effect on CRF in breast cancer patients; exercising for over 6 months (SMD = − 0.88 [95%CI − 1.59, − 0.17]; I2 = 42.7%; P = 0.0001), three times per week (SMD = − 0.77 [95%CI − 1.04, − 0.05]; I2 = 0%; P = 0.0001), and for 30 to 60 min per session (SMD = − 0.81 [95%CI − 1.15, − 0.47]; I2 = 42.3%; P = 0.0001) can contribute to a moderate improvement of CRF. Supervised exercise (SMD = − 0.48 [95%CI − 0.77, − 0.18]; I2 = 87%; P = 0.001) was shown to relieve CRF.
Conclusion
Exercise played a favorable role in alleviating CRF in breast cancer. Yoga was recommended as a promising exercise modality for CRF management in the majority of the included studies. Exercising for at least three times per week with 30 to 60 min per session could be recommended as a suitable dosage for achieving improvement in CRF. Supervised exercise was found to be more effective in alleviating CRF than unsupervised exercise. More rigorously designed clinical studies are needed to specify the exact exercise type, duration, frequency, and intensity to have an optimal effect on CRF in breast cancer patients.
Trial registration
ClinicalTrials.gov Identifier: CRD42020219866.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The Global Burden of Disease Study 2020 [1] indicated that breast cancer remains the leading cancer diagnosis among females [2]. Although survival rates of breast cancer are improving, patients still experience a series of adverse effects caused by cancer and its related treatment, such as depression, fatigue, sleep disturbance, and bone marrow suppression [3, 4]. Cancer-related fatigue (CRF) is one of the most familiar and often overlooked symptoms [5], referring to a general, persistent, and subjective feeling of fatigue caused by cancer or relevant treatment that cannot be improved by sleep or rest [6] and may persist for months or even years [7]. The incidence of CRF in breast cancer patients is higher than that in other types of cancers [1], among which up to 33% of patients experience fatigue five years after the end of breast cancer treatment [8]. CRF adversely affects breast cancer patients in multiple aspects [9], which severely not only affects their quality of sleep but also prolongs their length of hospital stay and can result in a reduction of physical, mental, and emotional function and poor quality of life [10].
Currently, some pharmaceutical agents such as stimulants, antidepressants, acetylcholinesterase inhibitors, and corticosteroids have been recommended for CRF management in breast cancer patients [10] However, those pharmacological approaches were reported to be associated with a range of undesirable side effects such as tumor protection [11], decreased appetite [12] and venthrombotic events [13, 14]. The unclear pathophysiological mechanism of CRF also makes it difficult to develop tailored pharmacological interventions for CRF management [6]. Non-pharmacological interventions such as exercise interventions [10], mindfulness-based decompression therapy [15], and cognitive behavioral therapy [16] have been explored as adjuvant approaches to pharmacological interventions to alleviate CRF. Exercise interventions refer to a physical activity treatment that is planned, structured, and repetitive and have a final or an intermediate objective of improving or maintaining physical fitness, which includes running, aerobics, tai chi, yoga, and resistance exercise [17]. Exercise interventions have been commonly utilized and recommended as an effective intervention for the alleviation of CRF by the American Society of Clinical Oncology (ASCO) [18] and Exercise and Sports Science Australia (ESSA) [19]. In addition, the Japan Breast Cancer Society (JBCS) [20], the German Gynecological Oncology Group (AGO) [21], and a previous systematic review [22] found that exercise interventions are an effective, low-risk modality for breast cancer patients in reducing morbidity and improving body functions and quality of life. However, most of the literature on exercise interventions [18, 19, 21] have not clearly stated the type, frequency, and duration of exercise for practice in breast cancer patients with CRF, leading to a gap in developing personalized and evidence-based exercise intervention protocols tailored to patients’ health conditions and needs.
With the rapid development of evidence-based medicine in the field of cancer supportive care, an increasing body of systematic reviews (SRs)/meta-analyses have provided much evidence on using exercise interventions for CRF management in breast cancer patients, but their conclusions were inconsistent [23, 24] and the methodological quality varied across studies, which are barriers to the transformation of research evidence to practice and the application of clinical decision-making. To our knowledge, no overviews of systematic reviews on the effects of exercise interventions on CRF in breast cancer patients have been conducted so far. Thus, the aim of this overview was to critically appraise and consolidate evidence from current SRs/meta-analyses on the effects of exercise interventions on CRF in breast cancer patients. Specifically, the study objectives were as follows: (1) to identify the effects of exercise interventions on relieving CRF in breast cancer patients; (2) to assess the methodological quality of as well as the level of evidence from current SRs/meta-analyses on the effects of exercise interventions for breast cancer patients with CRF; and (3) to identify the optimal modality, duration, and frequency of exercise interventions for CRF management in breast cancer patients.
Methods
This overview of systematic reviews was reported in accordance with the Preferred Reporting Items for OoSRs (PRIO-harms) checklist and the Preferred Reporting Items for OoSRs (PRIO) checklist. The protocol has been registered with PROSPERO (CRD42020219866). A pre-print version of this manuscript is also available at https://www.researchsquare.com/article/rs-1376171/v1
Data sources and searches
This overview included SRs/meta-analyses that focused on the effects of exercise therapy on CRF in breast cancer patients. Relevant SRs/meta-analyses were comprehensively searched until September 2021 through the following data sources: (1) PubMed, Cochrane Library, Excerpta Medica Database (EMBASE), Web of Science, China National Knowledge Infrastructure (CNKI), China Biology Medicine Disc (CBMdisc), Wan Fang Data, and China Science and Technology Journal Database, and The Lancet; (2) references of the included SRs/meta-analyses; and (3) grey literature from the National Institute for Health Research (NIHR) Centre, such as unpublished manuscripts and published reports. The search terms included “breast neoplasms”, “exercise therapies”, “fatigue”, “systematic review”, and “meta-analysis”. The search procedure in the databases above followed the text string “((Breast neoplasms) OR (Breast tumor) OR (Mammary cancer) OR (Breast cancer) OR (Carcinoma breast)) AND (Exercise OR (Physical activity) OR (Physical exercise) OR (Exercise training) OR (Exercise therapies)) AND (Fatigue OR CRF) AND ((Systematic review) OR meta-analysis)”. Taking PUBMED and EMBASE as examples, a full search strategy was summarized in Supplementary file A.
Inclusion and exclusion criteria
The inclusion criteria were developed in accordance with the Population, Intervention, Comparator, Outcome, Study (PICOS) framework: (1) types of studies: SRs/meta-analyses of randomized controlled trials (RCTs) that were published in either English or Chinese; (2) types of populations: adult breast cancer patients [5] with CRF [25], regardless of stages of cancer, age, gender, and nationality; (3) types of interventions: exercise interventions [17], such as aerobic exercise, tai chi, yoga, resistance training, dancing, and walking; (4) types of comparison: routine methods of treatment and care with no active exercise components or any other types of active treatments; and (5) types of outcomes: CRF as the primary outcome as measured by valid assessment tools, such as the Functional Assessment of Chronic Illness Therapy-Fatigue (FACIT-F) Scale, the Multidimensional Fatigue Inventory (MFI), or the Brief Fatigue Inventory (BFI). Exclusion criteria were the following: (1) proposals of SRs or meta-analyses; (2) study population was breast cancer mixed with other diseases or complications; (3) conference abstracts; (4) full text was not available after multiple search methods, including contacting the author.
Literature screening and data extraction
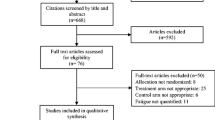
Duplications were identified and removed via reference management software (NoteExpress). The titles and abstracts of the rest of the SRs/meta-analyses were screened by two reviewers (HJZ and YZX) independently to determine the potentially eligible SRs/meta-analyses. Full texts of the potentially eligible SRs/meta-analyses were further screened and examined by the same two reviewers. If there were duplications, the latest version of the SR or meta-analysis was selected. Eligible SRs/meta-analyses were finally included after discussion between the reviewers. Any contradiction regarding study inclusion was resolved through consultation or arbitration by an experienced third reviewer (TW). Data from the included SRs/meta-analyses were extracted using a data extraction form predesigned by one reviewer (HJZ), which was verified by another reviewer (YZX). Disagreements between the two reviewers regarding data extraction were discussed by involving a third reviewer (TW). The extracted data included the author, publication year and country, number of studies and sample size of the participants, types of intervention and control, quality assessment (whether the included SRs/ meta-analyses evaluated the quality of their included studies and the tools used for the quality appraisal), measurement tools, main conclusion, and whether it included a meta-analysis. Moreover, relevant data for subgroup analysis including the exercise type, frequency, duration, and inclusion/absence of supervision were extracted and verified. The study selection process is presented in Fig. 1.
Quality appraisal of the included reviews
The methodological quality and the level of evidence of the included SRs/meta-analyses were independently assessed by two reviewers (HJZ and YZX) with two tools (see the “Methodological quality” and the “Evidence quality” sections). The final assessment results were cross-checked. Any disapprovals were discussed and decided by involving a third reviewer (JYT).
Methodological quality
A Measurement Tool to Assess systematic Reviews II (AMSTAR II) was used to comprehensively assess the methodological quality of the included SRs/meta-analyses [26], which is presently the most widely used methodological quality assessment tool [27, 28]. The AMSTAR II includes 16 items (www.amstar.ca), each of which can be answered “yes” or “no”, and some of the items can be answered “partially yes” [27, 28]. Seven items, including items 2, 4, 7, 9, 11, 13, and 15, that are considered to critically affect the validity of the included reviews and its conclusions are generally recommended as critical items [26]. The methodological quality of the included reviews was rated using the following criteria: (1) high quality: no or only one non-critical item flaw; (2) moderate quality: more than one non-critical item flaw but no critical item flaws; (3) low quality: one critical item flaw, with or without a non-critical item flaw; and (4) critically low quality: more than one critical item flaw, with or without a non-critical item flaw [26, 27, 29].
Evidence quality
Two reviewers (HJZ and YZX) used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) to rate the level of evidence of the included SRs/meta-analyses in five aspects, including limitations, inconsistencies, indirectness, imprecision, and publication bias [30]. Disagreements were addressed by involving a third author (TW) until consensus was achieved. For each aspect, the evidence was graded as high, moderate, low, or extremely low. Detailed grading criteria were as follows [31]: (1) high-level evidence: not downgraded, which represents the true effect estimates; (2) moderate-level evidence: downgraded one grade, which indicates that the true value is possible to come near to the estimate but is substantially different; (3) low-level evidence: downgraded two grades, which indicates that there is a significant difference between the actual and estimated values; and (4) extremely low-level evidence: downgraded three grades, which indicates that the true value is likely to be very different from the estimated value.
Data analysis
The characteristics of the included SRs, including author, publication year and country, number of studies and sample size, types of intervention and control, and main findings (i.e., effects of the exercise on CRF), are summarized in Table 1. The overlap across the included studies (only RCTs) of the analyzed SRs/meta-analyses was estimated using the corrected covered area (CCA) [32]. A lower CCA value indicated a lower likelihood of overlaps [32]. A CCA value of 5% or below was regarded as a “slight overlap”, 6–10% as a “moderate overlap”, 11–15% as a “high overlap”, while above 15% was regarded as a “very high overlap” [33]. For continuous variables, mean differences (MD) or standardized mean differences (SMD) with 95% confidence intervals (CI) was used for meta-analysis and effect size calculation (a P value of ≤ 0.05 was considered statistically significant). For continuous variables, the random-effects model was used to calculate the number of participants and RCTs included in the meta-analyses and to summarize the effect size [with 95% confidence intervals (CI) and P values ≤ 0.05 considered significant]. According to Cohen [33], 0.2 is considered a small effect, 0.2 to 0.8 a medium effect, and 0.8 or above a large effect. We also extracted and analyzed the data of included meta-analyses to better illustrate the effects of the duration of the interventions, exercise type, frequency, and duration of each session on CRF of breast cancer patients. Because of the lack of relevant data, direct comparisons between different interventions were impossible. I-square (I2) statistics were used to measure the heterogeneity of the included SRs/meta-analyses and explain the various thresholds by effect size and direction and the P-value from Cochran’s Q test [34]. An I2 value > 50% is regarded as a substantial level of heterogeneity [34]. Sub-group analyses are planned based on exercise type, frequency, and duration, and inclusion/absence of supervision. Statistical analysis was conducted with the Stata version 16.0 software.
Adapted from: Preferred reporting items for overviews of systematic reviews [35].
Results
Identification of the included reviews
A total of 369 records were searched, of which 135 were excluded due to duplication and 193 were screened by title or abstract and deemed irrelevant to the topic. Of the remaining 41 records, 12 were excluded after assessing the full text for eligibility. Twenty-nine SRs/meta-analyses [23, 24, 36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62] were finally included in the overview. The literature retrieval and selection process are shown in Fig. 1. A list of excluded reviews from full-text analysis with reasons is provided in supplementary file B.
Characteristics of the included reviews
The 29 SRs/meta-analyses included 402 studies, with a total of 33,655 patients, published between 2006 and 2021. Meta-analyses were carried out for all the included reviews. A total of 252 RCTs were included and analyzed across the 29 reviews, with a CCA of 3% indicating a slight overlap rate that reflected a low level of unnecessary duplications in the reviews and less biased results. Sixteen reviews [23, 24, 36,37,38,39,40,41,42,43,44,45,46,47,48,49] explored the effects of exercise therapy on CRF by including studies with different types of exercise, including aerobic exercise, yoga, resistance exercise, and Pilates. For the other 13 reviews, six [50,51,52,53,54,55] focused on yoga, five [56,57,58,59,60] on aerobic exercise, and two [61, 62] on tai chi. Routine methods of care and/or health education without any active exercise components were commonly utilized as the study comparisons. Seventeen reviews [23, 24, 36, 37, 39, 43, 44, 46, 50,51,52,53,54, 56,57,58, 61] used the Cochrane risk of bias (RoB) criteria. Other reviews were assessed using the Quality Assessment of Controlled Intervention Studies [38], the Norwegian Knowledge Centre for the Health Services (NOKC) Handbook for Systematic Reviews [41], Homemade Standard [42], the Revised Risk-of-Bias Tool for Randomized Trials (RoB 2.0) [55], Jadad Scores [59], the Newcastle–Ottawa Scale (NOS) [60], the Physiotherapy Evidence Databases (PEDro) Scale [40, 45, 62], and the Joanna Briggs Institute-Critical Appraisal for Randomized Controlled Trials (JBI-MAStARI) tool [47], respectively. Two reviews [48, 49] did not describe its methodological quality assessment process. The FACIT-F Scale [23, 36, 37, 39,40,41,42,43, 45,46,47,48,49, 51,52,53,54,55,56,57,58,59,60,61,62], the BFI [36, 37, 39, 40, 45, 46, 53,54,55, 58], the MFI [23, 24, 36, 38, 39, 41, 44, 45, 48, 54, 55, 58, 59], and the Piper Fatigue Scale (PFS) [24, 36, 39,40,41,42, 47, 48, 56,57,58,59,60] were the most commonly used instruments for CRF assessment in the 29 SRs/meta-analyses, the characteristics of which are presented in Table 1.
Quality appraisal of included reviews
Methodological quality
Regarding the methodological quality of the included SRs/meta-analyses, three reviews [40, 45, 53] were evaluated as high quality, 21 reviews [23, 24, 36, 37, 39, 41, 43, 44, 46,47,48, 50,51,52, 54, 56,57,58, 60,61,62] were rated as low quality, and the remaining five reviews [38, 42, 49, 55, 59] were assessed as critically low quality. Specifically, the critical items that had an effect on the quality of the reviews were item 2 (only five reviews [45, 46, 50, 55, 61] were evaluated as “yes” due to registered proposals in the early stage, and the remaining reviews only provided the research methods so they were assessed as “partly yes”, which means that the research methods could not be compared with the registered proposals approved by official organizations and may have caused a risk of bias), item 4 (whether to search for grey literature and counsel experts in the relevant field was not mentioned in 18 reviews[23, 24, 36, 37, 39, 43,44,45, 48,49,50,51, 54,55,56,57, 59, 60], suggesting that there may have been incomplete retrievals in the above, which may have led to results and conclusion errors), and item 7 (apart from three reviews[40, 45, 53], the list of excluded references and the causes for their exclusion were not provided and illustrated in the other reviews, which reduced the rigor of the study and the reliability of the results). In addition, non-critical item 10 also affected the methodological quality results since none of the 29 reviews reported the funding of their included RCTs, which indicated uncertainty about the possibility of commercial funding interference that might have made study results favorable to the commercial funder. All the reviews described the basic characteristics of and were able to scientifically discuss and analyze the included studies. Specific methodological quality assessment results are shown in Table 2.
Evidence quality
Eleven reviews [37, 38, 40, 43, 49,50,51,52, 59, 60, 62] were evaluated as having an extremely low level of evidence, 13 reviews [23, 24, 42, 44, 46,47,48, 53, 54, 56,57,58, 61] had a low level of evidence, and the remaining five [36, 38, 39, 41, 45] had a moderate level of evidence. Inconsistency (n = 22, 68.75%) was the most common reason for downgrading levels in the included reviews, followed by publication bias (n = 17, 53.12%), limitations (n = 15, 46.8%), imprecision (n = 10, 31.25%). Elaborating on the reasons for downgrading levels, the most common reason was the significant heterogeneity of the results [23, 36, 37, 39,40,41,42,43, 45,46,47, 49,50,51,52,53,54,55,56,57, 59, 61, 62] (n = 23) and the missing grey literature and manual retrieval [24, 37, 40, 42, 44, 50, 57,58,59, 61, 62] (n = 11). Other reasons included an unclear description of the blinding procedures [36, 40, 50, 52, 53, 55, 61, 62] (n = 8), inclusion of invalid values (RR = 1.0) within the confidence intervals [23, 42, 49, 51, 52, 59, 60, 62] (n = 8), failure to report publication bias [38, 42, 48, 49, 51, 62] (n = 6), unsatisfactory methodological quality of the included RCTs [24, 43, 44, 52, 53] (n = 5), unreported or incomplete report of outcomes such as adverse reactions [58] (n = 1), and the inclusion of only one RCT resulting in an inability to measure heterogeneity [42] (n = 1). The results of the evidence assessment are presented in Table 3.
Data synthesis and meta-analysis
The overall effects of the exercise interventions in the 29 SRs/meta-analyses indicated that exercise had a moderate effect on the reduction of fatigue in the breast cancer patients in the intervention groups (SMD = − 0.40 [95%CI − 0.58, − 0.22]; P = 0.0001) (see Fig. 2). However, due to the high heterogeneity among the 29 reviews (I2 = 95.4%), the overall effect size might be affected by various existing moderating variables. Therefore, the standardized mean differences of the moderating variables were further analyzed to determine the most effective intervention modalities based on the exercise type, frequency, intervention duration, and inclusion/absence of supervision in the intervention protocol. These results are shown in Table 4.
Exercise type
Seven exercise types were reported in the 29 SRs/meta-analyses, including aerobic exercise, resistance exercise, yoga, mind–body exercise (Pilates and gymnastics), combination exercise (home-based exercise and combined aerobic-resistance exercise), tai chi, and other exercises such as periodic rehabilitation exercise. The subgroup analyses of exercise type showed that both yoga (SMD = − 0.30 [95%CI − 0.56, − 0.05]; I2 = 28.7%; P = 0.021) and aerobic exercise (SMD = − 0.29 [95%CI − 0.56, − 0.02]; I2 = 16%; P = 0.048) had positive effects on improving CRF in breast cancer patients in the intervention groups compared with those in the control groups receiving routine methods of care. The remaining five exercise types—resistance exercise (SMD = − 0.01 [95%CI − 0.31, 0.29]; I2 = 48%; P = 0.958), tai chi (SMD = − 0.19 [95%CI − 1.13, 0.76]; I2 = 75.2%; P = 0.702), combination exercise (SMD = − 0.35 [95%CI − 0.76, 0.05]; I2 = 38.8%; P = 0.086), mind–body exercise (SMD = − 0.21 [95%CI − 0.63, 0.22]; I2 = 45.1%; P = 0.336), and other exercises (SMD = − 0.09 [95%CI − 0.45, 0.26]; I2 = 93.2%; P = 0.599)—showed no statistically significant differences between the intervention groups and the control groups using routine methods of care.
Duration of the intervention
Intervention duration among the included studies were categorized as: less than 2 months, 2 to 6 months, and more than 6 months. The effect magnitude of the moderating variable of duration had high heterogeneity (I2 = 94.8%; P < 0.000), indicating that the intervention duration could have affected the results of exercise for CRF management in breast cancer patients. The subgroup data indicated that only the intervention duration of more than 6 months had a beneficial effect (SMD = − 0.88 [95%CI − 1.59, − 0.17]; I2 = 42.7%; P = 0.000) on CRF compared with routine methods of care. The other intervention duration categories— less than 2 months (SMD = − 0.55 [95%CI − 1.15, 0.66]; I2 = 34.2%; P = 0.078) and 2 to 6 months (SMD = − 0.23 [95%CI − 0.65, 0.19]; I2 = 59.1%; P = 0.288)—resulted in no statistically significant improvement of CRF.
Frequency of exercise
The frequency of exercise included in the 29 SRs/meta-analyses can be categorized as 3 ≤ times per week and > 3 times per week, and the heterogeneity of the combined effect size between the two types was 95.3% (P = 0.0001), suggesting that the breast cancer patients’ CRF was affected by the frequency of exercise. The subgroup results of exercise frequency ≤ 3 times per week (SMD = − 0.75 [95%CI − 1.58, 0.08]; I2 = 91.6%; P = 0.076) indicated that its effect on CRF in the intervention groups was no statistically different from that in the control groups using routine methods of care, while exercise frequency > 3 times per week (SMD = − 0.77 [95%CI − 1.04, − 0.05]; I2 = 0%; P = 0.0001) indicated that the improvement effect was better in the intervention groups compared with the control groups receiving routine methods of care.
Duration of exercise
Subgroup analysis revealed that the duration of exercise that lasted 30 to 60 min per session (SMD = − 0.81 [95%CI − 1.15, − 0.47]; I2 = 42.3%; P = 0.0001) and 60 min per session (SMD = − 0.77 [95%CI − 1.04, − 0.50]; I2 = 0%; P = 0.0001) showed improvement effects on relieving CRF in breast cancer patients in the intervention groups compared with those in the control groups receiving routine methods of care.
Inclusion or absence of supervision during exercise
Relevant data on supervised and unsupervised exercise interventions were extracted from the included SRs/meta-analyses and the heterogeneity of effect size was 95.9%, indicating that supervision might impact the effects of exercise on CRF in breast cancer patients. Compared with routine methods of care, exercise interventions relieved the breast cancer patients’ CRF regardless of whether they were supervised while exercising; however, supervised exercise (SMD = − 0.48 [95%CI − 0.77, − 0.18]; I2 = 87%; P = 0.001) was shown to produce a larger effect on CRF compared with unsupervised exercise (SMD = − 0.21 [95%CI − 0.40, − 0.01]; I2 = 92.2%; P = 0.023).
Discussion
In this overview, we assessed the methodological and evidence quality of the included SRs/meta-analyses, and additional meta-analyses were performed for the 29 reviews to identify the effects of exercise on CRF in breast cancer patients. The intervention duration, exercise type, duration, and frequency, and whether the exercise intervention was supervised had varying degrees of influence on the effects of exercise on CRF. However, the unsatisfactory methodological quality and level of evidence of the included reviews might affect the reliability of the overview findings on the effects of exercise interventions on CRF in breast cancer survivors, which warrants a prudent interpretation of the study results.
Findings from this study suggested that exercise could be introduced as an effective intervention for CRF management in breast cancer patients. The findings supported the recommendations proposed in some clinical practice guidelines [18, 19, 68], in which exercise was rated and recommended as a beneficial approach to alleviating CRF. However, these guidelines [18, 19, 68] did not mention specific exercise plans. To help further detail the recommendations in the guidelines and facilitate healthcare professionals’ decision-making, subgroup analyses based on the type, frequency, duration, and intensity of exercise were conducted in this study. For exercise type, the study findings suggested that aerobic exercise and yoga were commonly recommended as promising approaches to improving CRF, which is consistent with Lin’s study [69], indicating that yoga can relieve patients’ tension and anxiety and help decrease their fatigue. Yoga is a convenient, easy-to-practice, and safe exercise modality that has been recommended as Grade I evidence by the U.S. National Comprehensive Cancer Network Guidelines [70]. The subgroup analyses results on the duration of exercise indicated that patients who had exercised for more than 6 months achieved the best improvement in fatigue. Our study findings showed that exercising more than three times per week for 30 to 60 min per session was beneficial for CRF alleviation, which is in line with previous research findings [71,72,73]. The included SRs/meta-analyses also indicated that patients should be encouraged to participate in supervised exercise when conditions permitted, which might lead to a better CRF outcome.
Although quantitative synthesis indicated that exercise interventions can alleviate CRF in breast cancer patients, the findings should be interpreted with caution given the unsatisfactory methodological quality (e.g., lack of reporting a list of exclusion studies and unclear funding resources) and level of evidence identified in the included reviews. For future studies, a list of excluded studies should be provided as an independent appendix to journals to facilitate readers’ understanding of the data selection process and further improve the reliability of the review findings [74]. Moreover, funding sources should be clearly declared in future publications to help readers determine whether funding bias existed. To achieve a comprehensive literature search, future systematic reviews are suggested to identify potential studies by searching not only the commonly used databases but also gray literature retrieval websites to minimize publication bias, for example, Greynet International (http://greynet.org/), the British Library (http://www.b1.uk), and other free grey literature sites such as PLoS. Conference abstracts, book chapters, academic theses and dissertations should also be sources of gray literature. In order to further improve the level of evidence of the included SRs, more original studies with rigorous study designs and detailed descriptions of the intervention protocols (e.g., type, frequency, intensity, and duration of the exercise) are necessary. Nevertheless, there is a need to acknowledge that the results of the unsatisfactory methodological quality of the included SRs may have been related to the selection of the quality appraisal tool. In this overview, nine of the included SRs were published before 2017, while the tool that we used for the quality appraisal (i.e., AMSTAR II) was also updated in 2017, which is an issue that needs to be considered for future overviews.
Study limitations
This overview has some limitations. Suboptimal methodological quality of some of the includes reviews (e.g., lack of registered protocols, unclear descriptions of data sources) may affect the strength of the evidence. language bias is possible given that only reviews published in Chinese and English were included. Due to the limited number of included reviews, within each current subgroup analysis, further subgroup analyses based on the intervention “dose” (e.g., intervention duration and frequency of each type of exercise intervention) were not conducted, which might, to some extent, limit the generalizability of the review findings to clinical practice.
Conclusion
Findings from this overview suggested that yoga and aerobic exercise with a long-term practice duration (over 6 months) might benefit CRF alleviation in breast cancer patients. Exercising for at least three times per week for 30 to 60 min per session might be an appropriate dose for alleviating CRF in breast cancer patients. Although existing evidence indicated that exercise interventions have a positive impact on CRF in breast cancer patients, the results should be interpreted with caution due to the limited quantity and unsatisfactory methodological quality and level of evidence of the included SRs/meta-analyses. More rigorously designed large-scale RCTs are needed to provide more robust evidence to specify the exact exercise type, duration, frequency, and intensity to have an optimal effect on CRF in breast cancer patients.
Data availability
All authors agreed that the data can be made public and used.
Code availability
Not applicable.
References
Siegel RL, Miller KD, Fuchs HE et al (2021) Cancer statistics. CA: A Cancer J Clin 71(1):7–33. https://doi.org/10.3322/caac.21654
Bai Y, Xu L, Duan X et al (2020) The Breast Cancer Cohort Study in Chinese Women: research design and preliminary results of clinical multi-center cohort. Chin J Epidemiol 41(12):2046–2052. https://doi.org/10.3760/cma.j.cn112338-20200507-00694
Gradishar WJ, Anderson BO, Abraham J et al (2020) Breast cancer, version 3. NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw 18(4):452–478. https://doi.org/10.6004/jnccn.2020.0016
Fu Z (2020) Comparison of the effect of different chemotherapy regimens in postoperative breast cancer. China Pract Med 15(35):148–150. https://doi.org/10.14163/j.cnki.11-5547/r.2020.35.063
Joly F, Lange M, Dos SM, et al (2019) Long-term fatigue and cognitive disorders in breast cancer survivors. Cancers (Basel) 11(12). https://doi.org/10.3390/cancers1112189
Schmidt ME, Wiskemann J, Armbrust P et al (2015) Effects of resistance exercise on fatigue and quality of life in breast cancer patients undergoing adjuvant chemotherapy: a randomized controlled trial. Int J Cancer 137(2):471–480. https://doi.org/10.1002/ijc.29383
Kim S, Han J, Lee MY et al (2020) The experience of cancer-related fatigue, exercise and exercise adherence among women breast cancer survivors: insights from focus group interviews. J Clin Nurs 29(5–6):758–769. https://doi.org/10.1111/jocn.15114
Lenja W, Hiensch AE, Velthuis MJ et al (2018) Four-year effects of exercise on fatigue and physical activity in patients with cancer. BMC Med 16(1):86. https://doi.org/10.1186/s12916-018-1075-x
Gelinas C, Fillion L (2004) Factors related to persistent fatigue following completion of breast cancer treatment. Oncol Nurs Forum 31(2):269–278. https://doi.org/10.1188/04.ONF.269-278
Fabi A, Bhargava R, Fatigoni S et al (2020) Cancer-related fatigue: ESMO Clinical Practice Guidelines for diagnosis and treatment. Ann Oncol 31(6):713–723. https://doi.org/10.1016/j.annonc
Candy B, Jones L, Williams R, et al (2008) Psychostimulants for depression. Cochrane Database Syst Rev (2):CD006722. https://doi.org/10.1002/14651858
Minton O, Richardson A, Sharpe M, et al (2010) Drug therapy for the management of cancer-related fatigue.Cochrane Database Syst Rev (7):D6704. https://doi.org/10.1002/14651858.
Mohandas H, Jaganathan SK, Mani MP et al (2017) Cancer-related fatigue treatment: an overview. J Cancer Res Ther 13(6):916–929. https://doi.org/10.4103/jcrt.JCRT_50_17
Tomlinson D, Robinson PD, Oberoi S et al (2018) Pharmacologic interventions for fatigue in cancer and transplantation: a meta-analysis. Curr Oncol 25(2):e152–e167. https://doi.org/10.3747/co.25.3883
Liu Y, Ouyang Y-Q, Huang Y et al (2019) Meta-analysis of intervention effect of mindfulness-based stress reduction therapy on breast cancer patients. J Nurs 26(8):31–36. https://doi.org/10.16460/j.issn1008-9969.2019.08.031
Eichler C, Pia M, Sibylle M et al (2015) Cognitive behavioral therapy in breast cancer patients—a feasibility study of an 8 week intervention for tumor associated fatigue treatment. Asian Pac J Cancer Prev 16(3):1063–1067. https://doi.org/10.7314/apjcp.2015.16.3.1063
Caspersen CJ, Christenson PGM (1985) Physical activity, exercise, and physical fitness: definitions and distinctions for health-related research. Public Health Rep (1974-) 100(2):126–131. https://doi.org/10.2307/20056429
Hassett MJ, Somerfield MR, Baker ER et al (2020) Management of male breast cancer: ASCO Guideline. J Clin Oncol 38(16):1849–1863. https://doi.org/10.1200/JCO.19.03120
Hayes SC, Newton RU, Spence RR et al (2019) The Exercise and Sports Science Australia position statement: exercise medicine in cancer management. J Sci Med Sport 22(11):1175–1199. https://doi.org/10.1016/j.jsams.2019.05.003
Kawate T, Yoshida A, Sugae S et al (2021) Recommendations for the management of breast cancer patients during the COVID-19 pandemic from the Japan Breast Cancer Society. Breast Cancer 28(2):247–253. https://doi.org/10.1007/s12282-020-01214-9
Ditsch N, Kolberg-Liedtke C, Friedrich M et al (2021) AGO Recommendations for the diagnosis and treatment of patients with early breast cancer: update 2021. Breast Care (Basel) 16(3):214–227. https://doi.org/10.1159/000516419
Belloni S, Arrigoni C, Caruso R (2021) Effects from physical exercise on reduced cancer-related fatigue: a systematic review of systematic reviews and meta-analysis. Acta Oncol 60(12):1678–1687. https://doi.org/10.1080/0284186X.2021.1962543
Zhu G, Zhang X, Wang Y et al (2016) Effects of exercise intervention in breast cancer survivors: a meta-analysis of 33 randomized controlled trails. Onco Targets Ther 9:2153–2168. https://doi.org/10.2147/OTT.S97864
Vannorsdall TD, Straub E, Saba C et al (2021) Interventions for multidimensional aspects of breast cancer-related fatigue: a meta-analytic review. Supp Care Cancer 29(4):1753–1764. https://doi.org/10.1007/s00520-020-05752-y
Thong M, van Noorden C, Steindorf K et al (2020) Cancer-related fatigue: causes and current treatment options. Curr Treat Options Oncol 21(2):17. https://doi.org/10.1007/s11864-020-0707-5
Shea BJ, Reeves BC, Wells G et al (2017) AMSTAR 2: a critical appraisal tool for systematic reviews that include randomised or non-randomised studies of healthcare interventions, or both. BMJ 358:j4008. https://doi.org/10.1136/bmj.j4008
Zhang F, Shen A, Zeng X et al (2018) An introduction to AMSTAR 2: a critical appraisal tool for systematic reviews. Chin J Evidence-Bases Cardiovasc Med 10(01):14–18. https://doi.org/10.3969/j.issn.1674-4055.2018.01.03
Pussegoda K, Turner L, Garritty C et al (2017) Systematic review adherence to methodological or reporting quality. Syst Rev 6(1):131. https://doi.org/10.1186/s13643-017-0527-2
Hu H, Xu A, Gao C et al (2015) The effect of physical exercise on rheumatoid arthritis: an overview of systematic reviews and meta-analysis. J Adv Nurs (2017) 77(2):506–522. https://doi.org/10.1111/jan.14574
Malmivaara A (2015) Methodological considerations of the GRADE method. Ann Med 47(1):1–5. https://doi.org/10.3109/07853890.2014.969766
Gordon HG, Andrew DO, Gunn EV et al (2009) GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. Chin J Evid Based Med 9(01):8–11. https://doi.org/10.1136/bmj.39489.470347.AD
Pieper D, Antoine SL, Mathes T et al (2014) Systematic review finds overlapping reviews were not mentioned in every other overview. J Clin Epidemiol 67(4):368–375. https://doi.org/10.1016/j.jclinepi.2013.11.007
Cohen J, Nee JC (1990) Robustness of type I error and power in set correlation analysis of contingency tables. Multivar Behav Res 25(3):341–350. https://doi.org/10.1207/s15327906mbr2503-6
Posadzki P, Pieper D, Bajpai R et al (2020) Exercise/physical activity and health outcomes: an overview of Cochrane systematic reviews. BMC Public Health 20(1):1724. https://doi.org/10.1186/s12889-020-09855-3
Bougioukas KI, Liakos A, Tsapas A et al (2017) Preferred reporting items for overviews of systematic reviews including harms checklist: a pilot tool to be used for balanced reporting of benefits and harms. J Clin Epidemiol 6(93):9–24. https://doi.org/10.1016/j.jclinepi.2017.10.002
Ehlers DK, Dubois K,Salerno EA (2020) The effects of exercise on cancer-related fatigue in breast cancer patients during primary treatment: a meta-analysis and systematic review. Expert Rev Anticancer Ther 1–13. https://doi.org/10.1080/14737140.2020.1813028
Shen Q, Yang H (2020) Impact of post-radiotherapy exercise on women with breast cancer: a systematic review and meta-analysis of randomized controlled trials. J Rehabil Med 52(10):m112. https://doi.org/10.2340/16501977-2740
Lee J, Lee MG (2020) Effects of exercise interventions on breast cancer patients during adjuvant therapy: a systematic review and meta-analysis of randomized controlled trials. Cancer Nurs 43(2):115–125. https://doi.org/10.1097/NCC.0000000000000682
Singh B, Spence RR, Steele ML et al (2018) A systematic review and meta-analysis of the safety, feasibility, and effect of exercise in women with stage II+ breast cancer. Arch Phys Med Rehabil 99(12):2621–2636. https://doi.org/10.1016/j.apmr.2018.03.026
Lipsett A, Barrett S, Haruna F et al (2017) The impact of exercise during adjuvant radiotherapy for breast cancer on fatigue and quality of life: a systematic review and meta-analysis. Breast 32:144–155. https://doi.org/10.1016/j.breast.2017.02.002
Juvet LK, Thune I, Elvsaas IKØ et al (2017) The effect of exercise on fatigue and physical functioning in breast cancer patients during and after treatment and at 6 months follow-up: a meta-analysis. Breast 33:166–177. https://doi.org/10.1016/j.breast.2017.04.003
McNeely ML (2006) Effects of exercise on breast cancer patients and survivors: a systematic review and meta-analysis. Can Med Assoc J 175(1):34–41. https://doi.org/10.1503/cmaj.051073
Gu L, Yu M, Xu D et al (2012) Effectiveness of physical exercise on health-related quality of life and psychological outcomes in breast cancer patients: a meta analysis. Chin J Pract Nurs 36:58–62. https://doi.org/10.3760/cma.j.issn.1672-7088.2012.36.139
van Vulpen JK, Peeters PHM, Velthuis MJ et al (2016) Effects of physical exercise during adjuvant breast cancer treatment on physical and psychosocial dimensions of cancer-related fatigue: a meta-analysis. Maturitas 85:104–111. https://doi.org/10.1016/j.maturitas.2015.12.007
Ramírez-Vélez R, Zambom-Ferraresi F, García-Hermoso A et al (2021) Evidence-based exercise recommendations to improve mental wellbeing in women with breast cancer during active treatment: a systematic review and meta-analysis. Cancers (Basel) 13(2):264. https://doi.org/10.3390/cancers13020264
Liu C, Qin M, Zheng X et al (2021) A meta-analysis: intervention effect of mind-body exercise on relieving cancer-related fatigue in breast cancer patients. Evidence-Based Complement Altern Med 2021:9980940. https://doi.org/10.1155/2021/9980940
Lin HP, Kuo YH, Tai WY et al (2021) Exercise effects on fatigue in breast cancer survivors after treatments: a systematic review and meta-analysis. Int J Nurs Pract 2021:e12989. https://doi.org/10.1111/ijn.12989
Duijts SFA, Faber MM, Oldenburg HSA et al (2011) Effectiveness of behavioral techniques and physical exercise on psychosocial functioning and health-related quality of life in breast cancer patients and survivors—a meta-analysis. Psychooncology 20(2):115–126. https://doi.org/10.1002/pon.1728
Liu X, Li H, Guo C et al (2017) Effects of home-based physical activities on the health outcomes in early-stage breast cancer survivors: a meta-analysis. Int J Clin Exp Med 10(11):15129–15139
Zheng F, Li C, Liu Y et al (2021) Meta-analysis of the effects of behavioral intervention in breast cancer patients. Chin J Gerontol 41(02):255–265. https://doi.org/10.3969/j.issn.1005-9202.2021.02.010
Wu Q, Yin Y, Chen L et al (2018) Effects of yoga on negative emotions and quality of life in patients with breast cancer: a meta-analysis. Chin J Med 53(05):559–564. https://doi.org/10.3969/j.issn.1008-1070.2018.05.026
Zhang Q, Piao L, Zhao D et al (2015) Effects of yoga on cancer-related fatigue in breast cancer patients: meta-analysis. Chin J Modern Nurs 21(28):3380–3386. https://doi.org/10.3760/cma.j.issn.1674-2907.2015.28.009
O’Neill M, Mina DS, Sabiston C, et al (2020) The effect of yoga interventions on cancer-related fatigue for breast cancer: a systematic review and meta-analysis of randomized controlled trials. J Clin Oncol 34. https://doi.org/10.1177/1534735420959882
Dong B, Xie C, Jing X et al (2019) Yoga has a solid effect on cancer-related fatigue in patients with breast cancer: a meta-analysis. Breast Cancer Res Treat 177(1):5–16. https://doi.org/10.1007/s10549-019-05278-w
Hsueh EJ, Loh EW, Lin JJ et al (2021) Effects of yoga on improving quality of life in patients with breast cancer: a meta-analysis of randomized controlled trials. Breast Cancer 28(2):264–276. https://doi.org/10.1007/s12282-020-01209-6
Xu H-Y, Zhang L-D, Yang H (2020) Meta analysis of the effects of aerobic exercise on cancer-related fatigue in breast cancer patients. Chin Med Pharm 10(08):23–27. https://doi.org/10.3969/j.issn.2095-0616.2020.08.006
Hu Y, Gu P, Zhang X (2009) Effectiveness of rehabilitation program on shoulder function of breast cancer patients after mastectomy: a systematic review. Chin J Evid Based Med 9(01):41–54. https://doi.org/10.3969/j.issn.1672-2531.2009.01.012
Yang D, Guo J-H, Zhang J (2019) Effects of aerobic exercise on cancer-related fatigue in breast cancer patients: a meta-analysis. J Modern Clin Med 45(04):288–292. https://doi.org/10.11851/j.issn.1673-1557.2019.04.016
Zhang Y, Liang M (2012) Meta-analysis of the effect of aerobic exercise on cancer-induced fatigue in breast cancer patients. Chin Med Modern Dist Educ China 10(13):122–123. https://doi.org/10.3969/j.issn.1672-2779.2012.13.077
Zou L, Yang L, He X et al (2014) Effects of aerobic exercise on cancer-related fatigue in breast cancer patients receiving chemotherapy: a meta-analysis. Chin Gen Pract 17(13):1524–1528. https://doi.org/10.3969/j.issn.1007-9572.2014.13.020
Luo X, Liu J, Fu J, et al (2020) Effect of tai chi chuan in breast cancer patients: a systematic review and meta-analysis. Front Oncol 10(607). https://doi.org/10.3389/fonc.2020.00607
Liu L, Tan H, Yu S, et al. (2020). The effectiveness of tai chi in breast cancer patients: a systematic review and meta-analysis. Complement Ther Clin Pract 38(101078). https://doi.org/10.1016/j.ctcp.2019.101078
Guyatt GH, Oxman AD, Vist G et al (2011) GRADE guidelines: 4. Rating the quality of evidence—study limitations (risk of bias). J Clin Epidemiol 64(4):407–415. https://doi.org/10.1016/j.jclinepi.2010.07.017
Guyatt GH, Oxman AD, Montori V et al (2011) GRADE guidelines: 5. Rating the quality of evidence—publication bias. J Clin Epidemiol 64(12):1277–1282. https://doi.org/10.1016/j.jclinepi.2011.01.011
Guyatt GH, Oxman AD, Kunz R et al (2011) GRADE guidelines: 6. Rating the quality of evidence—imprecision. J Clin Epidemiol 64(12):1283–1293. https://doi.org/10.1016/j.jclinepi.2011.01.012
Guyatt GH, Oxman AD, Kunz R et al (2011) GRADE guidelines: 7. Rating the quality of evidence—inconsistency. J Clin Epidemiol 64(12):1294–1302. https://doi.org/10.1016/j.jclinepi.2011.03.017
Guyatt GH, Oxman AD, Kunz R et al (2011) GRADE guidelines: 8. Rating the quality of evidence—indirectness. J J Clin Epidemiol 64(12):1303–1310. https://doi.org/10.1016/j.jclinepi.2011.04.014
Tumor Support and Rehabilitation Therapy Group the Oncology Committee of Chinese Medical Association (2021) Clinical practice guidelines for cancer-related fatigue in China (2021 edition). China Oncol 31(9):852–872. https://doi.org/10.19401/j.cnki.1007-3639.2021.09.012
Lin PJ, Kleckner IR, Loh KP et al (2019) Influence of yoga on cancer-related fatigue and on mediational relationships between changes in sleep and cancer-related fatigue: a nationwide, multicenter randomized controlled trial of yoga in cancer survivors. Integr Cancer Ther 18:1871073566. https://doi.org/10.1177/1534735419855134
Piercy KL, Troiano RP, Ballard RM et al (2018) The physical activity guidelines for Americans. JAMA 320(19):2020–2028. https://doi.org/10.1001/jama.2018.14854
Yao L, Ma J, Ji C et al (2021) Research progress of application of kinesitherapy in cancer-related fatigue in patients with breast cancer. Chin J Modern Nurs 27(02):159–163. https://doi.org/10.3760/cma.j.cn115682-20200630-04141
Mock V, Atkinson A, Barsevick A et al (2000) NCCN practice guidelines for cancer-related fatigue. Oncology (Williston Park, NY) 14(11A):151–161
Zhang Y, Zhang J (2016) Influence of aerobic exercise combined with relaxation therapy on symptom clusters and quality of life of breast cancer patients with chemotherapy. Chin Nurs Res 30(22):2764–276. https://doi.org/10.3969/j.issn.1009-6493.2016.22.0197
Wang H, Long Y, Chen X (2020) The thinking and suggestion of scientific research interest conflict. Public Commun Sci Technol 12(21):52–55. https://doi.org/10.16607/j.cnki.1674-6708.2020.21.018
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions
Author information
Authors and Affiliations
Contributions
HJZ and TW contributed to study conception and design, data extraction, analysis and interpretation, manuscript drafting and revision. JXC and JYT contributed to study conception and design, data interpretation, and manuscript revision. YZX, LJD, YNC, and CW supported study design, data extraction and/or double check, and manuscript revision. All authors approved the submitted version.
Corresponding authors
Ethics declarations
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
All authors agree that the data can be made public.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Hong-Juan Zhou and Tao Wang have contributed equally to this work and share the first authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhou, HJ., Wang, T., Xu, YZ. et al. Effects of exercise interventions on cancer-related fatigue in breast cancer patients: an overview of systematic reviews. Support Care Cancer 30, 10421–10440 (2022). https://doi.org/10.1007/s00520-022-07389-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-022-07389-5