Abstract
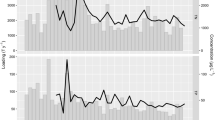
Spring algal development in deep temperate lakes is thought to be strongly influenced by surface irradiance, vertical mixing and temperature, all of which are expected to be altered by climate change. Based on long-term data from Lake Constance, we investigated the individual and combined effects of these variables on algal dynamics using descriptive statistics, multiple regression models and a process-oriented dynamic simulation model. The latter considered edible and less-edible algae and was forced by observed or anticipated irradiance, temperature and vertical mixing intensity. Unexpectedly, irradiance often dominated algal net growth rather than vertical mixing for the following reason: algal dynamics depended on algal net losses from the euphotic layer to larger depth due to vertical mixing. These losses strongly depended on the vertical algal gradient which, in turn, was determined by the mixing intensity during the previous days, thereby introducing a memory effect. This observation implied that during intense mixing that had already reduced the vertical algal gradient, net losses due to mixing were small. Consequently, even in deep Lake Constance, the reduction in primary production due to low light was often more influential than the net losses due to mixing. In the regression model, the dynamics of small, fast-growing algae was best explained by vertical mixing intensity and global irradiance, whereas those of larger algae were best explained by their biomass 1 week earlier. The simulation model additionally revealed that even in late winter grazing may represent an important loss factor during calm periods when losses due to mixing are small. The importance of losses by mixing and grazing changed rapidly as it depended on the variable mixing intensity. Higher temperature, lower global irradiance and enhanced mixing generated lower algal biomass and primary production in the dynamic simulation model. This suggests that potential consequences of climate change may partly counteract each other.









Similar content being viewed by others
References
Baretta JW, Ebenhöh W, Ruardij P (1995) The European-Regional-Seas-ecosystem-model, a complex marine ecosystem model. Neth Inst Sea Res 33:233–246
Bäuerle E, Gaedke U (1998) Lake Constance – characterization of an ecosystem in transition. In: Lampert W (ed) Archiv für hydrobiologie – advances in limnology, vol 53. E. Schweizbart’sche Verlagsbuchhandlung, Stuttgart
Bäuerle E, Ollinger D, Ilmberger J (1998) Some meteorological, hydrological and hydrodynamical aspects of Upper Lake Constance. Arch Hydrobiol Spec Issues Adv Limnol 53:31–83
Diehl S (2002) Phytoplankton, light, and nutrients in a gradient of mixing depths: theory. Ecology 83:386–398
Eilertsen HC (1993) Spring blooms and stratification. Nature 363:24
Erga SR, Heimdal BR (1984) Ecological-studies on the phytoplankton of Korsfjorden, Western Norway – the dynamics of a spring bloom seen in relation to hydrographical conditions and light regime. J Plankton Res 6:67–90
Gaedke U (1998a) Functional and taxonomical properties of the phytoplankton community of large and deep Lake Constance: interannual variability and response to re-oligotrophication (1979–1993). Arch Hydrobiol Spec Issues Adv Limnol 53:119–141
Gaedke U (1998b) The response of the pelagic food web to re-oligotrophication of a large and deep lake (L. Constance): evidence for scale-dependent hierarchical patterns? Arch Hydrobiol Spec Issues Adv Limnol 53:317–333
Gaedke U, Ollinger D, Bäuerle E, Straile D (1998a) The impact of interannual variability in hydrodynamic conditions on the plankton development in Lake Constance in spring and summer. Arch Hydrobiol Spec Issues Adv Limnol 53:565–585
Gaedke U, Ollinger D, Kirner P, Bäuerle E (1998b) The influence of weather conditions on the seasonal plankton development in a large and deep lake (L. Constance) − III. The impact of water column stability on spring algal development. In: George DG, Jones JG, Puncochár P, Reynolds CS, Sutcliffe DW (eds) Management of lakes and reservoirs during global climate change. Kluwer, Dordrecht, pp 71–84
Gaedke U, Hochstädter S, Straile D (2002) Interplay between energy limitation and nutritional deficiency: Empirical data and food web models. Ecol Monogr 72:251–270
Geider RJ (1992) Respiration: taxation without representation? In: Falkowski PG, Woodhead AD (eds) Primary productivity and biogeochemical cycles in the sea. Plenum Press, New York, pp 333–360
George DG, Hewitt DP (1999) The influence of year-to-year variations in winter weather on the dynamics of Daphnia and Eudiaptomus in Esthwaite Water. Cumbria Funct Ecol 13:45–54
Giorgi F, Bi XQ, Pal J (2004) Mean, interannual variability and trends in a regional climate change experiment over Europe. II: climate change scenarios (2071–2100). Climate Dynam 23:839–858
Güde H, Gries T (1998) Phosphorus fluxes in Lake Constance. Arch Hydrobiol Spec issues Adv Limnol 53:505–544
Hancke K, Glud RN (2004) Temperature effects on respiration and photosynthesis in three diatom-dominated benthic communities. Aquat Microbiol Ecol 37:265–281
Häse C, Gaedke U, Seifried A, Beese B, Tilzer MM (1998) Phytoplankton response to re-oligotrophication in large and deep Lake Constance: photosynthetic rates and chlorophyll concentrations. Arch Hydrobiol Spec Issues Adv Limnol 53:159–178
Hawes I (1990) The effects of light and temperature on photosynthate partitioning in antarctic fresh-water phytoplankton. J Plankton Res 12:513–518
Huisman J, van Oostveen P, Weissing FJ (1999a) Critical depth and critical turbulence: two different mechanisms for the development of phytoplankton blooms. Limnol Oceanogr 44:1781–1787
Huisman J, van Oostveen P, Weissing FJ (1999b) Species dynamics in phytoplankton blooms: incomplete mixing and competition for light. Am Nat 154:46–68
IPCC (2001) Climate change 2001: Impacts, adaptation, and vulnerability. In: McCarthy JJ, Canziano OF, Leary NA, Dokken DJ, White KS (eds) Contribution of working group II to the third assessment report of IPCC. Cambridge University Press, Cambridge
Knisely K, Geller W (1986) Selective feeding of 4 zooplankton species on natural lake phytoplankton. Oecologia 69:86–94
Kotzur S (2003) Ein pelagisches ökosystem-modell zur analyse von mesokosmos-experimenten. PhD thesis/dissertation, University of Oldenburg, Oldenburg, Germany
Leckebusch GC, Ulbrich U (2004) On the relationship between cyclones and extreme windstorm events over Europe under climate change. Glob Planet Change 44:181–193
Lehman JT (2002) Mixing patterns and plankton biomass of the St. Lawrence Great Lakes under climate change scenarios. J Great Lakes Res 28:583–596
Montagnes DJS, Franklin DJ (2001) Effect of temperature on diatom volume, growth rate, and carbon and nitrogen content: Reconsidering some paradigms. Limnol Oceanogr 46:2008–2018
Müller H (1989) The relative importance of different ciliate taxa in the pelagic food web of Lake Constance. Microbiol Ecol 18:261–273
Müller H, Schöne A, Pinto-Coelho RM, Schweizer A, Weisse T (1991) Seasonal succession of ciliates in Lake Constance. Microb Ecol 21:119–138
Müller-Navarra DC, Güss S, VonStorch H (1997) Interannual variability of seasonal succession events in a temperate lake and its relation to temperature variability. Glob Change Biol 3:429–438
Neale PJ, Talling JF, Heaney SI, Reynolds CS, Lund JWG (1991) Long-time series from the English Lake district – irradiance-dependent phytoplankton dynamics during the spring maximum. Limnol Oceanog 36:751–760
Ollinger D, Bäuerle E (1998) The influence of weather conditions on the seasonal plankton development in a large and deep lake (L. Constance). II. Water column stability derived from one-dimensional hydrodynamical models. In: George DG, Jones JG, Puncochár P, Reynolds CS, Sutcliffe DW (eds) Management of lakes and reservoirs during global climate change. Kluwer, Dordrecht, pp 57–70
Ptacnik R, Diehl S, Berger S (2003) Performance of sinking and nonsinking phytoplankton taxa in a gradient of mixing depths. Limnol Oceanogr 48:1903–1912
Quigg A, Beardall J (2003) Protein turnover in relation to maintenance metabolism at low photon flux in two marine microalgae. Plant Cell Environ 26:693–703
Ragueneau O, Queguiner B, Treguer P (1996) Contrast in biological responses to tidally-induced vertical mixing for two macrotidal ecosystems of Western Europe. Estuar Coast Shelf Sci 42:645–665
Reynolds CS (1988) Functional morphology and the adaptive strategies of freshwater phytoplankton. In: Sandgren CD (ed) Growth and reproductive strategies of freshwater phytoplankton. Cambridge University Press, Cambridge, pp 388–433
Reynolds CS (1997) Vegetation processes in the pelagic: a model for ecosystem theory. Ecology Institute, Oldendorf/Luhe
SAS OnlineDoc (1999) Ref type: computer program, ver. 8. SAS Institute, Cary, N.C.
Scheffer M, Straile D, van Nes EH, Hosper H (2001) Climatic warming causes regime shifts in lake food webs. Limnol Oceanogr 46:1780–1783
Sicko-Goad L, Stoermer EF, Fahnenstiel G (1986) Rejuvenation of Melosira-Granulata (Bacillariophyceae) resting cells from the anoxic sediments of Douglas Lake, Michigan. 1. Light-microscopy and C-14 uptake. J Phycol 22:22–28
Sommer U, Gliwicz ZM, Lampert W, Duncan A (1986) The PEG-model of seasonal succession of planktonic events in fresh waters. Arch Hydrobiol 106:433–471
Steele JH (1962) Environmental control of photosynthesis in the sea. Limnol Oceanogr 7:137–150
Straile D (2000) Meteorological forcing of plankton dynamics in a large and deep continental European lake. Oecologia 122:44–50
Straile D, Geller W (1998) Crustacean zooplankton in Lake Constance from 1920 to 1995: response to eutrophication and re-oligotrophication. Arch Hydrobiol Spec Issues Adv Limnol 53:255–274
Straile D, Jöhnk K, Rossknecht H (2003) Complex effects of winter warming on the physicochemical characteristics of a deep lake. Limnol Oceanogr 48:1432–1438
Sverdrup HU (1953) On conditions for the vernal blooming of phytoplankton. J Conserv Explor Mer 18:287–295
Tian RC, Deibel D, Thompson RJ, Rivkin RB (2003) Modeling of climate forcing on a cold-ocean ecosystem, Conception Bay, Newfoundland. Mar Ecol Prog Ser 262:1–17
Tilzer MM (1984) Estimation of phytoplankton loss rates from daily photosynthetic rates and observed biomass changes in Lake Constance. J Plankton Res 6:309–324
Tilzer MM, Beese B (1988) The seasonal productivity cycle of phytoplankton and controlling factors in Lake Constance. Schweiz Z Hydrol 50:1–39
Tilzer MM, Elbrachter M, Gieskes WW, Beese B (1986) Light-temperature interactions in the control of photosynthesis in antarctic phytoplankton. Polar Biol 5:105–111
Tirok K, Gaedke U (2006) Spring weather determines the relative importance of ciliates, rotifers and crustaceans for the initiation of the clear-water phase in a large, deep lake. J Plankton Res 28:361–373
Townsend DW, Keller MD, Sieracki ME, Ackleson SG (1992) Spring phytoplankton blooms in the absence of vertical water column stratification. Nature 360:59–62
Vasseur DA, McCann KS (2005) A mechanistic approach for modeling temperature-dependent consumer-resource dynamics. Am Nat 166:184–198
Waniek JJ (2003) The role of physical forcing in initiation of spring blooms in the northeast Atlantic. J Mar Syst 39:57–82
Weisse T, Müller H (1998) Planktonic protozoa and the microbial food web in Lake Constance. Arch Hydrobiol Spec Issues Adv Limnol 53:223–254
Winder M, Schindler DE (2004) Climatic effects on the phenology of lake processes. Global Change Biol 10:1844–1856
Acknowledgements
We thank Wolfgang Ebenhöh, Cora Kohlmeier and Stefan Kotzur for assistance with model development; Erich Bäuerle, Veronika Huber and Kai Wirtz for their helpful remarks; David Vasseur for comments and correcting the English. We are grateful to two anonymous referees for detailed and constructive comments. K.T. was funded by the German Research Foundation (DFG) within the priority program 1162 “The impact of climate variability on aquatic ecosystems (AQUASHIFT)” (GA 401/7-1). Data acquisition was, for the most part, performed within the Special Collaborative Program (SFB) 248 “Cycling of Matter in Lake Constance” supported by the German Research Foundation (DFG).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Ulrich Sommer.
Priority program of the German Research Foundation—contribution 1.
Appendix: model equations
Appendix: model equations
Parameters are indicated by \(\widetilde{},\;\hbox{e.g}.,\;\widetilde{r}.\) Their values are provided in Table 2. Variables taken from the time-series are indicated by (t) and are the following: water temperature (°C), T(t); global irradiance (W m−2), Globirad(t); vertical mixing intensity (day−1) in the upper 20 m, mix(t)0–20; deep vertical mixing intensity (day−1), mix(t)0–100 and mix(t)8–100; chlorophyll a concentration (μg Chla l−1) in the euphotic layer, chla(t)0–20, and in the aphotic layer, chla(t)20–100.
The functional response of primary production to light and temperature is written as being dependent on regulating factors. As a general rule, the regulating factors are non-dimensional and are 1 under optimum conditions and tend toward 0 when phytoplankton is in a limiting situation. The following indices were used:
- i::
-
we, le, tot referring to edible (we), less-edible (le) and total phytoplankton (tot), respectively;
- j::
-
20, 100 referring to the euphotic layer (0–20 m) and the aphotic layer (20–100 m), respectively;
- k::
-
A, H referring to autotrophic processes (A) and heterotrophic processes (H), respectively.
Equations referring to method section “Analysis of the impact of deep vertical mixing and global irradiance on algal growth”
Production rate (day−1):
with light regulation factor (eI) (see below).
Net algal losses (day−1):
Deep vertical mixing intensity (day−1):
Vertical algal gradient (measured):
Equations of the primary production module providing eI, the light regulation factor [adopted from Baretta et al. (1995) and Kotzur (2003)]
Primary production of algal group i per day (prod i ), averaged over the water column, is calculated as:
with
- p i (I(z))::
-
production at depth z of algal group i;
- I(z)::
-
photosynthetic active irradiance at depth z;
- I(z) = I(0) × e− κ × z:
-
κ: vertical extinction coefficient (m−1).
Substitution results in
For p i (I) the formulation of Steele (1962) was chosen:
The resulting function of the primary production is:
Integration results in:
Photosynthetic active radiation at the surface (W m−2):
Extinction coefficient (m−1):
Radiation integrated over the water column (W m−2):
Optimum irradiance (W m−2):
Equations to describe algal dynamics
Algae in the euphotic layer: A i,20 (mg C m−3):
Algae in the aphotic layer: A i,100 (mg C m−3):
Production rate (day−1):
Activity dependent respiration rate (day−1):
Activity dependent exudation rate (day−1):
Basal respiration rate (d−1):
Dynamic mortality rate (day−1):
Mimicking grazers with algal dependent growth and first order mortality.
Sedimentation rate (day−1):
It is assumed that sedimentation depends on the mixing intensity (turbulence) within the euphotic layer if the deep vertical mixing intensity is small. Otherwise sedimentation plays no role, as mix(t)0–100 > 0.1 implies high values of mix(t)0–20. During the winter and spring, 50% of the values of mix(t)0–20 fell into the range of 0.05 and 0.43, resulting in a sedimentation rate between 13 and 4% if mix(t)0–100 ≤ 0.1. This is consistent with the sedimentation rates reported by Güde and Gries (1998) and Tilzer (1984) (maximum values 10 and 15%, respectively).
Temperature regulation factor:
Vertical algal gradient (modeled):
Rights and permissions
About this article
Cite this article
Tirok, K., Gaedke, U. The effect of irradiance, vertical mixing and temperature on spring phytoplankton dynamics under climate change: long-term observations and model analysis. Oecologia 150, 625–642 (2007). https://doi.org/10.1007/s00442-006-0547-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-006-0547-4