Abstract
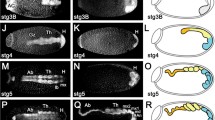
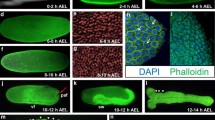
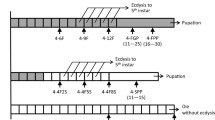
Metamorphosis is one of the most common, yet dramatic of life history strategies. In insects, complete metamorphosis with morphologically distinct larval stages arose from hemimetabolous ancestors that were more direct developing. Over the past century, several ideas have emerged that suggest the holometabolous pupa is developmentally homologous to the embryonic stages of the hemimetabolous ancestor. Other theories consider the pupal stage to be a modification of a hemimetabolous nymph. To address this question, we have isolated an ortholog of the pupal determinant, broad (br), from the hemimetabolous milkweed bug and examined its role during embryonic development. We show that Oncopeltus fasciatus br (Of’br) is expressed in two phases. The first occurs during germ band invagination and segmentation when Of’br is expressed ubiquitously in the embryonic tissues. The second phase of Of’br expression appears during the pronymphal phase of embryogenesis and persists through nymphal differentiation to decline just before hatching. Knock-down of Of’br transcripts results in defects that range from posterior truncations in the least-affected phenotypes to completely fragmented embryonic tissues in the most severe cases. Analysis of the patterning genes engrailed and hunchback reveal loss of segments and a failure in neural differentiation after Of’br depletion. Finally, we show that br is constitutively expressed during embyrogenesis of the ametabolous firebrat, Thermobia domestica. This suggests that br expression is prominent during embryonic development of ametabolous and hemimetabolous insects but was lost with the emergence of the completely metamorphosing insects.







Similar content being viewed by others
References
Bergot BJ, Baker FC, Cerf DC, Jamieson G, Schooley DA (1981) Qualitative and quantitative aspects of juvenile hormone titers in developing embryos of several insect species: discovery of a new JH-like substance extracted from eggs of Manduca sexta. In: Pratt GE, Brooks GT (eds) Juvenile Hormone Biochemistry. Elsevier, Amsterdam, pp 35–45
Buszczak M, Freeman MR, Carlson JR, Bender M, Cooley L, Segraves WA (1999) Ecdysone response genes govern egg chamber development during mid-oogenesis in Drosophila. Development 126:4581–4589
Butt FH (1949) Embryology of the milkweed bug, Oncopeltus fasciatus (Hemiptera). Cornell Experiment Station Memoir 238
DiBello PLR, Withers DA, Bayer CA, Fristrom JW, Guild GM (1991) The Drosophila broad-complex encodes a family of related proteins containing zinc fingers. Genetics 129:385–397
Dorn A (1976) Ultrastructure of embryonic envelopes and integument of Oncopeltus fasciatus Dallas (Insecta, Heteroptera). I. Chorion, amnion, serosa, integument. Zoomorphologie 85:111–113
Dorn A (1983) Hormones during embryogenesis in the milkweed bug, Oncopeltus fasciatus. Entomol Gen 8:193–214
Dorn A, Buhlmann KJ (1982) Exogenous makisterone A accelerates early embryonic development in the milkweed bug Oncopeltus fasciatus. Experientia 38:367–368
Dorn A, Hoffman P (1981) The ‘embryonic molts’ of the milkweed bug as seen by the S.E.M. Tiss Cell 13:461–473
Dorn A, Romer F (1976) Structure and function of prothoracic glands and oenocytes in embryos and last larval instars of Oncopeltus fasciatus Dallas (Insecta, Heteroptera). Cell Tissue Res 171:331–350
Erezyilmaz, D.F. (2004). The genetic and endocrine bases for the origins of insect metamorphosis. PhD thesis, University of Washington, Seattle, WA
Erezyilmaz DF (2006) Imperfect eggs and oviform nymphs: a history of ideas about the origins of insect metamorphosis. Integ Comp Biol 46:795–807
Erezyilmaz DF, Riddiford LM, Truman JW (2006) The pupal specifier broad directs progressive morphogenesis in a direct-developing insect. Proc Nat Acad Sci U S A 103:6925–6930
Erezyilmaz DF, Kelstrup HC, Riddiford LM (2009) The nuclear receptor E75A has a novel pair-rule-like function in patterning the milkweed bug, Oncopeltus fasciatus. Dev Biol 334:300–310
Feldlaufer MF, Svoboda JA (1986) Makisterone A: a 28-carbon insect ecdysteroid. Insect Biochem 16:45–48
Hemming BS (2003) Insect Development and Evolution. Cornell University Press, Ithaca
Hoffmann JA, Lagueux M (1985) Endocrine aspects of embryonic development in insects. In: Kerkut GA, Gilbert LI (eds) Comprehensive Insect Physiology, Biochemistry and Pharmacology, vol 1. Pergamon, Oxford, pp 435–460
Hughes CL, Kaufman TC (2000) RNAi analysis of Deformed, proboscipedia, and Sex combs reduced in the milkweed bug Oncopeltus fasciatus: novel roles for Hox genes in the hemipteran head. Development 127: 3683-3694
Karim FD, Guild GM, Thummel CS (1993) The Drosophila Broad-Complex plays a role in controlling ecdysone-regulated gene expression at the onset of metamorphosis. Development 118:977–988
Konopova B, Jindra M (2008) Broad-complex acts downstream of Met in juvenile hormone signaling to coordinate primitive holometabolan metamorphosis. Development 135:559–568
Konopova B, Zrzavy J (2005) Ultrastructure, development, and homology of insect embryonic cuticles. J Morphol 264:339–362
Lagueux M, Hetru C, Goltzene F, Kappler C, Hoffmann JA (1979) Ecdysone titre and metabolism in relation to cuticulogenesis in embryos of Locusta migratoria. J Insect Physiol 25:709–723
Lanot R, Dorn A, Gunster B, Thiebold J, Lagueux M, Hoffmann JA (1989) Functions of ecdysteroids in oocyte maturation and embryonic development of insects. In: Koolman J (ed) Ecdysone. From Chemistry to Mode of Action. Georg Thieme, Stuttgart, pp 262–269
Liu PZ, Kaufman TC (2004a) hunchback is required for suppression of abdominal identity, and for proper germband growth and segmentation in the intermediate germband insect, Oncopeltus fasciatus. Development 131:1515–1527
Liu PZ, Kaufman TC (2004b) Krüppel is a gap gene in the intermediate germband insect Oncopeltus fasciatus and is required for development of both blastoderm and germband-derived segments. Development 131:4567–4579
Liu PZ, Kaufman TC (2005) even-skipped is not a pair-rule gene but has segmental and gap-like functions in Oncopeltus fasciatus, an intermediate germband insect. Development 132:2081–2092
Novak VJA (1966) Insect Hormones. Methuen, London
Parthasarathy R, Tan A, Bai H, Palli SR (2008) Transcription factor broad suppresses precocious development of adult structures during larval–pupal metamorphosis in the red flour beetle, Tribolium castaneum. Mech Dev 125:299–313
Patel NH, Hayward DC, Lall S, Pirkl NR, DiPietro D, Ball EE (2001) Grasshopper hunchback expression reveals conserved and novel aspects of axis formation and segmentation. Development 128:3459–3472
Riddiford LM (1996) Juvenile hormone: the status of its “status quo” action. Arch Insect Biochem Molec Biol 32:271–286
Suzuki Y, Truman JW, Riddiford LM (2008) The role of Broad in the development of Tribolium castaneum: implications for the evolution of the holometabolous insect pupa. Development 135:569–577
Sbrenna-Micciarelli A, Sbrenna G (1972) The embryonic apolyses of Schistocerca gregaria (Orthoptera). J Insect Physiol 18:1027–1037
Temin G, Zander M, Roussel J-P (1986) Physio-chemical (GC–MS) measurements of juvenile hormone III titres during embryogenesis of Locusta migratoria. Internat J Invert Reprod Dev 9:105–112
Truman JW, Riddiford LM (1999) The origins of insect metamorphosis. Nature 401:447–452
Truman JW, Riddiford LM (2002) Endocrine insights into the evolution of metamorphosis in insects. Ann Rev Entomol 47:467–500
Truman JW, Riddiford LM (2007) The morphostatic actions of juvenile hormone. Insect Biochem Mol Biol 37:761–770
Uhlirova M, Foy BD, Beaty BJ, Olson KE, Riddiford LM, Jindra M (2003) Use of Sindbis virus-mediated RNA interference to demonstrate a conserved role of Broad-Complex in insect metamorphosis. Proc Natl Acad Sci U S A 100:15607–15612
Zhou B, Riddiford LM (2001) Hormonal regulation and patterning of the broad-complex in the epidermis and wing discs of the tobacco hornworm, Manduca sexta. Dev Biol 231:125–137
Zhou X, Riddiford LM (2002) Broad specifies pupal development and mediates the 'status quo' action of juvenile hormone on the pupal–adult transformation in Drosophila and Manduca. Development 129:2259–2269
Zhou B, Hiruma K, Shinoda T, Riddiford LM (1998) Juvenile hormone prevents ecdysteroid-induced expression of Broad Complex RNAs in the epidermis of the tobacco hornworm, Manduca sexta. Dev Biol 203:233–244
Zhou B, Williams DW, Altman J, Riddiford LM, Truman JW (2009) Temporal patterns of Broad isoform expression during the development of neuronal lineages in Drosophila. Neural Dev 4:39
Zollman S, Godt D, Prive GG, Couderc J-L, Laski FA (1994) The BTB domain, found primarily in zinc finger proteins, defines an evolutionarily conserved family that includes several developmentally regulated genes in Drosophila. Proc Natl Acad Sci U S A 91:10717-10721
Acknowledgments
We thank Dr. Paul Liu and Thom Kaufman (Indiana University, Bloomington) for a gift of the digoxigenin-labeled Of’en probe as well as cDNAs for Of’Kr, Of’hb, and Of’eve. We also thank Bilal Ahmad and Shaunna Harris for the initial isolation of the Thermobia broad gene, and Dr. Takashi Koyama for consultation on molecular techniques. This study was supported by NSF grant IBN-9904959 to JWT and NIH grant GM60122 to LMR.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Simpson
Rights and permissions
About this article
Cite this article
Erezyilmaz, D.F., Rynerson, M.R., Truman, J.W. et al. The role of the pupal determinant broad during embryonic development of a direct-developing insect. Dev Genes Evol 219, 535–544 (2009). https://doi.org/10.1007/s00427-009-0315-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00427-009-0315-7