Abstract
The High Arctic region has experienced marked climate fluctuations within the past decades strongly affecting tundra shrub growth. However, the spatial variability in dwarf shrub growth responses in this remote region remains largely unknown. This study characterizes temperature sensitivity of radial growth of two willow dwarf shrub species from two distinct High Arctic sites. The dwarf shrub Salix arctica from Northern Greenland (82°N), which has a dry continental High Arctic climate, is linked with Salix polaris from central Svalbard (78° N), which experiences a more oceanic High Arctic climate with relatively mild winters. We found similar positive and significant relationships between annual growth of both Salix dwarf shrub species and July–August air temperatures (1960–2010), despite different temperature regimes and shrub growth rates at the two sites. Also, Salix dwarf shrub growth was significantly negatively correlated with Arctic and North Atlantic Oscillation (AO/NAO) indices; S. arctica from Northern Greenland was negatively correlated with previous autumn (AO index) and current summer AO and NAO indices, and S. polaris with the summer NAO index. The results highlight the importance of both local and regional climatic drivers for dwarf willow shrub growth in harsh polar desert habitats and are a step in the direction of identifying and scaling changes in plant growth across the High Arctic.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent decades, tundra vegetation greenness has increased at many Arctic sites, which has been documented by remotely-sensed proxies, as the normalized difference vegetation index (NDVI) (Myneni et al. 1997; Jia et al. 2003; Walker et al. 2012). This increase has mainly been associated with increased shrub growth in response to warmer summers (Forbes et al. 2010; Blok et al. 2011; Macias-Fauria et al. 2012; Tape et al. 2012; Weijers et al. 2018) and has furthermore been confirmed by site-specific experimental and monitoring observations on tundra shrubs, typically over relatively short (less than two decades) time-scales (e.g., Walker et al. 2006; Elmendorf et al. 2012; Bjorkman et al. 2015; Prevéy et al. 2017). Large-scale patterns under field conditions are more difficult to confirm and growth response studies of shrubs of the same species across large regional and pan-regional scales and longer time-scales are rare (but see Weijers et al. 2012).
Recent studies reported a heterogeneous pattern in shrub growth responses to a changing climate (Elmendorf et al. 2012; Tape et al. 2012; Myers-Smith et al. 2015; Ropars et al. 2015) including evidences of shrub growth decline (Gamm et al. 2018). Climate sensitivity of shrub growth was found to be greatest at the boundary between the Low and High Arctic, and greater for taller than shorter shrub species (Myers-Smith et al. 2015). Additionally, Arctic vegetation is getting taller (Bjorkman et al. 2018) which will further influence albedo changes and global heat flux (Pearson et al. 2013). This inconsistent growth response of tundra shrubs across the Arctic requires detailed studies, at both site- and species-specific levels, to gain insight in the mechanisms controlling shrub growth over longer time scales. However, extreme northern sites are especially understudied (Metcalfe et al. 2018), although these areas are expected to experience the largest climatic changes in the decades to come (Kirtman et al. 2013). Therefore, it is important to test the impact of climate warming on High Arctic shrub growth, especially as secondary growth of these shrubs may not only reflect annual growth rates of the respective species, but also past fluctuations in annual vascular plant production at the community level (Le Moullec et al. 2018).
Many studies have recently concluded that tundra shrub growth at high latitudes is limited primarily by growing season temperatures (for example: Forbes et al. 2010; Blok et al. 2011; Buchwal et al. 2013; Myers-Smith et al. 2015; Weijers et al. 2017). In contrast, Salix arctica growth was found to be related with snow precipitation in NE Greenland (74° N), suggesting that shrub growth may not be exclusively related to air temperatures (Schmidt et al. 2006, 2010). In addition, winter temperatures in the Arctic have been shown to be an important driver for Betula nana shrub growth (Hollesen et al. 2015). Therefore, comparisons of shrub growth sensitivity to climate between sites across climate gradients are an important new step in terms of linking site-specific observations to regional vegetation responses to climate changes (Prevéy et al. 2017).
Very few chronologies covering recent decades are available from the extreme High Arctic (> 75° N; see Weijers et al. 2010, Weijers et al. 2017). This is both due to the remoteness of those sites as well as the numerous growth constraints caused by the harsh environmental conditions that northernmost woody plants represent (cf. Schweingruber et al. 2013), which often lead to incomplete or missing rings and cross-dating difficulties. Willow shrubs are among the most widespread tundra woody plants, but their growth has not yet been compared across genus and locations in the extreme High Arctic. Specifically, Salix arctica (Pall.) is among the most northerly occurring woody plant species in the world and its dendrochronological potential in the High Arctic was already recognized in the nineteenth century (Kraus 1874). S. arctica exhibits clear but irregular growth rings and is known to reach ages of about 130 years (Kraus 1874; Polunin 1955; Polunin 1958). Studies on that species were conducted for example on Axel Heiberg Island (ca. 79°N) (Beschel and Webb 1963), Cornwallis Island (74° N) (Warren Wilson 1964), and Ellesmere Island (82° N and 81° N; Woodcock and Bradley 1994, Savile 1979), all in the Canadian High Arctic, as well as in Eastern Greenland, Zackenberg (74° N) (Schmidt et al. 2006, 2010). In parallel, in the European High Arctic (Spitsbergen, Svalbard 77°–78° N), the dendroclimatological potential of another willow shrub was recently recognized and described for the dwarf willow shrub Salix polaris Wahlenb. (Buchwal et al. 2013; Owczarek and Opała 2016). Thus, with the spatial distribution of Salicaceae shrubs in the northernmost regions of the Arctic, regional variability in climatic controls on that genus’ growth can be assessed. This is further relevant for assessing Salix dwarf shrubs potential and role in future snow redistribution, water balance, permafrost thaw and forage availability (cf. Myers-Smith et al. 2011) in the most extreme and climatically diverse High Arctic ecosystems.
This study aims to characterize the temperature sensitivity of two dominant dwarf willow shrub species from two strongly contrasting High Arctic sites (continental vs. oceanic). We tested weather radial growth of different dwarf shrub species from the same genus, is characterized by similar temperature sensitivity, despite potential species-specific growth requirements and site-specific conditions in two remote High Arctic locations. Additionally, to account for a part of variance in shrub growth not explained by temperature and in relation to the recent findings highlighting strong relationship between High Arctic dwarf shrub growth and large-scale atmospheric circulation patterns (Weijers et al. 2017), we compared our two Salix chronologies with the Arctic and North Atlantic Oscillation (AO/NAO) indices (Hurrell 1995). Although the two indices are highly correlated, the AO index is expected to influence weather patterns in Northern Greenland more, whereas NAO index signature is more prominent in the lower polar latitudes. Specifically, the NAO index relates to a redistribution of atmospheric mass between the Arctic and the subtropical Atlantic, and swings from one phase to another, producing large changes in, for example, heat and moisture transport between the Atlantic and neighboring continents (Hurrel et al. 2003). The positive phase of the NAO index was found to be associated with a significant increase in storminess in the Spitsbergen region (Hurrell 1995; Hurrell and Van Loon 1997), as well rain-on-snow events (Putkonen and Roe 2003). The related AO index accounts for a significant larger fraction of the variance in Northern Hemisphere surface air temperatures and has been identified as the dominant empirical orthogonal function of winter sea-level pressure variability for the Northern Hemisphere poleward of 20° N (Thompson and Wallace 1998; Deser 2000). Moreover, signatures of both NAO and AO index are reflected in numerous tree (D’Arrigo et al. 2003) and selected shrub (Aanes et al. 2002; Welker et al. 2005; Weijers et al. 2017) growth chronologies.
We hypothesized (1) a similar climate-growth response of Salix dwarf shrubs with respect to temperature despite the differences in climate between the two High Arctic sites and (2) that dwarf shrub growth of both species is controlled indirectly and in a similar manner by larger-scale climatic features, such as the AO and NAO atmospheric circulation patterns.
Material and methods
Study sites and shrub sampling
Jørgen Brønlund Fjord is located on Peary Land, which is a peninsula in northern Greenland about 700 km south of the North Pole and thereby the most northerly ice-free region in the world. Peary Land reaches from Independence Fjord in the south to the northernmost point of Greenland’s mainland and the Arctic Ocean in the North.
Peary Land is mountainous polar desert with generally a growing season lasting 1 month (July) and an annual precipitation sum usually less than 100 mm per year (Bennike 1987). The mean annual air temperature in the central part of the fiord is − 16.2 °C (sd = 1.1 °C) as computed for the period 1960–2010 (Table 2). Mean winter temperature (previous December - current February, pDJF) is - 29.1 °C (sd = 1.7 °C). The warmest month is July and the coldest February with average temperatures of 3.7 °C (sd = 1.5 °C) and − 30.1 °C (sd = 2.9 °C), respectively. All temperature averages for Peary Land were computed based on CRUTEM4 and DMI datasets (with gaps, see below).
The summer (JJA) is relatively mild with typically 40–60 days without frost, a low relative humidity and periods with strong and dry foehn-like winds. Due to the very limited precipitation, the area is characterized as “desert-like” with very sparse vegetation developed only in limited micro-sites where melting snowdrifts and creeks supply water in summer (Holmen 1957). In lowlands and on slopes with running meltwater from snowdrifts, a thin soil layer up to 20 cm, but mostly less than 5 cm, is formed.
S. arctica is characterized as one of the earliest (mid-June) flowering plant in Peary Land, growing in micro-habitats such as snow-beds, slopes, and riversides (Holmen 1957). At the study site, mainly isolated individuals are found that prostrate from a central stem and are usually less than 15 cm in height.
Shrub sampling in Peary Land, Northern Greenland, was conducted along a transect beside the 25 km long Jørgen Brønlund Fjord (82°07′ N; 33° 04′ W to 82° 03′ N; 32° 00′ W), which consists of an inner 15 km long and 2 km wide part running east-west and an outer 10 km long and up to 3 km wide section running northwest-southeast. Here 11 intact S. arctica individuals were collected at five locations in July 2014. Sampling locations consisted of relatively moist plots, i.e., along the creeks or downstream from snowbed areas where individual willow shrubs were observed (Fig. 1a). Samples were taken within valley bottom sites and alluvial fans at elevations below 200 m above sea level (Suppl. Table 1). Maximum distance between the sampling sites in Northern Greenland was 18 km and one to three shrubs were collected from each site. Only mature and apparently healthy individuals were sampled. Additionally, to investigate the shrubs’ temperature sensitivity over a longer time-scale, we attempted to sample relatively old individuals.
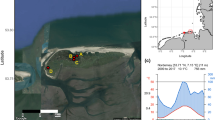
The High Arctic study locations and species (a and b), and climate (c and d). aSalix arctica shrub and cross-section from Northern Greenland (Peary Land) - vicinity of Jørgen Brønlund Fjord (82°N); and bSalix polaris shrub and cross-section from central Spitsbergen, Svalbard - vicinity of Petuniabukta (78°N). Cross-section black scale bars indicate 2 mm. Satellite images: Google Maps (Peary Land)/ Norwegian Polar Institute (Spitsbergen). Mean temperatures (c and d): annual temperature (black solid line), winter temperature (previous December-current February; blue solid line) and summer temperature (July–August; red solid line). Dashed lines represent linear trends. See “Material and Methods” for temperature data sources
S. polaris samples from the Svalbard archipelago were collected in central Spitsbergen (78° 44′ N; 16° 27′ E to 78° 42′ N; 16° 40′ E), in the Petuniabukta area located in the northernmost part of Billefjorden, which is the northeastern part of Isfjorden (Fig. 1b). According to the long-term and homogenized climate data from Svalbard Airport (Nordklim dataset 1.0, 08/01 KLIMA 2001; DNMI 2018; www.eklima.no), mean annual temperature for central Spitsbergen is − 5.8 °C (sd = 1.8 °C), as computed for the period 1960–2010. Mean winter temperature (previous December - current February, pDJF) for central Spitsbergen is − 13.7 °C (sd = 3.6 °C). The warmest month is July and the coldest February with average temperatures of 6.2 °C (sd = 1.0 °C) and − 15 °C (sd = 4.2 °C), respectively. The climate in the Petuniabukta area is characterized by more continental conditions than Svalbard Airport, where the long-term climate station is located (Rachlewicz and Styszyńska 2007; Láska et al. 2012), but is still under influence of the West Spitsbergen Current, which brings warm water and shapes relatively mild winters in this part of the High Arctic, much warmer than those in Peary Land. Mean annual precipitation in the study location is usually not higher than 200 mm (DNMI 2018), which is twice as high as that at our site in Northern Greenland.
S. polaris is a mat-forming prostrate dwarf shrub species, usually smaller than 10 cm in height. The species commonly dominates the middle and northern arctic tundra zones. It often grows on fine texture substrates (www.svalbardflora.no). S. polaris is one of the most widely distributed woody species on Svalbard and its stands can be found in harsh polar desert habitats, including glacier moraines.
Sampling in central Spitsbergen was conducted at three locations in Petuniabukta vicinity: i) an edge of the Hørbye glacier forefield (two shrubs), ii) Ebbadalen valley bottom prone to aeolian and niveo-aeolian activity (four shrubs) and iii) gentle slope of an alluvial fan in Ebbadalen prone to seasonal wash-out (four shrubs) (Suppl. Table 1). Maximum distance between any two sampling locations was 6 km (Fig. 1b). Shrubs were selected from a broader sample pool (i.e., more than 30 shrubs) collected in central Spitsbergen in mid-August 2010. For this study, ten shrubs were selected by choosing the oldest individuals from each location that cover the study period (1960–2010). Six individuals were previously sectioned, measured, cross-dated and incorporated into the S. polaris site chronology, as published in Buchwal et al. (2013); however, in the current study, these shrubs were used for the calculation of climate-growth relationships over a longer time scale.
Thin-sections preparation and growth rings measurements
Although a limited number of shrubs were collected from each study area, individuals were carefully inspected and sectioned at both below- and aboveground plant segments in accordance with basic principles of serial sectioning (Kolishchuk 1990). The sectioning consisted of the extraction of 1-cm long fragments from the main root and up to two main aboveground shoots at a mean distance of 2–3 cm apart from each other. Bent or injured shrub parts were not sectioned. From each plant fragment, a thin section of 15–20 μm of thickness was cut with a sledge GSL1-microtome and later stained by a mixture of Safranin and Astra Blue (Gärtner et al. 2014). This was done to highlight lignified and unlignified tissues, and to enable the identification of narrow and irregular ring boundaries. As S. polaris from central Svalbard (Fig. 1b) was characterized by narrower rings and greater growth irregularities, a larger amount of cross-sections were prepared per shrub for this species than for S. arctica from Greenland. In total 30 stained micro-slides were prepared from Northern Greenland and 117 from central Svalbard material following a standard protocol (Schweingruber and Poschlod 2005). Sequential images were taken from each micro-slide under 25× magnification for regular cross-sections and under × 50 magnification for irregular individuals. This was done using a Leica DFC420C camera connected to a Leica DM4500B microscope. Images were stitched together in Adobe Photoshop (Adobe Systems Incorporated, USA) to form a single high-resolution image for each cross-section, required for accurate annual ring-border recognition and ring-width measurements. The latter were performed along a minimum of three radii for each cross-section, using manual path in WinCell (Regent Instruments, Canada).
Cross-dating and chronology development
Cross-dating of raw ring width measurements of the two willow shrub species was performed separately for each species in four independent steps: Firstly, radial measurement series were cross-dated within cross-sections. This was supplemented by careful visual inspection of irregular and partially missing rings within complete cross-section. Secondly, the cross-dated radial growth curves were averaged for each cross-section and then cross-dated and averaged per shrub individual. In a third step, the resulting mean shrub series were cross-dated and averaged per sample location. In the final step, the resulting mean growth series were cross-dated at site level, which resulted in a final willow shrub chronology for each High Arctic site. Quality check of visual cross-dating was performed during the third and fourth steps using COFECHA (Holmes 1983; Grissino-Mayer 2001). Additionally, both locally missing rings (i.e., missing in some part of the shrub) and completely missing rings (rings that were not detected in a particular shrub but found in another shrub) were quantified for each shrub individual (Table 1, Suppl. Table 1). Furthermore, recognition of continuously missing outer rings (CMOR; Wilmking et al. 2012) was performed for both dwarf shrub species.
For the Peary Land site, ten S. arctica shrubs from five locations, 24 cross-sections (from one to four cross-section per plant) and 77 radii (from four to 12 radii per shrub) were successfully cross-dated (Table 1). Based on their low inter-series correlations with other shrubs, one shrub with clear growth suppression in the outermost rings and six cross-sections were excluded from further analyses.
For central Spitsbergen, all 117 S. polaris cross-sections prepared from ten willow shrubs were successfully cross-dated and further analyzed. In total, two to 19 thin-sections were prepared per shrub and a total of 420 radii measured (i.e., from eight to 74 radii per shrub) (Table 1). Ring-widths were measured along three to six radii within each S. polaris cross-section to support the detection of incomplete (wedging) and locally absent rings, which are frequent in this species (Buchwal et al. 2013).
After cross-dating, two raw site chronologies were computed, one for each willow shrub, using an arithmetic mean.
Data standardization
Raw growth ring-width measurements after cross-dating were standardized to remove possible non-climatic age-related and/or biologically induced growth trends. The juvenile phase growth trend, i.e., a sharp decline in ring-widths with increasing stem diameter during early stage of shrub growth, was visible in S. arctica growth curves, but not in S. polaris (Fig. 2; Suppl. Fig. S1). To evaluate climate-growth responses of shrubs from two distant High Arctic sites in a comprehensive way both chronologies were standardized.
The High Arctic dwarf shrub chronologies: a raw (red) and b standardized (black) Salix arctica Pall. ring-width chronology from Northern Greenland (Peary Land, 82°N); c raw (red) and d standardized (black) Salix polaris Wahlenb. ring-width chronology from central Spitsbergen, Svalbard (Petuniabukta, 78°N); RWI: ring-width index after regional curve standardization (RCS). Sample depth refers to total number of cross-sections. The vertical dashed lines in both graphs mark the onset of the common period, i.e., 1960, used in the climate-growth relationship analyses
To retain the low-frequency component in our growth data, we used a regional curve standardization (RCS) method to standardize raw tree-ring series by calculating an empirical age-related growth curve and by dividing each mean cross-section series by this growth trend (Briffa et al. 1992; Briffa and Melvin 2011). The single-curve RCS chronologies are often affected by what is known as “modern sample bias” (Melvin 2004; Sullivan et al. 2016), which can emerge when a population of trees (here shrubs) is composed of both young fast-growing and older slow-growing individuals. This artifact was not present in either chronology (Suppl. Fig. S8), most likely due to relatively homogeneous age distribution within the shrub individuals of both species.
As a result of RCS detrending ring-width indices (RWI) were generated. These were then averaged at the individual shrub level. Shrub means were than averaged at the site level using bi-weight robust means to calculate two standardized site chronologies, one for each shrub species. Autocorrelation was removed from the chronologies through whitening of the time-series using autoregressive coefficients prior to averaging. For comparison results for both raw and standardized data are shown.
We calculated the following descriptive statistics, commonly used in dendrochronology, for the quality evaluation of both chronologies: i) the mean correlation (i.e., inter-series correlation) between individual growth curves and a master chronology (r), ii) mean pairwise correlation between all cross-sections (rbar.tot), iii) mean correlations between mean cross-section series within individual shrub (rbar.wt), and iv) mean inter-series correlation between all mean shrub series (rbar.bt). Analyses were computed in R (version 3.2.5) using the dplR package (version 1.6.4) (Bunn 2008; Bunn et al. 2016). Other statistics included computation of: v) the expressed population signal (EPS), vi) signal-to-noise ratio (snr), vii) mean sensitivity (MS), viii) Gini coefficient (Gini) as well ix) first-order autocorrelation, i.e., AR(1) (Table 1).
Climate-growth relationship analyses
The period 1960–2010 (50 years) was used for climate-growth relationship analyses as this period was characterized by at least three individual shrubs in both study areas (Table 1) with a stable common signal (i.e., EPS > 0.85). Climate-growth relationship analyses for both willow species were performed only with air temperature data and AO/NAO indices, as precipitation records from Peary Land are neither representative for our site, nor available for the entire study period.
To test climate sensitivity of both species while accounting for potential variation in growing season lengths between the study sites and influence of climate during and outside of the growing season, various temporal scales and climate-growth relationship analyses were performed. Following common procedures in dendroclimatology, we primarily calculated Pearson’s correlation coefficients to investigate relations between radial growth of each willow shrub species and monthly climate. Specifically, correlations with air temperature and AO/NAO indices were computed on monthly, i.e., from previous June to September of the year of growth, and seasonal basis, i.e., using a combination of two to three following months for which the highest monthly correlation was revealed. These analyses enable the comparison with earlier dendrochronological studies. To all correlations performed, p values were adjusted for multiple comparisons using the false discovery rate (Benjamini and Hochberg 1995) and Bonferoni (Bland and Altman 1995) corrections. In the main text, the results of the first method are reported. Complete comparisons between raw and adjusted p values (two methods), together with additional bootstrapped correlation analyses performed between the chronologies and summer temperature as well as AO/NAO indices, are presented in the Supplementary Materials (Suppl. Table 7–9; Suppl. Fig. S10-S16). Also, linear mixed effect (LME) modeling was performed to account for potential site-based variability of shrubs’ climate sensitivity. The full methods and results of LME modeling are shown in the Supplementary Materials (Suppl. Table 1–6).
To account for variations in temperatures over shorter time-scales, and therefore differences in lengths of growing seasons at both study areas, moving correlations were calculated between average 10-days mean air temperatures offset by 1-day and standardized chronologies of both Salix species. For these analyses homogenized daily temperature data (see below) were used for the common period 1976–2010. Also relationships between growth rings and summer temperature (at monthly and seasonal resolution) of both species were tested over two periods, i.e., an early (1960–1985) and a recent (1986–2010) period. This was done by fitting a linear model and conducting a regression analysis between standardized mean annual growth (RWI) and mean monthly (July, August) and seasonal (July–August) temperature, respectively, for each studied species. This comparison of climate sensitivity of shrub growth across two periods was performed to evaluate trends in growth responses as a function of changing temperature. Additionally, temporal trends in temperature were assessed by fitting linear models.
Climate data
Mean monthly temperature data for Peary Land, northern Greenland, were obtained from CRUTEM4 gridded land-surface air temperature dataset (Jones et al. 2012; Osborn and Jones 2014) and based mainly on records obtained from Station Nord climatic station (81° 43′ N, 17° 47′ W) for the period 1960–2008, supplemented for last 2 years by data obtained from Danish Meteorological Institute (DMI 2018). Nevertheless, the following data gaps have been identified for our final temperature dataset: June1972–September 1975, February 1976, January–March and May 1977, January–November 1981, April–June 2007, February–March 2009. Mean monthly temperatures from Svalbard Airport (78° 15′ N 15° 28′ E) were obtained for the period 1960–2001 through the package “nordklimdata1” in R (08/01 KLIMA, 2001) and supplemented with DNMI (2018) data for the same station up to year 2010.
For running correlations with mean daily air temperatures, the following climate station data were used from period 1976–2010: Station Nord for Peary Land, Northern Greenland (source: DMI 2018; 04310 Station Nord for period 1976–2006 and 04312 Station Nord AWS for period 2007–2010), and homogenized Svalbard Lufthavn data from central Spitsbergen (source: DNMI 2018).
Monthly Arctic Oscillation (AO) and North Atlantic Oscillation (NAO) index values were obtained from Climate Prediction Center (CPC), a part of National Oceanic and Atmospheric Administration’s (NOAA) National Weather Service (CPC 2016, www.cpc.ncep.noaa.gov).
Results
Air temperature characteristics and trends
Table 2 and Fig. 1c, d summarize the results of climate data, mainly temperature, from the two sites. Linear regression analyses over 1960–2010 revealed that two strongest positive trends in mean monthly temperature were recorded in Peary Land for April (0.08 °C year−1, p = 0.003) and November (0.07 °C year−1, p = 0.005), whereas for central Spitsbergen for February (0.15 °C year−1, p < 0.001) and January (0.13 °C year−1, p = 0.007). The trend in mean annual temperature, although not statistically different, was higher for central Spitsbergen (0.08 °C year−1, p < 0.001, df = 49) than for Peary Land (0.05 °C year−1, p < 0.001, df = 40). Seasonal temperature trends both for winter (pDJF) and summer (JulAug) were higher for central Spitsbergen (0.12 °C year−1, p < 0.001 and 0.03 °C year−1, p < 0.001 respectively) than for Peary Land (0.04 °C year−1, p = 0.008 and 0.03 °C year−1, p = 0.031 respectively) (Fig. 1c, d). In Peary Land, the strongest positive trend in summer air temperature was observed for July (r2 = 0.129, p < 0.05, slope = 0.035, df = 44), whereas in central Spitsbergen for July (r2 = 0.256, p < 0.001, slope = 0.034, df = 49) and August (r2 = 0.253, p < 0.001, slope = 0.031, df = 49). Also, for central Spitsbergen considerable increase of a positive trend in August temperature was observed in the recent period, i.e., from 1986 to 2010 (r2 = 0.254, p < 0.05, slope = 0.053, df = 23). Trends estimated for summer months temperature were not significant for Peary Land over that period (i.e., for June: p = 0.402; for July: p = 0.082; for August: p = 0.856).
Shrub species chronologies and growth ring patterns
Growth ring measurements and successful cross-dating of ten shrubs per High Arctic site resulted in two willow shrub ring-width chronologies (Fig. 2). The S. arctica chronology from Peary Land covered 90 years (1925–2014), whereas the S. polaris chronology from central Spitsbergen was 70 years long (1941–2010). Mean age of S. arctica individuals was 58 years (max = 90, sd = 25), whereas mean age of S. polaris was 48 years (max = 70, sd = 9). The S. polaris chronology was represented by higher mean sensitivity and mean inter-series correlation, but lower first-order autocorrelation than S. arctica (Table 1).
Among 1187 growth rings measured in S. arctica and 4289 rings analyzed in S. polaris there were in total 5.3% (i.e., 64 rings) and 6.4% (i.e., 275 rings) missing rings detected respectively, which represents locally missing rings (i.e., missing in some part of the shrub) together with completely missing rings (i.e., missing entirely in a shrub individual) (Table 2). Despite different amounts of cross-sections per species and different ages of the willow shrubs analyzed, the total number of completely missing rings, i.e., rings that were not detected in a particular shrub but found in another shrub, was similar between the species. The number of missing rings equalled 2.1% (i.e., 12 rings) and 1.9% (i.e., 9 rings) for S. arctica and S. polaris, respectively (Suppl. Table 1). Continuously missing outer rings (CMOR) were not observed in S. polaris, but were detected in four (i.e., 17%) S. arctica cross-sections. A maximum of six consecutive CMOR were detected in a single S. arctica shrub.
Mean annual radial growth over the 1960–2010 period measured 0.122 mm (sd = 0.04) for S. arctica (Greenland) and 0.056 mm (sd = 0.02) for S. polaris (Svalbard). Maximum mean annual ring width was registered for S. arctica chronology in 2003 (0.234 mm) and for S. polaris chronology in 2002 (0.111 mm).
Temperature-dependent growth
Both willow species from the two High Arctic sites revealed a significant summer temperature signal over the 50-year study period (1960–2010) (Fig. 3a, b, Suppl. Table 7). The raw S. arctica ring-widths from Peary Land were found to correlate with mean July (r = 0.397, p = 0.016) and July–August (JULAUG) air temperature (r = 0.424, p = 0.016), whereas the correlations between the standardized S. arctica chronology and both July and July–August were nearly significant (p = 0.054). Standardized S. polaris radial growth at our site in central Spitsbergen was found to be strongly correlated with mean July (r = 0.486, p = 0.002), August (r = 0.502, p = 0.001), and mean July–August temperature (r = 0.550, p < 0.001). Additionally, the raw S. polaris chronology revealed a positive correlation with previous September air temperature (r = 0.305, p = 0.017) and current June temperature (p = 0.006). Summer temperature signal was additionally observed in both species with bootstrapped confidence intervals computed for correlation coefficients (Suppl. Fig. S11–S12).
Pearson’s correlation coefficients between raw (black) and RCS standardized (gray) growth rings chronologies of Salix arctica (a, c, e) from Peary Land (82° N), Northern Greenland and Salix polaris (b, d, f) from central Spitsbergen, Svalbard (78° N), and monthly and seasonal temperatures (a, b), AO indices (c, d), and NAO indices (e, f). Correlations were computed over the common period 1960–2010. Significance levels are marked with dashed lines (raw p-values: p < 0.05, p < 0.01, p < 0.001, respectively) and asterisks indicate significant correlation coefficients obtained with p-values adjusted for the false discovery rate. Lowercase letters represent previous year months, uppercase letters current year months. JJ: current June and July; JJA: current June–August; ndJ: previous November-current January; son: previous September–November). See Material & Methods for climatic data sources. Degrees of freedom (df) differs between A (df = 44) and B (df = 49), due to gaps in the Greenland temperature record
Growth increase for S. polaris estimated between a recent (1986–2010) and an early (1960–1985) period equalled 23.1 μm for raw tree ring widths (33.6%) and 0.113 for detrended RWI (11.6%), whereas for S. arctica it was 11.2 μm (8.7%) and 0.153 (14.9%), respectively. In addition, linear regression analyses revealed a positive linear trend in annual growth of the two willow shrub species in relation to summer temperature at both High Arctic locations. Stronger positive relationships between Salix growth and summer temperature were found in the recent period, i.e., 1986–2010, as compared to the early period (i.e., 1960–1985) at both locations (Fig. 4; Suppl. Fig. S2 and S5). Linear mixed effect (LME) modeling (Suppl. Mat.) confirmed the results obtained using more traditional dendrochronological methods and indicated that the detected summer temperature-growth relationships at both sites and both species were largely consistent when accounting for variability across sites and years (Suppl. Table 2–3).
Linear regression analyses of Salix spp. ring-width index (RWI, standardized chronologies) to summer air temperature for two periods: (1960–1985)—open circles; (1986–2010)—filled circles. Salix arctica chronology (blue) from Peary Land (82° N), Northern Greenland; bSalix polaris chronology (green) from central Spitsbergen (78° N); Climatic data source: see Fig. 2 caption
The correlation analysis with moving 10-day-windows of mean air temperatures and standardized Salix ring-width growth revealed that among temperature variables S. arctica (Peary Land) secondary growth was mainly influenced by temperatures during the beginning of July, whereas secondary growth of S. polaris (central Spitsbergen) was mainly influenced by August temperatures (Fig. 5a). The highest correlations with 10-day mean air temperature for S. arctica was with mean temperatures between 1st and 10th of July (r = 0.62, p < 0.05) and between 10th and 20th of August for S. polaris at (r = 0.64, p < 0.01).
a Moving correlations between the standardized Salix arctica (Northern Greenland; blue line) and Salix polaris (Spitsbergen; green line) chronologies and 10-day mean temperatures (offset: 1 day) over the period 1976–2010. The horizontal dashed lines indicate significance levels (p < 0.05 and p < 0.01). b, c Mean daily air temperatures (black lines) during the growing season over the period 1976–2010 at b Station Nord, Northern Greenland (blue lines represent mean daily temperatures for each year), and c Svalbard Airport, Spitsbergen (green lines represent mean daily temperatures for each year). Dashed red lines represent 5 °C, a common thermal threshold for cambial activity; doy - day of year. See “Material and Methods” for temperature data sources
AO/NAO indices versus growth
We found significant correlations (Fig. 3c–f, Suppl. Fig. S6, Suppl. Table 8) between radial growth of S. arctica dwarf shrubs and regional atmospheric circulation pattern reflected in monthly and seasonal AO indices over a half century-long period (1960–2010). Specifically, both species demonstrated reduced growth with positive AO and/or NAO summer indices. The raw S. arctica chronology from Peary Land was negatively correlated with June–July-August (JJA) AO index (r = − 0.341, p = 0.011). In addition, the correlation between autumn AO index of the previous year (i.e., September–October-November, son) and raw S. arctica growth at our site in Northern Greenland was nearly significant (r = − 0.318, p = 0.058). Correction of p values according to the false discovery rate suggested no further significant relationships between Salix growth and AO/NAO indices. However, correlation coefficients with bootstrapped confidence intervals suggest significant correlations between the standardized S. arctica chronology and previous November and current June AO index, as well previous September, current June and current September NAO index (Suppl. Fig. S13-S14). Furthermore, bootstrapped confidence intervals suggest significant correlation coefficients between the standardized S. polaris chronology and previous August NAO index (Suppl. Fig. S16).
Discussion
Temperature sensitivity of two willow shrub species from two distinct High Arctic sites
Despite differences in site-specific conditions and regional climate, the two willow shrub species studied, each from another remote High Arctic location, were characterized by summer temperature sensitivity recorded in their ring-width chronologies spanning a half-century (1960–2010). This significant summer temperature signal found for both species is in line with observations for non-dwarf Salix spp. shrubs from other Arctic sites, such as: Salix pulchra in Northeastern Siberia (70° N) (Blok et al. 2011) and in Southwest Yukon Territory (61° N) (Weijers et al. 2018), Salix lanata in Northwest Russian Arctic (68° N) (Forbes et al. 2010), and Salix glauca in Western Greenland (67° N) (Young et al. 2016). Correspondingly, summer temperature signals were found in the evergreen dwarf shrub Cassiope tetragona in the Canadian High Arctic (Johnstone and Henry 1997; Rayback et al. 2012) and Northern Peary Land, Greenland (Weijers et al. 2017), approx. 130 km from our S. arctica study site in northern Greenland, as well as in central Spitsbergen, Svalbard (Weijers et al. 2010), approx. 65 km from our S. polaris study site. Additionally, the correlations with daily temperatures performed (Fig. 5) detailed the summer temperature sensitivity of both species obtained using monthly values. The results highlight that peak correlations between S. arctica (Northern Greenland) and temperature emerge much earlier in the growing season than it was noted for S. polaris (Spitsbergen). This difference is most likely linked to contrasting length of the growing season at two study sites (Fig. 5b, c), favoring growth of S. polaris shrubs from Spitsbergen during much warmer August in this part of the High Arctic.
As expected, air temperature is an important environmental parameter explaining radial growth at both sites, but relatively low correlation coefficients suggest that other factors, besides air temperature, may also be important for the growth of willow dwarf shrubs in the High Arctic. As indicated in previous studies conducted on shrubs in the Low Arctic, snow cover (Hallinger et al. 2010) and soil moisture (Myers-Smith et al. 2015; Ackerman et al. 2017) could be potential drivers for favorable Salix spp. growth. However, verification of such relationships is hampered by lack of other-than-temperature climate data in situ from High Arctic locations, where wind-drifted snow cover is challenging to measure (cf. Haas et al. 2017).
In contrast, Schmidt et al. (2006) found no correlation between S. arctica ring growth and summer temperature in Zackenberg in Eastern Greenland (74°N), but a negative correlation with early spring snow cover instead. Similarly, Owczarek and Opała (2016) found no correlation between S. polaris growth and summer temperature in Southwestern Spitsbergen and Zalatan and Gajewski (2006) found no temperature signal in Salix alaxensis at Victoria Island in the Western Canadian Arctic (71° N). This further indicates that other potentially more local and micro-site conditions such as topography (cf. Ropars et al. 2015) counter the effects of temperature and therefore might limit upscaling of shrub growth sensitivity from plot to regional scales. Hence, the incoherence in various shrubs species growth responses to summer temperature requires further investigations, most likely at the regional level, to assess both species-specific and site-specific growth relations to various climatic regimes in the Arctic.
AO/NAO indices signature in willow shrubs’ chronologies
Specific relationships revealed between seasonal and monthly atmospheric oscillation indices and shrub annual growth, especially for S. arctica, indicate that AO/NAO indices serve as a potential measure of climatic conditions important for dwarf willow shrubs growth in the High Arctic. Since both AO/NAO indices and temperature are only weakly correlated (Suppl. Fig. S3), such relationships may suggest that not only local air temperature but also regional climatic patterns captured in monthly or seasonal AO/NAO indices may be relevant for scaling shrub radial growth over a regional scale. Variation in precipitation, rain-on-snow events, and cloudiness, are most likely strongly related to pressure differences and thus exhibited in seasonal AO/NAO indices (Putkonen and Roe 2003; Hurrel et al. 2003). Thus, the specific relationships found between shrubs’ growth and AO/NAO indices can be an important predictor of plant growth in remote high arctic areas which lack long-term climatic records (see Weijers et al. 2017).
The strongest pairwise correlation between AO/NAO indices and study sites temperatures was in this study found for negative NAO index and Station Nord (Northern Greenland) in June (r2 = 0.218, df = 43, p = 0.002) (Suppl. Fig. S3). This relationship can be explained by the link between negative NAO index and warm weather associated with a high pressure system, which peaks in June in this section of the Arctic (Overland et al. 2012). This may further justify the negative June NAO signature found in S. arctica chronology (Fig. 3e). However, considering the overall relatively weak correlations between both study site monthly temperatures and regional AO/NAO indices (Suppl. Fig. S3) the significant seasonal relationship revealed between both shrub species and regional climate (reflected in both indices) remains somehow puzzling. One way of a possible inference could link a negative phase of AO/NAO indices with a reduction in cloudiness (Hofer et al. 2017; Lim et al. 2016) and therefore with drier and more favorable for dwarf shrubs growth conditions during short arctic growing season.
Only a few studies have revealed AO/NAO indices fingerprints in Arctic shrub growth, exclusively with Cassiope tetragona (Aanes et al. 2002; Welker et al. 2005; Weijers et al. 2017). Specifically, the latter study conducted in Northern Greenland (Johannes V. Jensen Land, Northern Peary Land) revealed that growth of that species was significantly correlated with June NAO and previous October AO. This is in line with the correlations obtained for S. arctica and AO/NAO indices in this study. Whereas in Svalbard, a negative correlation with summer AO (June–September) index was revealed for Cassiope tetragona growth from Spitsbergen (Aanes et al. 2002) and no correlation with NAO index. In contrast, our study on S. polaris from a nearby location showed no correlation with AO summer index but a negative correlation with NAO June–July index instead. This suggests species-specific responses to climate reflected by AO/NAO indices for the Svalbard region, despite high correspondence between those two indices (Suppl. Fig. S4).
The correlation found between a negative AO index during the dormant period (previous September - previous November, son) and S. arctica growth in Northern Greenland suggests that willow shrub growth might be related to variations in seasonal snow cover, as snow cover at higher latitudes is positively coupled with a negative AO phase (Bamzai 2003; Saito and Cohen 2003; Allen and Zender 2011). This might indicate that growth of S. arctica in one of the northernmost High Arctic site relies on snow cover, which can be explained by the importance of meltwater during the growing season and snow cover insulation during dormancy. Positive relationship between enhanced snow cover and S. arctica growth was found in other High Arctic located in North-Western Greenland (Leffler and Welker 2013), as well for Cassiope tetragona in Svalbard (Blok et al. 2015). We conclude that further exploration is required to gain understanding of these arctic teleconnections, i.e., importance of AO/NAO signatures in the High Arctic dwarf shrubs. The results from two sites in this study are important as these sites represent contrasting environmental conditions across the High Arctic, but current exploration is hampered by a lack of high resolution in situ data (including dormant period) such as precipitation or snow depths at multiple sites across the High Arctic.
Chronology quality
Although we analyzed plants, which were sampled across different habitat types, we obtained relatively high, for dwarf shrub series, inter-series correlation coefficients (r) and a consistent summer temperature signal for both species. Yet, the S. arctica chronology was represented by overall lower descriptive statistic values (Table 1), such as a lower inter-series correlation between shrubs (rbar.bt) and EPS, which most likely resulted from both lower sample depth per study plot and relatively higher distance between the sampling plots than applied for S. polaris shrubs in central Spitsbergen. Inter-series correlation between individual S. arctica shrubs is likely to increase when a higher number of shrubs is sampled per study.
Missing rings are a common phenomenon in arctic shrubs and might prevent successful cross-dating of shrubs growing under harsh conditions (Woodcock and Bradley 1994; Zalatan and Gajewski 2006). The relative low number of shrubs studied from both sites in our study might potentially increase the risk of not detecting missing rings. However, we believe that missing rings were successfully detected through the application of serial sectioning. The inclusion of shrubs in our study from different micro-sites, both in terms of geographical location and environmental settings, most likely increased the chance of missing rings detection, which occurrence might be highly site-specific. In addition, the significant relations with summer temperatures obtained in our study strengthens the notion that the elimination of missing rings for our study period has been fulfilled. Our study thus reaffirms that i) only a careful study of annual growth rings performed on dyed and complete thin-sections, followed by ii) thorough cross-dating within and between the shrubs, enables reliable, i.e. calendar dating of High Arctic willow shrub rings and construction of ring-width chronologies.
Summary and perspectives
This study presents the first comparison of willow dwarf shrubs radial growth chronologies from two remote High Arctic sites. Despite the differences in local climates, both studied species, i.e., the northernmost S. arctica ring width chronology constructed for Peary Land in Northern Greenland (82°N) and S. polaris from central Svalbard (78°N), showed similarity and consistency in growth responses to summer temperatures. Moreover, both species highlighted significant relationships with large-scale atmospheric circulation patterns, such as AO/NAO indices at both locations.
Through the application of shrub sampling across a relatively large area, supplemented by serial sectioning and careful detection of locally and completely missing rings, we were able to successfully cross-date relatively old shrubs from different micro-sites in two contrasting sites in the High Arctic. We suggest that extension of the chronology lengths and associated climatic signals is possible through a more extensive sampling campaign in the northernmost polar deserts, which are lacking high resolution in situ climate data. This is especially true for S. arctica shrubs from Northern Greenland, which, despite more extreme climatic conditions, expressed more advanced ages than shrubs from central Spitsbergen.
References
Aanes R, Sæther B-E, Smith FM, Cooper EJ, Wookey PA, Øritsland NA (2002) The Arctic oscillation predicts effects of climate change in two trophic levels in a high-arctic ecosystem. Ecol Lett 5:445–453
Ackerman D, Griffin D, Hobbie SE, Finlay JC (2017) Arctic shrub growth trajectories differ across soil moisture levels. Glob Chang Biol 23(10):4294–4302
Allen RJ, Zender CS (2011) Forcing of the Arctic oscillation by Eurasian snow cover. J Clim 24:6528–6539
Bamzai AS (2003) Relationship between snow cover variability and Arctic Oscillation index on a hierarchy of time scales. Int J Climatol 23:131–142
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate – a practical and powerful approach to multiple testing. J R Stat Soc Ser B Methodol 57:289–300
Bennike O (1987) Quaternary geology and biology of the Jørgen Brøndlund Fjord area. Northern Greenland Meddr Grønland Geosci 18:6–15
Beschel R, Webb D (1963) Growth ring studies on Arctic willows. Axel Heiberg Island: preliminary report, Montreal, pp 189–198
Bjorkman AD, Elmendorf SC, Beamish AL, Vellend M, Henry GHR (2015) Contrasting effects of warming and increased snowfall on Arctic tundra plant phenology over the past two decades. Glob Chang Biol 21:4651–4661. https://doi.org/10.1111/gcb.13051
Bjorkman AD, Myers-Smith IH, Elmendorf SC, Normand S, Rüger N, Beck PSA, Blach-Overgaard A, Blok D, Cornelissen JHC, Forbes BC, Georges D, Goetz SJ, Guay KC, Henry GHR, HilleRisLambers J, Hollister RD, Karger DN, Kattge J, Manning P, Prevéy JS, Rixen C, Schaepman-Strub G, Thomas HJD, Vellend M, Wilmking M, Wipf S, Carbognani M, Hermanutz L, Lévesque E, Molau U, Petraglia A, Soudzilovskaia NA, Spasojevic MJ, Tomaselli M, Vowles T, Alatalo JM, Alexander HD, Anadon-Rosell A, Angers-Blondin S, te Beest M, Berner L, Björk RG, Buchwal A, Buras A, Christie K, Cooper EJ, Dullinger S, Elberling B, Eskelinen A, Frei ER, Grau O, Grogan P, Hallinger M, Harper KA, Heijmans MMPD, Hudson J, Hülber K, Iturrate-Garcia M, Iversen CM, Jaroszynska F, Johnstone JF, Jørgensen RH, Kaarlejärvi E, Klady R, Kuleza S, Kulonen A, Lamarque LJ, Lantz T, Little CJ, Speed JDM, Michelsen A, Milbau A, Nabe-Nielsen J, Nielsen SS, Ninot JM, Oberbauer SF, Olofsson J, Onipchenko VG, Rumpf SB, Semenchuk P, Shetti R, Siegwart Collier L, Street LE, Suding KN, Tape KD, Trant A, Treier UA, Tremblay JP, Tremblay M, Venn S, Weijers S, Zamin T, Boulanger-Lapointe N, Gould WA, Hik DS, Hofgaard A, Jónsdóttir IS, Jorgenson J, Klein J, Magnusson B, Tweedie C, Wookey PA, Bahn M, Blonder B, van Bodegom PM, Bond-Lamberty B, Campetella G, Cerabolini BEL, Chapin FS III, Cornwell WK, Craine J, Dainese M, de Vries FT, Díaz S, Enquist BJ, Green W, Milla R, Niinemets Ü, Onoda Y, Ordoñez JC, Ozinga WA, Penuelas J, Poorter H, Poschlod P, Reich PB, Sandel B, Schamp B, Sheremetev S, Weiher E (2018) Plant functional traits change across a warming tundra biome. Nature 562:57–62
Bland JM, Altman DG (1995) Multiple significance tests: the Bonferroni method. Br Med J 310:170. https://doi.org/10.1136/bmj.310.6973.170
Blok D, Sass-Klaassen U, Schaepman-Strub G, Heijmans MMPD, Sauren P, Berendse F (2011) What are the main climate drivers for shrub growth in northeastern Siberian tundra? Biogeosciences 8:1169–1179. https://doi.org/10.5194/bg-8-1169-2011
Blok D, Weijers S, Welker JM, Cooper EJ, Michelsen A, Löffler J, Elberling B (2015) Deepened winter snow increases stem growth and alters stem δ13C and δ15N in evergreen dwarf shrub Cassiope tetragona in high-arctic Svalbard tundra. Environ Res Lett 10:44008. https://doi.org/10.1088/1748-9326/10/4/044008
Briffa KR, Jones PD, Bartholin TS, Eckstein D, Schweingruber FH, Karlén W, Zetterberg P, Eronen M (1992) Fennoscandian summers from AD 500: temperature changes on short and long timescales. Clim Dyn 7:111–119
Briffa KR, Melvin TM (2011) A closer look at regional curve standardization of tree-ring records: justification of the need, a warning of some pitfalls, and suggested improvements in its application. In: Hughes MK, Swetnam T.W., Diaz HF (eds) Dendroclimatology, Springer Netherlands, pp 113–145
Buchwal A, Rachlewicz G, Fonti P, Cherubini P, Gärtner H (2013) Temperture modulates intra-plant growth of Salix polaris from a high Arctic site (Svalbard). Polar Biol 36(9):1305–1318
Bunn AG (2008) A dendrochronology program library in R (dplR). Dendrochronologia 26(2):115–124. https://doi.org/10.1016/j.dendro.2008.01.002
Bunn AG, Korpela M, Biondi F, Campelo F, Mérian P, Qeadan F, Zang C, Buras A, Cecile J, Mudelsee M, Schulz M (2016) dplR: Dendrochronology program library in R. R package version 16.4, https://cran.r-project.org/web/packages/dplR/dplR.pdf
CPC (2016) NOAA Climate Prediction Center (CPC), www.cpc.ncep.noaa.gov. Accessed 5 February 2016
D’Arrigo RD, Cook ER, Mann ME, Jacoby GC (2003) Tree-ring reconstructions of temperature and sea-level pressure variability associated with the warm-season Arctic oscillation since AD 1650. Geophys Res Lett 30(11):1549
Deser C (2000) On the teleconnectivity of the “Arctic oscillation”. Geophys Res Lett 27:779–782
DMI (2018) Danish Meterological Institute, https://www.dmi.dk/groenland/arkiver/vejrarkiv/
DNMI (2018) eKlima, free access to weather- and climate data from Norwegian Meteorological Institute from historical data to real time observation. http://eklima.met.no. Accessed 5 February 2018
Elmendorf SC, Henry GH, Hollister RD, Björk RG, Bjorkman AD, Callaghan TV, Collier LS, Cooper EJ, Cornelissen JH, Day TA, Fosaa AM, Gould WA, Grétarsdóttir J, Harte J, Hermanutz L, Hik DS, Hofgaard A, Jarrad F, Jónsdóttir IS, Keuper F, Klanderud K, Klein JA, Koh S, Kudo G, Lang SI, Loewen V, May JL, Mercado J, Michelsen A, Molau U, Myers-Smith IH, Oberbauer SF, Pieper S, Post E, Rixen C, Robinson CH, Schmidt NM, Shaver GR, Stenström A, Tolvanen A, Totland O, Troxler T, Wahren CH, Webber PJ, Welker JM, Wookey PA (2012) Global assessment of experimental climate warming on tundra vegetation: heterogeneity over space and time. Ecol Lett 15:164–175
Forbes BC, Fauria MM, Zetterberg P (2010) Russian Arctic warming and ‘greening’ are closely tracked by tundra shrub willows. Glob Chang Biol 16:1542–1554. https://doi.org/10.1111/j.1365-2486.2009.02047.x
Gamm CM, Sullivan PF, Buchwal A, Dial RJ, Young AB, Watts DA, Cahoon SMP, Welker JM, Post E (2018) Declining growth of deciduous shrubs in the warming climate of continental western Greenland. J Ecol 106:640–654. https://doi.org/10.1111/1365-2745.12882
Gärtner H, Lucchinetti S, Schweingruber FH (2014) New perspectives for wood anatomical analysis in dendrosciences: the GSL1-microtome. Dendrochronologia 32:47–51
Grissino-Mayer HD (2001) Evaluating crossdating accuracy: a manual and tutorial for the computer program COFECHA. Tree-Ring Res 57:205–221
Haas C, Beckers J, King J, Silis A, Stroeve J, Wilkinson J, Notenboom B, Schweiger A, Hendricks S (2017) Ice and snow thickness variability and change in the high arctic ocean observed by in situ measurements. Geophys Res Lett 44(20):10,462–10,469
Hallinger M, Manthey M, Wilmking M (2010) Establishing a missing link: warm summers and winter snow cover promote shrub expansion into alpine tundra in Scandinavia. New Phytol 186(4):890–899
Hofer S, Tedstone AJ, Fettweis X, Bamber JL (2017) Decreasing cloud cover drives the recent mass loss on the Greenland ice sheet. Sci Adv 3:e1700584
Hollesen J, Buchwal A, Rachlewicz G, Hansen BU, Hansen MO, Stecher O, Elberling B (2015) Winter warming as an important co-driver for Betula nana growth in western Greenland during the past century. Glob Chang Biol 21:2410–2423
Holmen K (1957) The vascular plants of Peary Land, North Greenland. A list of the species found between Victoria Fjord and Danmark Fjord. Dansk Pearyland Ekspedition 1947-50. Leader: Eigill Knuth. Medd Grønland 124(9):1–149
Holmes R (1983) Computer-assisted quality control in tree-ring dating and measurement. Tree-Ring Bull 43:69–78
Hurrell JW (1995) Decadal trends in the North Atlantic oscillation region temperatures and precipitation. Science 269:676–679
Hurrell JW, van Loon H (1997) Decadal variations in climate associated with the North Atlantic oscillation. Clim Chang 36:301–326
Hurrel JW, Kushnir Y, Ottersen G, Visbeck M (2003) An overview of the North Atlantic oscillation. The North Atlantic oscillation - climatic significance and environmental impact. Geophys Monogr 134:1–35
Johnstone JF, Henry GH (1997) Retrospective analysis of growth and reproduction in Cassiope tetragona and relations to climate in the Canadian high Arctic. Arct Alp Res 29:459–469
Jia GJ, Epstein HE, Walker DA (2003) Greening of arctic Alaska, 1981–2001. Geophys Res Lett 30:2067
Jones PD, Lister DH, Osborn TJ, Harpham C, Salmon M, Morice CP (2012) Hemispheric and large-scale land surface air temperature variations: an extensive revision and an update to 2010. J Geophys Res 117:D05127. https://doi.org/10.1029/2011JD017139
Kirtman B, Power SB, Adedoyin JA, Boer GJ, Bojariu R, Camilloni I, Doblas-Reyes FJ, Fiore AM, Kimoto M, Meehl GA, Prather M, Sarr A, Schär C, Sutton R, van Oldenborgh GJ, Vecchi G, Wang HJ (2013) Near-term Climate Change: Projections and Predictability. In: Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgley P (eds) Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change. Cambridge University Press, Cambridge, United Kingdom and New York, NY, USA, pp 953–1028
Kolishchuk V (1990) Dendroclimatological study of prostrate woody plant. In: Cook ER, Kairiukstis LA (eds) Methods of dendrochronology applications in the environmental sciences. Kluwer Academic Publishers, Dordrecht, pp 51–55
Kraus G (1874) Einige Bemerkungen über Alter und Wachsthumsverhältnisse ostgrönländischer Holzgewächse. In: die zweite deutsche Nordpolarfahrt in den jahren 1869 und 1870 unter Führung des Kapitän Karl Koldewey. Zweiter Band. Wissenschaftliche Ergebnisse. F.A. Brockhaus, Lepizig. pp 133–137
Láska K, Witoszowá D, Prošek P (2012) Weather patterns of the coastal zone in Petuniabukta, Central Spitsbergen in the period 2008-2010. Pol Polar Res 33(4):297–318
Leffler AJ, Welker JM (2013) Long-term increases in snow pack elevate leaf N and photosynthesis in Salix arctica: responses to a snow fence experiment in the High Arctic of NW Greenland. Environ Res Lett 8. https://doi.org/10.1088/1748-9326/8/2/025023
Le Moullec M, Buchwal A, Van der Wal R, Sandal L, Hansen BB (2018) Annual ring growth of a widespread high-arctic shrub reflects past fluctuations in community-level plant biomass. J Ecol, 1–16. doi: https://doi.org/10.1111/1365-2745.13036
Lim Y-K, Schubert SD, Nowicki SM, Lee JN, Molod AM, Cullather RI, Zhao B, Velicogna I (2016) Atmospheric summer teleconnections and Greenland ice sheet surface mass variations: insights from MERRA-2. Environ Res Lett 11:024002
Macias-Fauria M, Forbes BC, Zetterberg P, Kumpula T (2012) Eurasian Arctic greening reveals teleconnections and the potential for structurally novel ecosystems. Nat Clim Chang 2:613–618
Melvin TM (2004) Historical growth rates and changing climate sensitivity of boreal conifers. PhD Dissertation, University of East Anglia
Metcalfe DB, Hermans TDG, Ahlstrand J, Becker M, Berggren M, Björk RG, Björkman MP, Blok D, Chaudhary N, Chisholm C, Classen AT, Hasselquist NJ, Jonsson M, Kristensen JA, Kumordzi BB, Lee H, Mayor JR, Prevéy J, Pantazatou K, Rousk J, Sponseller RA, Sundqvist MK, Tang J, Uddling J, Wallin G, Zhang W, Ahlström A, Tenenbaum DE, Abdi AM (2018) Patchy field sampling biases understanding of climate change impacts across the Arctic. Nature Ecology and Evolution 2:1443–1448
Myers-Smith IH et al (2011) Shrub expansion in tundra ecosystems: dynamics, impacts and research priorities. Environ Res Lett 6:45509
Myers-Smith IH, Elmendorf S, Beck P, Wilmking M, Hallinger M, Blok D, Tape KD, Rayback SA, Macias-Fauria M, Forbes BC, Speed JDM, Boulanger-Lapointe N, Rixen C, Lévesque E, Schmidt NM, Baittinger C, Trant A, Hermanutz L, Siegwart Collier L, Dawes M, Lantz T, Weijers S, Jørgensen RH, Buchwal A, Buras A, Naito A, Ravolainen V, Schaepman-Strub G, Wheeler J, Wipf S, Hik D, Guay K, Vellend M (2015) Climate sensitivity of shrub growth across the tundra biome. Nat Clim Chang 5(9):887–891
Myneni RB, Keeling CD, Tucker CJ, Asrar G, Nemani RR (1997) Increased plant growth in the northern high latitudes from 1981 to 1991. Nature 386:698–702
Nordklim dataset (2001) 1.0 - description and illustrations Norwegian meteorological institute, 08/01 KLIMA
Osborn TJ, Jones PD (2014) The CRUTEM4 land-surface air temperature dataset: construction, previous versions and dissemination via Google Earth. Earth Syst Sci Data 6:61–68. https://doi.org/10.5194/essd-6-61-2014
Owczarek P, Opała M (2016) Dendrochronology and extreme pointer years in the tree-ring record (AD 1951-2011) of polar willow from southwestern Spitsbergen (Svalbard, Norway). Geochronometria 43:84–95. https://doi.org/10.1515/geochr-2015-0035
Overland JE, Francis JA, Hanna E, Wang M (2012) The recent shift in early summer Arctic atmospheric circulation. Geophys Res Lett 39:L19804. https://doi.org/10.1029/2012GL053268
Pearson RG, Phillips SJ, Loranty MM, Beck PSA, Damoulas T, Knight AJ, Goetz SJ (2013) Shifts in Arctic vegetation and associated feedbacks under climate change. Nat Clim Chang 3:673–677
Polunin N (1955) Attempted dendrochronological dating of ice island T-3. Science 122:1184–1186. https://doi.org/10.1126/science.122.3181.1184
Polunin N (1958) The botany of ice-island T-3. J Ecol 46:323–347
Prevéy J, Vellend M, Rüger N, Hollister RD, Bjorkman AD, Myers-Smith IH, Elmendorf SC, Clark K, Cooper EJ, Elberling B, Fosaa AM, Henry GHR, Høye TT, Jónsdóttir IS, Klanderud K, Lévesque E, Mauritz M, Molau U, Natali SM, Oberbauer S, Panchen ZA, Post E, Rumpf SB, Schmidt NM, Schuur EAG, Semenchuk PR, Troxler T, Welker JM, Rixen C (2017) Greater temperature sensitivity of plant phenology at colder sites: implications for convergence across northern latitudes. Glob Chang Biol 23:2660–2671. https://doi.org/10.1111/gcb.13619
Putkonen J, Roe G (2003) Rain-on-snow events impact soil temperatures and affect ungulate survival. Geophys Res Lett 30(4):1188
Rachlewicz G, Styszyńska A (2007) Porównanie przebiegu temperatury powietrza w Petuniabukta i Svalbard-Lufthavn (Isfjord, West Spitsbergen) w latach 2001-2003. Problemy Klimatologii Polarnej 17:121–134
Rayback SA, Henry GH, Lini A (2012) Multiproxy reconstructions of climate for three sites in the Canadian high Arctic using Cassiope tetragona. Clim Chang 114:593–619
Ropars P, Lévesque E, Boudreau S (2015) How do climate and topography influence the greening of the forest-tundra ecotone in northern Québec? A dendrochronological analysis of Betula glandulosa. Glob Chang Biol 103:679–690
Saito K, Cohen J (2003) The potential role of snow cover in forcing interannual variability of the major northern hemisphere mode. Geophys Res Lett 30(6):1302. https://doi.org/10.1029/2002GL016341
Savile DBO (1979) Ring counts in Salix arctica from northern Ellesmere Island. Can Field Nat 93(1):81–82
Schmidt NM, Baittinger C, Forchhammer MC (2006) Reconstructing century-long snow regimes using estimates of high arctic Salix arctica radial growth. Arct Antarct Alp Res 38:257–262
Schmidt NM, Baittinger C, Kollmann J, Forchhammer MC (2010) Consistent dendrochronological response of the dioecious Salix arctica to variation in local snow precipitation across gender and vegetation types. Arct Antarct Alp Res 42(4):471–475. https://doi.org/10.1657/1938-4246-42.4.471
Schweingruber FH, Poschlod P (2005) Growth rings in herbs and shrubs: life span, age determination and stem anatomy. For Snow Landsc Res 79:195–415
Schweingruber FH, Hellmann L, Tegel W, Braun S, Nievergelt D, Büntgen U (2013) Evaluating the wood anatomical and dendroecological potential of arctic dwarf shrub communities. IAWA J 34(4):485–497
Sullivan PF, Pattison RR, Brownlee AH, Cahoon SMP, Hollingsworth TN (2016) Effect of tree-ring detrending method on apparent growth trends of black and white spruce in interior Alaska. Environ Res Lett 11:114007
Tape K, Hallinger M, Welker JM, Ruess R (2012) Landscape heterogeneity of shrub expansion in Arctic Alaska. Ecosystems 15(5):711–724
Thompson DW, Wallace JM (1998) The Arctic oscillation signature in the wintertime geopotential height and temperature fields. Geophys Res Lett 25:1297–1300
Walker MD, Wahren CH, Hollister RD, Henry GHR, Ahlquist LE, Alatalo JM, Bret-Harte S, Calef MP, Callaghan TV, Carroll AB, Epstein HE, Jónsdóttir IS, Klein JA, Magnússon B, Molau U, Oberbauer SF, Rewa SP, Robinson CH, Shaver GR, Suding KN, Thompson CC, Tolvanen A, Totland Ø, Turner PL, Tweedie CE, Webber PJ, Wookey PA (2006) Plant community responses to experimental warming across the tundra biome. Proc Natl Acad Sci U S A 103:1342–1346
Walker DA, Bhatt US, Epstein HE, Bieniek P, Comiso J, Frost GV, Pinzon J, Raynolds MK, Tucker CJ (2012) Changing Arctic tundra vegetation biomass and greenness. Bull Am Meteorol Soc 93:138–139
Warren Wilson J (1964) Annual growth of Salix arctica in the High Arctic. Ann Bot 28:71–76
Weijers S, Broekman R, Rozema J (2010) Dendrochronology in the High Arctic: July air temperatures reconstructed from annual shoot length growth of the circumarctic dwarf shrub Cassiope tetragona. Quat Sci Rev 29(27–28):3831–3842. https://doi.org/10.1016/j.quascirev.2010.09.003
Weijers S, Alsos IG, Eidesen PB, Broekman R, Loonen MJJE, Rozema J (2012) No divergence in Cassiope tetragona: persistence of growth response along latitudinal temperature gradient and under multi-year experimental warming. Ann Bot 110:653–666
Weijers S, Buchwal A, Blok D, Löffler J, Elberling B (2017) High Arctic summer warming tracked by increased Cassiope tetragona growth in the world’s northernmost polar desert. Glob Chang Biol 23(11):5006–5020. https://doi.org/10.1111/gcb.1374
Weijers S, Pape R, Löffler J, Myers-Smith I (2018) Contrasting shrub species respond to early summer temperatures leading to correspondence of shrub growth patterns. Environ Res Lett 13:34005. https://doi.org/10.1088/1748-9326/aaa5b8
Welker JM, Rayback SA, Henry GHR (2005) Arctic and North Atlantic oscillation phase changes are recorded in the isotopes (δ18O & δ13C) of Cassiope tetragona plants. Glob Chang Biol 11:997–1002
Wilmking M, Hallinger M, van Bogaert R, Kyncl T, Babst F, Hahne W, Juday GP, de Luis M, Novak K, Völlm C (2012) Continuously missing outer rings in woody plants at their distributional margins. Dendrochronologia 30(3):213–222. https://doi.org/10.1016/j.dendro.2011.10.001
Woodcock H, Bradley R (1994) Salix arctica (Pall.): its potential for dendroclimatological studies in the High Arctic. Dendrochronologia 12:11–22
Young AB, Watts DA, Taylor AH, Post E (2016) Species and site differences influence climate-shrub growth responses in West Greenland. Dendrochronologia 37:69–78
Zalatan R, Gajewski K (2006) Dendrochronological potential of Salix alaxensis from the Kuujjua river area, Western Canadian Arctic. Tree-Ring Res 62(2):75–82
Acknowledgements
We thank two anonymous reviewers for their constructive comments and suggestions. We thank John Cappelen (Danish Meteorological Institute) for assistance with Station North climate data.
Funding
Field work in Greenland (BE) was funded by a grant from the Danish National Research Foundation (CENPERM DNRF100) and the field work in central Spitsbergen (AB) was funded by Polish Ministry of Science and Higher Education (grant number N N306 009139). Additional funding (AB) was received from Scientific Exchange Program Sciex (grant number 09.045).
Author information
Authors and Affiliations
Contributions
BE conceived the study and collected the Salix arctica samples, AB collected Salix polaris samples, constructed growth rings chronologies for both Salix species, and analyzed the data, AB and BE wrote the manuscript with input from all co-authors.
Corresponding authors
Electronic supplementary material
ESM 1
(DOCX 3878 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Buchwal, A., Weijers, S., Blok, D. et al. Temperature sensitivity of willow dwarf shrub growth from two distinct High Arctic sites. Int J Biometeorol 63, 167–181 (2019). https://doi.org/10.1007/s00484-018-1648-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00484-018-1648-6