Abstract
Purpose
This study aims to evaluate adherence to guidelines of antiemetic prophylaxis and frequency of chemotherapy-induced nausea and vomiting (CINV) in the palliative first-line treatment of colorectal cancer (CRC) patients in Northern Bavaria.
Methods
We collected detailed information on chemotherapy and supportive drugs in 103 patients within a prospective observational study. The study was conducted to determine quality of care within an interdisciplinary context (first endpoint) and direct costs of palliative treatment for patients with CRC between 2006 and 2010 (second endpoint, Emmert et al. (Eur J Health Econ, 2012) [1]). In this paper, we evaluate adherence to Multinational Association of Supportive Care in Cancer (MASCC) 2006 recommendations for prophylaxis of CINV during the first administration of chemotherapy as well as incidence and grade of CINV within 120 h thereafter.
Results
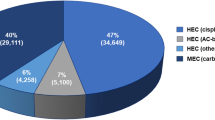
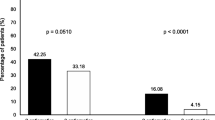
Of the patients studied, 95 patients (92 %) received moderately emetogenic (oxaliplatin- and/or irinotecan-containing combined chemotherapy treatment) and eight (8 %) received low emetogenic chemotherapy (either 5-fluorouracil (5-FU) or capecitabine monotherapy). Antiemetic prophylaxis could be assessed in 101 out of 103 (98 %) of patients. MASCC-recommended antiemetic prophylaxis was prescribed in three patients (3 %). Nonadherence was mainly caused by omission of dexamethasone. Nausea and/or vomiting occurred in 18 patients (18 %) within a 120-h period. All documented episodes were grade 1 or 2 according to the Common Toxicity Criteria of the National Cancer Institute. None of these patients received the recommended prophylaxis for CINV. In only one patient, antiemetic prophylaxis was intensified during the next chemotherapy application.
Conclusions
In the Integrated Health Care in the Palliative Treatment of Colorectal Carcinoma (IVOPAK) I Project, adherence to the MASCC clinical recommendations was very poor. Extent of CINV in this patient population seems to be underestimated. There is an urgent need to improve clinicians' awareness of this patient-relevant side effect.

Similar content being viewed by others
References
Emmert M, Pohl-Dernick K, Wein A et al. (2012) Palliative treatment of colorectal cancer in Germany: cost of care and quality of life. Eur J Health Econ. doi:10.1007/s10198-012-0408-5
Statistisches Bundesamt (2012) Gesundheit: Todesursachen in Deutschland 2010, Fachserie 12 Reihe 4. Wiesbaden
Schmiegel W, Pox C, Reinacher-Schick A et al (2010) S3 guidelines for colorectal carcinoma: results of an evidence-based consensus conference on February 6/7, 2004 and June 8/9, 2007 (for the topics IV, VI and VII). Z Gastroenterol 48(1):65–136
Basch E, Prestrud AA, Hesketh PJ et al (2011) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29(31):4189–4198
Roila F, Herrstedt J, Aapro M et al (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21(Suppl 5):v232–v243
Kaiser R (2005) Antiemetic guidelines: are they being used? Lancet Oncol 6(8):622–625
Griffin AM, Butow PN, Coates AS et al (1996) On the receiving end. V: patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol 7(2):189–195
Roila F, Hesketh PJ, Herrstedt J (2006) Prevention of chemotherapy- and radiotherapy-induced emesis: results of the 2004 Perugia International Antiemetic Consensus Conference. Ann Oncol 17(1):20–28
DCTD N, NIH, DHHS (1999) Cancer Therapy Evaluation Program Common Toxicity Criteria, version 2.0.
Burmeister H, Aebi S, Studer C et al (2012) Adherence to ESMO clinical recommendations for prophylaxis of chemotherapy-induced nausea and vomiting. Support Care Cancer 20(1):141–147
Drug Utilization Review Team in Oncology (2003) Antiemetic prescription in Italian breast cancer patients submitted to adjuvant chemotherapy. Support Care Cancer 11(12):785–789
Fabi A, Barduagni M, Lauro S et al (2003) Is delayed chemotherapy-induced emesis well managed in oncological clinical practice? An observational study. Support Care Cancer 11(3):156–161
The Italian Group for Antiemetic Research (1998) Transferability to clinical practice of the results of controlled clinical trials: the case of antiemetic prophylactic treatment for cancer chemotherapy-induced nausea and vomiting. Ann Oncol 9(7):759–765
Brugnatelli S, Gattoni E, Grasso D et al (2011) Single-dose palonosetron and dexamethasone in preventing nausea and vomiting induced by moderately emetogenic chemotherapy in breast and colorectal cancer patients. Tumori 97(3):362–366
Abbrederis K, Lorenzen S, Rothling N et al (2009) Chemotherapy-induced nausea and vomiting in the treatment of gastrointestinal tumors and secondary prophylaxis with aprepitant. Onkologie 32(1–2):30–34
Cabana MD, Rand CS, Powe NR et al (1999) Why don't physicians follow clinical practice guidelines? A framework for improvement. JAMA 282(15):1458–1465
Grunberg S (2012) Patient-centered management of chemotherapy-induced nausea and vomiting. Cancer Control 19(2 Suppl):10–15
Bero LA, Grilli R, Grimshaw JM et al (1998) Closing the gap between research and practice: an overview of systematic reviews of interventions to promote the implementation of research findings. The Cochrane Effective Practice and Organization of Care Review Group. BMJ 317(7156):465–468
Dranitsaris G, Leung P, Warr D (2001) Implementing evidence based antiemetic guidelines in the oncology setting: results of a 4-month prospective intervention study. Support Care Cancer 9(8):611–618
Mertens WC, Higby DJ, Brown D et al (2003) Improving the care of patients with regard to chemotherapy-induced nausea and emesis: the effect of feedback to clinicians on adherence to antiemetic prescribing guidelines. J Clin Oncol 21(7):1373–1378
Majek O, Gondos A, Jansen L et al (2012) Survival from colorectal cancer in Germany in the early 21st century. Br J Cancer 106(11):1875–1880
Grunberg S (2012) Improving control of chemotherapy-induced nausea and vomiting. Cancer Control 19(2 Suppl):2
Molassiotis A, Coventry PA, Stricker CT et al (2007) Validation and psychometric assessment of a short clinical scale to measure chemotherapy-induced nausea and vomiting: the MASCC antiemesis tool. J Pain Symptom Manage 34(2):148–159
Acknowledgments
We would firstly like to thank the participating study sites for their cooperation. We are also indebted to Mrs. Gudrun Maennlein, who considerably made a contribution to the success of IVOPAK by coordinating the project. We acknowledge with particular gratitude Mrs. Melanie Hempel for her valuable comments. The IVOPAK project was supported by AOK Bayern, Fresenius, Medac, Merck Serono, Pfizer, and Roche.
Conflict of interest
All authors have completed and submitted the Disclosure Form of Potential Conflicts of Interest. Dr. Dörje reports being a member of the Pfizer National Advisory Board Germany. No other conflict of interest disclosures were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Koch, S., Wein, A., Siebler, J. et al. Antiemetic prophylaxis and frequency of chemotherapy-induced nausea and vomiting in palliative first-line treatment of colorectal cancer patients: the Northern Bavarian IVOPAK I Project. Support Care Cancer 21, 2395–2402 (2013). https://doi.org/10.1007/s00520-013-1801-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00520-013-1801-z