Abstract
Fatigue of single mouse fibers during repeated high-frequency stimulation results initially from decreased Ca2+ sensitivity while free myoplasmic calcium concentration ([Ca2+]m) increases, followed by decreasing [Ca2+]m. Recovery of active force with low-frequency stimulation is slow and persistent fatigue results from low [Ca2+]m. However, the consequences of intermittent submaximal contractions are not known. The aim of the present study was to investigate the changes in [Ca2+]m and active force during intermittent submaximal contractions and subsequent recovery. Single fibers of mouse flexor digitorum brevis muscles at 32 °C were stimulated with 40 or 50 Hz, for 350 ms every 2 s for 2 min and then every 1 s until < 40% of initial force. Values obtained during the intermittent stimulation were compared with a control force-[Ca2+]m relationship. A “P”-shaped pattern in the force-[Ca2+]m relationship was observed during intermittent stimulation. Early in the intermittent stimulation, [Ca2+]m increased while active force decreased. Subsequent force potentiation was accompanied by increased Ca2+ sensitivity. Later, as active force declined, [Ca2+]m decreased significantly (p < 0.001). This was followed, in the final phase, by a significant decrease in Ca2+ sensitivity determined by [Ca2+]m at half-maximal force (Ca50) (p = 0.001). Low-frequency fatigue persisted during recovery while Ca50 was not significantly different from prefatigue (p > 0.5). In conclusion, the main mechanism of fatigue is due to decreases in both [Ca2+]m and Ca2+ sensitivity following the initial force potentiation. The intermittent submaximal contractions resulted in persistent low-frequency fatigue seen during recovery, which was explained by depressed [Ca2+]m with no change in Ca2+ sensitivity.
Similar content being viewed by others
Introduction
The force exerted by a muscle expressed relative to myoplasmic free Ca2+ concentration ([Ca2+]m) gives a sigmoidal relationship [1, 2]. Observations of contractions under different conditions (frequency of stimulation, activity-dependent potentiation, force depression due to prior activation, weakness due to aging or disuse) can effectively be considered on the force-[Ca2+]m relationship to provide clues to the underlying mechanisms effecting change in active force [3]. A change in active force results from one of the following: a change in [Ca2+]m, a change in Ca2+ sensitivity, or a change in maximal force generating capacity [4, 5]. Ca2+ sensitivity can be represented by a change in half-maximal [Ca2+] (Ca50), where a shift of the curve to higher [Ca2+]m represents a decrease in Ca2+ sensitivity [1].
The [Ca2+]m is dependent on the amount of Ca2+ released with each activation and the amount of buffering of Ca2+ that is available [6]. We also know that the [Ca2+]m is dependent on the frequency of activation [7] and that during intermittent stimulation at a fixed high-frequency, there is initially an increase in [Ca2+]m in the early stages and then a decrease in the late stages of fatigue [8]. On the other hand, force decreases when [Ca2+]m increases in the early stages, followed by a simultaneous decrease in both force and [Ca2+]m in the late stages of fatigue. However, the pattern of change in [Ca2+]m is not known for intermittent low-frequency stimulation in mammalian muscles, a situation that is more compatible with in vivo muscle activation.
Activation of muscle for locomotion and general movements typically does not require maximal activation. A common fatigue protocol that takes this into consideration was first used by Burke et al. [9] for the purpose of identifying contractile properties of specific muscle fiber types. Burke and colleagues stimulated the cat gastrocnemius muscle with 40 Hz trains lasting 300 ms at 1-s intervals. When whole muscle is stimulated in situ with this intermittent submaximal low-frequency pattern, there is a characteristic change in active force that typically includes a down, then up, then down change in active force [10]. Considering the multitude of factors that can contribute to increases and decreases in active force, it is of interest to see which factors dominate through this pattern of change in active low-frequency force. We know of several factors that can contribute to enhancement and depression (see below) of low-frequency force in response to intermittent stimulation. Some of these factors will influence the [Ca2+]m and some will affect force by altering Ca2+ sensitivity, but either way, changes to both [Ca2+]m and Ca2+ sensitivity will particularly affect low- as opposed to high-frequency force since contraction resulting from this stimulation lie on the steep portion of the force-[Ca2+]m relationship.
An enhanced contractile response due to previous activity is thought to occur as a result of increased Ca2+ sensitivity [11] but could also result from increased [Ca2+]m. The mechanism of potentiation is generally thought to be dependent on increased Ca2+ sensitivity in association with regulatory light chain (RLC) phosphorylation. This RLC phosphorylation is thought to increase the rate of attachment of cross-bridges due to the increased mobility of the myosin heads [12]. This increase in probability of attachment results in a faster rate of rise in force and higher submaximal active force, at any given [Ca2+]m. This form of potentiation is effective up to a frequency of stimulation corresponding to that which yields 60% of maximal active force [10].
Ca2+ sensitivity is altered by factors that change cross-bridge kinetics, which can alter the number of simultaneous force-generating cross-bridges at submaximal [Ca2+]m. Common mechanisms of change in cross-bridge kinetics affecting Ca2+ sensitivity include temperature, pH, myosin RLC phosphorylation, [Pi], and sarcomere length [13]. A decrease in pH which is often reported to occur during repeated stimulation can contribute to decreased Ca2+ sensitivity, but the level of acidosis observed in single fibers is minor due to lactate transporters and a relatively large extracellular volume [14]. Furthermore, the impact of acidosis in depressing active force is reduced at physiological temperatures, relative to room temperature and lower [15, 16]. Finally, an increase in [Pi] has been suggested to decrease Ca2+ sensitivity, mainly during early fatigue [17]. In late fatigue, increased [Pi] is thought to cause a decrease in Ca2+ release from the sarcoplasmic reticulum (SR). This occurs because Pi can migrate into the SR and when [Pi] is high enough in relation to free [Ca2+] in the SR, a precipitate of Ca-Pi will result, which decreases the amount of Ca2+ available to be released [18].
Considering that potentiation due to RLC phosphorylation should occur early in a series of repeated submaximal contractions [11] and that changes in Ca2+ sensitivity due to acidosis will be attenuated at physiological temperature [16], it was hypothesized that sensitivity to Ca2+ will decrease briefly, then increase and that any fatigue-induced decline in active force will be primarily dependent on decreases in [Ca2+]m. It was also hypothesized that the fibers would fully recover force at high frequencies with persistent reduced force at low frequencies (low-frequency fatigue). Considering that most factors that depress Ca2+ sensitivity will recover within minutes [19, 20], it was hypothesized that persistent depression of active force at low but not high frequencies would be due to decreased [Ca2+]m.
Methods
Animal treatment
Healthy, adult female C57bl/6J (Janvier Labs, France) mice were used for this study. All mice were given standard care. Mice were killed by rapid cervical disarticulation and the whole flexor digitorum brevis (FDB) muscle was dissected to isolate single muscle fibers (see below).
Solutions
Tyrode solution was used during dissection and for superfusion during the experiments [3]. The solution consists of the following (mM): 121 NaCl, 5.0 KCl, 1.8 CaCl2, 0.5 MgCl2, 0.4 NaH2PO4, 24.0 NaHCO3, 0.1 EDTA, 5.5 glucose, and 0.2% fetal calf serum. The solution was bubbled with 5% CO2–95% O2 to keep pH at 7.4. The temperature of the Tyrode solution in the tissue bath was kept at 32 °C throughout the procedures. Indo-1 (#0146, TEFLABS, Austin, TX, USA), a [Ca2+]m indicator, and 5 mM caffeine were also used in the experiments [3].
Dissection and fiber preparation
The FDB muscles from a mouse were dissected down to a single, intact fiber using the method described by Cheng and Westerblad [3]. Tendons were folded into aluminum T-clips, and suspended between two hooks: one affixed to a Kronex Technologies AE801 force transducer and one that allowed length adjustment. The contractile bath was transferred to the stage of an inverted fluorescence microscope (Nikon Instruments, Eclipse Ti-S) with constant flow of Tyrode solution.
The single fiber was set to optimal length, determined with sequential 100 Hz stimulation for 350 ms, and was lengthened or shortened between contractions until the greatest isometric force was obtained. Each fiber was then microinjected with a [Ca2+]m indicator (< 100 μM), Indo-1, which was first dissolved in 150 mM KCl and 10 mM HEPES. Injection was followed by rest for 20–30 min to let the Indo-1 diffuse throughout the fiber. The fiber diameter was recorded as the average measured at both ends of the fiber. Diameter was used to calculate cross-sectional area, assuming a circular fiber. Excitation of Indo-1 was achieved by Xenon light source, passing through a monochromator to selectively illuminate the fiber at 360 nm. Emitted light was filtered and quantified at 405 ± 5 and 495 ± 5 nm with dual photomultiplier tubes (Photon Technology International). A FeliX32 Analysis Module (Photon Technology International) was used for data collection (sampling rate of 500 Hz) and for analysis.
Standard protocol
To establish the control relationship between force and [Ca2+]m, each fiber (n = 12) was stimulated at increasing frequencies (15, 20, 30, 40, 50, 70, 100, 120, 150, 200 Hz) for 350 ms at 1-min intervals. A 5-min recovery period was allowed following the last contraction before the intermittent stimulation was initiated. In some cases, a longer rest interval was allowed because it was noticed that active force for 50 Hz stimulation was depressed relative to the value obtained during the above contractions. This extra rest (up to 30 min) was not sufficient to restore active force. An additional three fibers were tested with prolonged rest (5–10 min) between contractions at a reduced number of frequencies (30, 40 50, 70, 100, and 200 Hz). This was done to evaluate the impact of potentiation or fatigue affecting the control force-[Ca2+]m relationship.
Intermittent contractions were elicited at 2-s intervals at a frequency of either 40 Hz (n = 8) or 50 Hz (n = 4) stimulation for 350 ms, whichever gave an active force closest to 50% of maximum force. After 2 min, the interval was changed to 1 s, if necessary, until force was < 40% of the starting force. Force and fluorescence were continuously recorded and were assessed every 10% of the time course. A 200 Hz stimulus for 350 ms was given immediately afterwards to determine maximal force and corresponding [Ca2+]m.
Recovery started immediately after the 200 Hz contraction. At 5, 15, 25, and 35 min of recovery, the fiber was stimulated at three increasing frequencies (30, 50, 200 Hz), each for 350 ms at 1-min intervals to allow for the periodic assessment of the force-[Ca2+]m relationship during the recovery period. After the last stimulation given at 37 min, some of the fibers (n = 4) were superfused for 2 min with Tyrode solution containing 5 mM of caffeine, then stimulated at 200 Hz to assure maximal activation. After seeing four consecutive fibers producing similar force as when stimulated at 200 Hz at 37 min, the use of caffeine was stopped.
Control protocol
Three fibers (n = 3) were tested as above, except they were allowed to rest for a duration of 3 min during the time the fiber would have been stimulated intermittently (intermittent contractions protocol). At the end of 3 min, the fiber was still stimulated at 200 Hz for 350 ms and recovery was monitored as above. This control protocol allowed the determination of the impact of the contractions during the recovery period on the recovery of active force.
Analysis
Quantification of active force
The force transducer was calibrated with known weights. Active force is presented as force per fiber cross-sectional area (kPa). Peak and passive force was measured for contractions at 10% (time) intervals during intermittent stimulation. Passive force was measured just before each contraction. Active force was calculated as peak force minus passive force. Active force will also be presented as relative force using the control 200 Hz contraction as denominator.
Quantification of [Ca2+]
Free myoplasmic [Ca2+], ([Ca2+]m) was determined by measuring the ratio (R) of fluorescence at the two emission wavelengths (405 and 495 nm) after background subtraction [21]. The ratio, calculated as an average across the plateau of the measured response, was used in the following equation derived by Grynkiewicz and colleagues [22] to calculate [Ca2+]m:
where KD is the apparent dissociation constant of Indo-1 [22]; Rmin is the ratio at very low [Ca2+]m (obtained by injecting 0.5 M EGTA into the fibers to get resting [Ca2+]m); Rmax is the ratio at saturating [Ca2+]m (obtained by injecting 1 mM calcium into the fibers to allow high [Ca2+] m); and ß is estimated (see Eq. (2) with the ratio of the 495 nm signals at low and saturating [Ca2+]m [22]. These values are found using the method described by Bakker and colleagues (1993).
Where m is the slope using linear regression from plotting 495 vs. 405 nm [23].
The Force-[Ca2+]m relationship
Once [Ca2+]m was calculated for contractions at various frequencies, the force-[Ca2+]m relationship can be plotted and Hill’s equation (Eq. (3) can be fit to the data (4):
where F is force, Fmax is the force at saturating [Ca2+]m, Ca50 is [Ca2+]m at 50% of Fmax, and N is a constant to describe steepness of the function. It was assumed that the contraction at 200 Hz gave saturating [Ca2+]m. The force-[Ca2+] curve for each fiber was used to compare changes in [Ca2+]m during fatigue and recovery.
Determination of Ca50
[Ca2+] at half the maximum force (Ca50) was one of the constants yielded by fitting the data to Eq. (3. This value was used as an estimate of the calcium sensitivity. An estimate of Ca50 was obtained at the beginning and end of the intermittent contractions, and at each measurement time during recovery. This estimate used the same Hill coefficient as the control force-[Ca2+] relationship, and Ca50 and Fmax were adjusted to fit through the available data.
Statistics
Data are presented as a mean ± SEM or SD as indicated and compared using one-way repeated measures ANOVA to evaluate the Ca50 values obtained at the following times: first contraction of intermittent stimulation, the end of fatigue, and at 5 and 15 min recovery times. It was noted that Ca50 did not change much after 15 min, so these times were compared without consideration of longer recovery times to reduce the number of comparisons for improved statistical power. The ANOVA was followed by multiple comparisons with the Tukey’s post-hoc analysis to detect significant differences over time. Results were considered significant when p < 0.05.
Results
Fiber characteristics
Average fiber diameter was 32.0 ± 4.4 μm. Maximal isometric force prior to intermittent contractions was 451 ± 60 kPa.
Control force-frequency and force-[Ca2+]
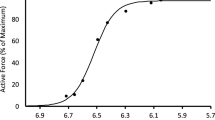
The force-frequency data obtained during the first part of the standard protocol gave a sigmoidal relationship between active force and [Ca2+]m as illustrated in Fig. 1. The steepest part of the slope occurred between 30 and 70 Hz, where there was a substantial increase in force with small increases in frequency and corresponding small increases in [Ca2+]m. Fitting these data to Eq. (3 yielded a Ca50 of 0.304 μM. When we compared this control force-[Ca2+]m curve to the first contraction of the intermittent stimulation protocol that followed, we noted that the first contraction occurred to the right of this control curve and at lower [Ca2+]m. This observation could be a result of potentiation of the original force-frequency data, and/or persistent fatigue resulting from completion of these contractions.
Mean ± SEM force-[Ca2+]m relationship. Relative force with the myoplasmic free [Ca2+] for n = 12 fibers during increasing frequencies (15–200 Hz) stimulated every minute during the initial standard protocol. The line is best fit through the data with half-maximal [Ca2+]m 3.14E-7M. Hill coefficient was 4.1
Initial potentiation caused by standard protocol
Additional experiments (n = 3) were completed to see if the control force-[Ca2+]m relationship reflected fatigue and/or activity-dependent potentiation, explaining the difference in apparent Ca50. Fewer contractions at longer (5–10 min) intervals were obtained (30, 40, 50, 70, 100, and 200 Hz). When this was done, the first contraction of the intermittent contractions was closer to or superimposed on the control force-[Ca2+]m relationship so we concluded that the control force-[Ca2+]m relationship, obtained with 1-min intervals in the original fibers, reflected activity-dependent potentiation. For this reason, further comparisons during and following the intermittent contractions are between the apparent Ca50 for the first contraction of the intermittent contractions. This was assumed to represent the non-potentiated condition. The Ca50 for this contraction was obtained using the same maximum, minimum (fixed at zero), and slope for the control Hill equation. Ca50 was adjusted until the curve went through the value for the first contraction. The first contraction was consistently weaker than the control contraction at the same frequency. We attribute this to long-lasting fatigue resulting from the contractions used to establish the control force-[Ca2+]m relationship.
Intermittent stimulation
It took a fiber on average 77 ± 34 (mean ± SD) contractions to decrease force to 40% of initial force.
Staircase potentiation (phase 1 and 2)
During the time course of intermittent stimulation, expressed as percent of total time, an initial decrease in active force was observed (phase 1), followed by an increase in active force until about 30% into the duration (phase 2). Following this, there was a slow decline, which usually was not sufficient to reach the 40% force target within 2 min. This target was achieved shortly after changing the frequency of contractions to one per second. The initial down then up staircase potentiation is shown in Fig. 2, where force increases on average 27 kPa from the initial force (194 kPa). Not every fiber demonstrated the initial decrease in force when looking at the time course of fatigue in 10% increments. However, in every case of the standard protocol, there was a decrease in active force before the increase in force that typically peaked at ~ 30% of elapsed time.
Time course for relative force during standard intermittent stimulation protocol. Active force typically decreased from the initial value (phase 1), then increased until 30% of time (phase 2). This was followed by decreasing active force (phase 3 and 4). Values are mean ± SEM (n = 12). Missing error bars are within the size of the symbol
Intermittent stimulation-induced fatigue (phase 3 and 4)
Following this peak, force decreased in two phases to 40% of initial force (phase 3 and 4, see below). Raw data for one fiber during the fatigue protocol is shown in Fig. 3, with examples of both force and [Ca2+]m on an expanded time scale at different time points throughout the period of intermittent stimulation.
Time course of active force for one fiber. a Stimulated at 50 Hz every 2 s for 2 min, then every 1 s until force was < 40% of initial force. b Sample contractions on expanded time scale are shown illustrating [Ca2+]m (upper tracing) and force (lower tracing) at start of intermittent stimulation, 40% of duration, 70% of duration, and end of intermittent stimulation where < 40% of initial force is reached, respectively. Horizontal black line corresponds with average resting myoplasmic free [Ca2+] for the first contraction. Horizontal gray line corresponds with average myoplasmic free [Ca2+] during the first contraction (0.3 μM). Vertical calibration bar is 200 kPa. Horizontal calibration bar is 200 ms
Changes in fusion index throughout intermittent stimulations
The fusion index (FUI) was calculated at 10% time increments throughout the time course of intermittent stimulation, to identify a potential mechanism for change in Ca2+ sensitivity. Accelerated relaxation is assumed to result in less summation of force for a given [Ca2+]m, reflecting a decreased Ca2+ sensitivity. Figure 4 shows sample contractions with the close-up of fusion index estimation at peak force for one fiber at three different time points during intermittent stimulation. Although there was a trend for fusion index to decrease by 10% of duration of the intermittent stimulation, then increase, there were no significant differences observed for the fusion index (data not presented).
Sample estimation of fusion index (FUI) during intermittent contractions. The left column shows the force for 350 ms contractions at specific times during repetitive stimulation. The dotted horizontal line indicates peak force and the arrow points at the trough (minimum value) that was used to calculate the fusion index. The right column demonstrates a close-up view of the trough and peak for each contraction and the magnitude of fusion index
A “P” pattern of changes in force and [Ca2+]m
From the first contraction to the end of the intermittent stimulation, the average [Ca2+]m and force appeared to change in a “P”-shaped pattern (Fig. 5). Initially, force decreased while [Ca2+]m increased (phase 1). From here, force increased first without a change in [Ca2+]m then with a corresponding decrease in [Ca2+]m (phase 2). Following the potentiation, force decreased, first in parallel with the initial force-[Ca2+]m relationship (phase 3), then without further change in [Ca2+]m (phase 4). Considering the small change in [Ca2+]m during phase 3, we evaluated the change to see if it reached statistical significance. A paired t test confirmed that this small change in [Ca2+]m from 3.722E-7 ± 5.5E-8M at 30% time to 3.31E-7 ± 4.7E-8M (mean ± SE) at 80% time was significant (p < 0.001). However, there was not a difference in average tetanic [Ca2+]m when comparing the start (3.2E-7M) and the end (3.2E-7M) of the intermittent contractions.
Force-[Ca2+]m relationship during intermittent stimulation. Values during the intermittent stimulation (n = 12) are shown in purple, beginning with the large purple dot. The control force-[Ca2+]m curve was fit through the first contraction and the control 200 Hz contraction, using the same Hill coefficient as the control force-[Ca2+] shown in Fig. 1. The large purple diamond indicates the last contraction of the intermittent stimulation. The black square is the average force-[Ca2+]m value when stimulated at 200 Hz at the end of fatigue. The curve through these two values represents the force-[Ca2+]m relationship at the end of intermittent contractions (Ca50 = 4.65E-7M)
Force-[Ca2+]m curves from start to end of intermittent stimulations
Two force-[Ca2+]m curves have been superimposed on the data in Fig. 5: one to illustrate the initial conditions and one to show how these intermittent contractions have altered the relationship between force and [Ca2+]m by the time the target force had been reached (< 40% of initial force). This latter curve was produced using the same Hill coefficient as the initial curve, but changing Ca50 and the maximal value. This maximal value for active force was obtained by stimulation at 200 Hz as soon as the target force was reached. Half-maximal [Ca2+]m had increased to 3.45E-7M. Resting [Ca2+]m increased throughout the period of intermittent contractions, but recovered within seconds when stimulation was stopped (see Fig. 6). There was no indication that this affected resting force.
Resting myoplasmic free [Ca2+]m. Concentrations shown were measured prior to first and last of the intermittent contractions, the 200 Hz contraction at end of intermittent stimulation and contractions obtained throughout 35-min recovery. Clearly resting [Ca2+]m increased during the intermittent stimulation, but recovered between the end of this stimulation and the 200 Hz contraction (within seconds). Vertical bars represent SEM
Recovery phase
During recovery, force and [Ca2+]m at 30 and 50 Hz exhibited persistent fatigue (see Fig. 7). Force and [Ca2+]m of these contractions continued to increase from 5 to 35 min but did not fully recover to prefatigue force. The 200 Hz force recovered to prefatigue condition by 5 min of recovery with [Ca2+]m increasing throughout the recovery period without further change in maximum force.
Force-[Ca2+]m during recovery. Contractions at 30, 50, and 200 Hz were obtained during recovery at 5, 15, 25, and 35 min. First recovery values were measured at 5–7 min (open circles). The prefatigue force-[Ca2+]m curve (red line) passes through the first contraction of the intermittent stimulation (red circle) for reference. Force and [Ca2+]m tended to increase throughout recovery from 5 to 35 min. Data points represent the mean of n = 12. Horizontal bars represent SEM
Control experiment
The control experiment included the recovery contractions without prior intermittent contractions. The results of this experiment are shown in Fig. 8. It can be seen here that these “recovery” contractions progressively decreased in amplitude, particularly at the low frequencies. These control tests were completed to evaluate the impact of collecting these contractions on our assessment of recovery. These results indicate that the recovery contractions would have slowed the actual recovery following the intermittent contractions. In the experiments with 5–10 min rest between the control contractions at different frequencies, recovery was tested only at 35–37 min. In these experiments, active force at low frequency was still depressed relative to high-frequency force, demonstrating that persistent low-frequency fatigue was not simply a consequence of regular monitoring of contractions during the recovery period.
Force-[Ca2+]m relationship during recovery without prior intermittent stimulation. Relative active force and [Ca2+]m at 5, 15, 25, and 35 min representing recovery (open circles) without prior intermittent stimulation. Values are compared to control force-[Ca2+]m relationship (red line) as in Figs. 5 and 7. Circles from small to large demonstrate values from 5 to 35 min. Data points presented as the mean of n = 3
Changes in Ca50 from prefatigue until end of recovery
The Ca50 was compared across four times: prefatigue, postfatigue, 5 min of recovery, and 15 min of recovery. As seen in Fig. 9, a significant effect was seen (p < 0.001), with post-hoc analysis showing a significant difference of Ca50 between start of fatigue and end of fatigue (p = 0.001). No significant difference was found when comparing Ca50 prefatigue to Ca50 at 5 and 15 min (p > 0.5). Values later in fatigue were not greater than the values observed at 5 min (data not shown).
Ca50 values before and after intermittent contractions. The average ± SEM Ca50 for prefatigue (through first contraction), end of fatigue (last contraction), 5 min recovery, and 15 min recovery. Asterisk shows significant difference when compared to postfatigue (p < 0.05). Value at 5 min recovery was not different from any other values (n = 12)
Changes in sarcomere length
Sarcomere length was determined (n = 3) at the beginning of the test and again at the end to see if a decrease in force or change in Ca2+ sensitivity occur as a consequence of shortening of sarcomeres which could be a consequence of tendon stretch or repositioning of the aluminum clips. There was only a 0.1 μm shortening in the three fibers from start of test to the end (2.5, 2.9, 2.9 μm to 2.4, 2.8, 2.8 μm respectively). This length change would not be expected to decrease active force in a mouse fiber in which optimal length of active force is achieved at a plateau from 2.4 to 2.8 μm [24].
Discussion
The key observations of this study during fatigue and recovery from submaximal intermittent contractions are (1) [Ca2+]m increased then decreased during intermittent submaximal contractions; (2) Ca50 increased then decreased and finally increased again during intermittent submaximal contractions; (3) depression of force at the end of the intermittent contractions was primarily due to decreased Ca2+ sensitivity, but this recovered quickly and low-frequency fatigue (LFF) persisted into the recovery period due to low [Ca2+]m; and (4) only small changes in Ca50 were observed during the intermittent contractions. Each of these observations will be discussed below.
Changes of force and Ca2+ during early intermittent stimulation (phase 1 and 2)
It was observed that the fibers were seemingly already “fatigued” before the intermittent contraction protocol started. The first measurement of force was lower than the force measured at the same frequency in the prefatigue force-frequency data and the first contraction was to the right of the control force-[Ca2+]m relationship. A potential reason for this lower force is that the measure of force at 40 or 50 Hz in the force-frequency data was actually potentiated from previous stimulations (15, 20, 30 Hz), because the intervals between these contractions were only 1 min. Additional experiments confirmed this theory as the apparent force-[Ca2+]m relationship through the first contraction of the intermittent stimulation was very close to that of the force-frequency data when the force-frequency data were collected with intervals of at least 5 min. However, the active force for the first contraction of the intermittent stimulation was still suppressed relative to that obtained with the 5-min intervals due to lower [Ca2+]m. Apparently, eliciting the contractions for the control force-[Ca2+]m relationship already induces some long-lasting fatigue.
Early in the period of intermittent contractions (0–10% of duration), active force decreased (see Figs. 2 and 3), or remained fairly constant. In association with the initial decline, and before active force increased, there was an increase in [Ca2+]m (evident in Fig. 5). Assuming maximal force has not yet changed, this increase in [Ca2+]m, with no increase in active force must represent a decrease in Ca2+ sensitivity. This increase in [Ca2+]m while Ca2+ sensitivity decreased is similar to the change reported with maximal stimulation [8]. The mechanism of the decreased Ca2+ sensitivity is not known, but could relate to increased [Pi] which has been identified previously as a factor decreasing Ca2+ sensitivity [25, 26]. Research has indicated that acidosis does not contribute to decreased Ca2+ sensitivity when physiological temperatures are used [27]. It is important to realize that an increase in [Ca2+]m observed here is not evidence of increased SR Ca2+ release. Ca2+ is strongly buffered in skeletal muscle. During repeated contractions, these buffers will become progressively occupied. With decreased availability of buffers, less of the released Ca2+ will be bound, so more will remain free, creating a higher free [Ca2+]m.
Between 10 and 30% of total time (phase two), the fibers generally increased in force while [Ca2+]m remained constant then decreased (Fig. 5). A similar down (phase 1) then up (phase 2) staircase response was reported by MacIntosh and Willis [10], with intermittent stimulation at 50, 60, and 70 Hz in rat whole gastrocnemius muscle in situ. It has historically been accepted that the positive response during a staircase of this nature is the result of increased RLC phosphorylation [11], which is known to increase Ca2+ sensitivity. Remarkably, there was no evidence of an increase in Ca2+ sensitivity (shift to the left for the force-[Ca2+]m relationship) beyond the position of the initial contraction during the intermittent stimulation, as would have been expected during the staircase response consistently observed in our experiments. The change in active force during staircase is partly due to an increased Ca2+ sensitivity, so this mechanism would be consistent with RLC phosphorylation, but this change occurs relative to the already decreased Ca2+ sensitivity. This small relative change in Ca2+ sensitivity can only be explained by coexistence of a mechanism in the opposite direction [28]; some factor was decreasing Ca2+ sensitivity while RLC phosphorylation and possibly S-glutathionylation of troponin I [29] increased Ca2+ sensitivity. This combination of factors increasing and decreasing Ca2+ sensitivity has probably contributed to the fact that we observed only minor changes in Ca50 in this study (observation 4 above).
Changes in [Ca2+]m and Ca50 during decline in active force (phase 3 and 4)
From the peak of the staircase response (~ 30% time in Fig. 2), force continually decreased. The decrease in active force occurred initially (phase 3) in parallel with the control force-[Ca2+]m relationship (Fig. 5), indicating that the decline in active force was primarily due to decreasing [Ca2+]m with no change in Ca50. A decrease in [Ca2+]m would require decreased Ca2+ release since increasing occupation of Ca2+ buffers would otherwise result in greater [Ca2+]m if release remained the same. It should be kept in mind that SR Ca2+ release was not actually measured in this study. Dutka and Lamb [30] proposed that the decrease in [Ca2+]m during late fatigue is due, in part, to the combination of reduced [ATP] with increased [Mg2+], inhibiting RyR activation thereby decreasing release of Ca2+. The reduced [ATP] may be a consequence of intramuscular glycogen depletion. Glycogen depletion is also known to cause decreased SR Ca2+ release [31, 32]. Fryer and colleagues [33] proposed another mechanism of reducing Ca2+ release is due to precipitation of Ca2+ and Pi in the SR, reducing the concentration of free Ca2+ available to be released. This mechanism has been supported by others [18]. It is possible that all of these mechanisms were effective, yet [Ca2+]m was not depressed by much because more of the Ca2+ buffers were occupied, allowing a higher free [Ca2+]m than would otherwise be expected.
In spite of possible factors impairing Ca2+ release, the final decrease in active force (phase 4) occurred with no further change in [Ca2+]m, indicating that the decline was due to a decrease in Ca2+ sensitivity, possibly in combination with a decrease in maximal force, which was evident at the end of the period of intermittent contractions (Fig. 5). In this respect, the fatigue observed with submaximal contractions is different from that reported for intermittent high-frequency contractions. Westerblad and Allen [5] observed a late decrease in force that corresponded with a decrease in [Ca2+]m when stimulated with repeated maximal tetani.
[Ca2+]m at the end of the intermittent contractions observed here was not less than that for the first contraction (Fig. 5). Therefore, the final phase of the decrease in active force was entirely due to decreases in Ca2+ sensitivity and/or maximal force. This occurred in spite of the likelihood that RLC phosphorylation and possibly other potentiating factors, would be expected to persist at this time. This observation emphasizes the simultaneous impact of fatigue and potentiation [28]. Apparently, the mechanisms decreasing Ca2+ sensitivity were more effective than the mechanisms increasing Ca2+ sensitivity. Two possible mechanisms that are thought to decrease Ca2+ sensitivity include altered cross-bridge kinetics by increased [Pi] [34] and/or increased production of reactive oxygen-nitrogen species [35, 36]. It should be noted that a decrease in force generating capacity will contribute to an apparent shift to the right in the force-[Ca2+]m relationship. However, this mechanism will not affect Ca2+ sensitivity.
Slowed relaxation
The fusion index of a contraction can be a good indication of the rate of relaxation (Fig. 4). It has been observed that a higher fusion index corresponds with a prolonged half-relaxation time [37]. A slowed relaxation time is typically seen during intermittent contractions leading to fatigue, and contributes to an increase in Ca2+ sensitivity. The mechanism associated with this increase in Ca2+ sensitivity can be due to either the slowing of Ca2+ dissociating from troponin C, or the slowing of detachment of cross-bridges [38]. The lowest FUI on average occurred at 10% of the time course of intermittent contractions and FUI increased from 30 to 80% of the time course of fatigue. However, differences in FUI were not significant. The reported changes in Ca2+ sensitivity during the intermittent contractions were not likely associated with mechanisms altering the FUI.
Resting [Ca2+]m during fatigue
The resting [Ca2+]m during the intermittent stimulation demonstrates a linear relationship over time, increasing [Ca2+]m from start to end of these contractions (Fig. 6). Westerblad and Allen [5] also observed an increase in resting [Ca2+]m throughout their sequence of fatiguing maximal stimulation. This increase can be due to either increased leakage of Ca2+ from the SR or due to slowed Ca2+ re-uptake into the sarcoplasmic reticulum. The subcellular distribution of [Ca2+]m was not measured during this study, preventing a more precise description of the mechanism for this increase in resting [Ca2+]m. It is interesting to note that the increase in resting [Ca2+]m could have caused persistent active force but the decrease in Ca2+ sensitivity prevented this during this period of elevated resting [Ca2+]m. Resting [Ca2+]m returned to the prefatigue level by the time the 200 Hz contraction was elicited.
Ca50 during recovery
After the fibers were fatigued, force and [Ca2+]m were measured periodically during a 35-min period of recovery (Fig. 7). Ca50 values were calculated at early recovery times (5 and 15 min) and compared to the Ca50 from prefatigue and the end of intermittent stimulation (postfatigue). Ca2+ sensitivity had decreased at the end of the intermittent contractions but had recovered such that Ca50 was not different from prefatigue values at both 5 and 15 min of recovery. During intense intermittent stimulation [35], a ROS-related reduction in Ca2+ sensitivity can be observed during recovery since ROS generation increases with increasing contraction intensity [39]. The results of the current study, however, suggest that a ROS-induced alteration in Ca2+ sensitivity following low-frequency contractions was unlikely since Ca50 was not affected during recovery. Thus, lower [Ca2+]m was the primary mechanism for the persistent LFF following low-frequency contractions.
LFF was also observed by Westerblad et al. [2] and Chin and Allen [40] and it was suggested that the persistent fatigue during recovery was caused by decreased [Ca2+]m, not decreased Ca2+ sensitivity [7, 40]. Allen et al. [41] suggested that the mechanism for decreased [Ca2+]m in LFF is due to disrupted excitation-contraction coupling [41]. More specifically, the decrease in [Ca2+]m is likely due to impaired Ca2+ release from the SR as the amount of free [Ca2+]m in the SR should not be affected after a few minutes of recovery. Further research will be required to confirm this possibility.
Some researchers have indicated that increased ROS during repeated stimulation can inhibit the RyR, disrupting the release of Ca2+ and/or that depletion of glycogen can cause decreased Ca2+ release [31, 36, 42,43,44]. Four fibers were stimulated at 200 Hz with added caffeine at the end of testing where maximal force was observed (101 ± 2% of force measured at 200 Hz prefatigue), confirming that the contractile proteins were not disrupted or impaired and the amount of free [Ca2+]m in the SR was not affected. This was also seen by Westerblad et al. [2]. Cheng et al. [31] have shown that recovery from low-frequency fatigue corresponds with glycogen resynthesis and recovery can be arrested by preventing glycogen resynthesis. Figure 7 demonstrates the slow increase in [Ca2+]m at 30, 50, and 200 Hz during the recovery period. In agreement with Westerblad et al. [2], the decrease in force with slightly reduced [Ca2+]m observed throughout recovery indicates that decreased [Ca2+]m was the likely cause of low-frequency fatigue. This is consistent with the absence of change in Ca2+ sensitivity.
Novel findings
Novel findings of this study include identifying that the fatigue-induced changes in force and [Ca2+]m differ during low-frequency compared with high-frequency intermittent stimulation reported previously. The resulting P-shaped change of force and [Ca2+]m has not been observed during high-frequency stimulation probably because low-frequency forces, which lie on the steepest portion of the force-[Ca2+]m relationship, are particularly sensitive to alterations in SR Ca2+ release and myofibrillar Ca2+ sensitivity that can increase as well as decrease force. Another novel observation was that force depression during the recovery period after fatigue induced by low-frequency stimulation was completely explained by impaired SR Ca2+ release, which is different from the consequences of high-frequency stimulation where impaired myofibrillar Ca2+ sensitivity also contributes to the persistent impairment of low-frequency force production. Thus, the mechanisms underlying fatigue and recovery of force generation are clearly task dependent.
Limitations
The measurement of [Ca2+]m during intermittent submaximal contractions and subsequent recovery permits assessment of the primary mechanisms of fatigue. However, the underlying cause of the change in [Ca2+]m and Ca50 has not been determined. The potential impact of changes in Pi concentration and Ca2+ release represent best guesses at these mechanisms. The use of Indo-1 for detection of [Ca2+]m cannot detect quickly changing concentrations, but is suitable for resting and average concentrations during tetanic stimulation [21].
Conclusion
In conclusion, results suggest that the mechanism of fatigue during submaximal intermittent contractions is a combination of changes to both [Ca2+]m and Ca2+ sensitivity. Early in the intermittent contractions, the reduced force is due to decreased Ca2+ sensitivity. This is followed by an increased Ca2+ sensitivity while force potentiates above the original force, creating the loop part of the “P” force-[Ca2+]m pattern. During late fatigue, the decline in force is first due to decreased [Ca2+]m, changing to a decreased Ca2+ sensitivity, both of which create the shaft part of the “P” pattern. During recovery, the persistent depression of low-frequency force was completely explained by decreased [Ca2+]m. Clearly, there are different patterns of change in the relationship between force and Ca2+ during intermittent low-frequency contractions than have been reported for high-frequency contractions.
References
Stephenson DG, Williams DA (1981) Calcium-activated force responses in fast-and slow-twitch skinned muscle fibres of the rat at different temperatures. J Physiol 317:281–302
Westerblad H, Duty S, Allen DG (1993) Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol 75:382–388
Cheng AJ, Westerblad H (2017) Mechanical isolation, and measurement of force and myoplasmic free [Ca2+] in fully intact single skeletal muscle fibers. Nat Protoc 12:1763–1776
Cheng AJ, Place N, Westerblad H (2018) Molecular basis for exercise-induced fatigue: the importance of strictly controlled cellular Ca2+ handling. Cold Spring Harb Perspect Med 8
Westerblad H, Allen DG (1991) Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol 98:615–635
Baylor SM, Hollingworth S (2003) Sarcoplasmic reticulum calcium release compared in slow-twitch and fast-twitch fibres of mouse muscle. J Physiol 551:125–138
Westerblad H, Duty S, Allen DG (1993) Intracellular calcium concentration during low-frequency fatigue in isolated single fibres of mouse skeletal muscle. J Appl Physiol 93:382–388
Westerblad H, Allen DG (1991) Changes of myoplasmic calcium concentrations during fatigue in single mouse muscle fibers. J Gen Physiol 98:615–635
Burke RE, Levine DN, Tsairis P, Zajac FE (1973) Physiological types and histochemical profiles in motor units of the cat gastrocnemius. J Physiol 234:723–748
MacIntosh BR, Willis JC (2000) Force-frequency relationship and potentiation in mammalian skeletal muscle. J Appl Physiol 88:2088–2096
MacIntosh BR, Grange RW, Cory CR, Houston ME (1993) Myosin light chain phosphorylation during staircase in fatigued skeletal muscle. Pflügers Archiv: European J Physiology 425:9–15
Levine RJC, Kensler RW, Yang ZH, Stull JT, Sweeney HL (1996) Myosin light chain phosphorylation affects the structure of rabbit skeletal muscle thick filaments. Biophys J 71:898–907
MacIntosh BR (2003) Role of calcium sensitivity modulation in skeletal muscle performance. News Physiol Sci 18:222–225
Westerblad H, Allen DG (1993) The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. J Physiol 466:611–628
Westerblad H, Allen DG (1992) Changes of intracellular pH due to repetitive stimulation of single fibres from mouse skeletal muscle. J Physiol 449:49–71
Westerblad H, Bruton J, Lännergren J (1997) The effect of intracellular pH on contractile function of intact, single fibres of mouse muscle declines with increasing temperature. J Physiol 500:193–204
Dahlstedt AJ, Katz A, Westerblad H (2001) Role of myoplasmic phosphate in contractile function of skeletal muscle: studies on creatine kinase-deficient mice. J Physiol 533:379–388
Allen DG, Westerblad H (2001) Role of phosphate and calcium stores in muscle fatigue. J Physiol 536:657–665
Harris RC, Edwards RHT, Hultman E, Nordesjî LO, Nylind B, Sahlin K (1976) The time course of phosphorylcreatine resynthesis during recovery of the quadriceps muscle in man. Pflugers Archiv: European J Physiology 367:137–142
Sahlin K, Ren JM (1989) Relationship of contraction capacity to metabolic changes during recovery from a fatiguing contraction. J Appl Physiol 67:648–654
Bruton JD, Cheng AJ, and Westerblad H. Methods to detect Ca2+ in living cells. In: Calcium Signaling, edited by Islam MS. Dordrecht: Springer Netherlands, 2012, p. 27–43
Grynkiewicz G, Poenie M, Tsien RYA (1985) New generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 260:3440–3450
Bakker AJ, Head SI, Williams DA, Stephenson DG (1993) Ca2+ levels in myotubes grown from the skeletal muscle of dystrophic (mdx) and normal mice. J Physiol 460:1–13
Stephenson DG, Williams DA (1982) Effects of sarcomere length on the force-pCa relation in fast- and slow-twitch skinned muscle fibres from the rat. J Physiol 333:637–653
Coupland ME, Puchert E, Ranatunga KW (2001) Temperature dependence of active tension in mammalian (rabbit poses) muscle fibres: effect of inorganic phosphate. J Physiol 536:879–891
Debold EP, Romatowski J, Fitts RH (2006) The depressive effect of Pi on the force-pCa relationship in skinned single muscle fibers is temperature dependent. AJP Cell Physiology 290:C1041–C1050
Pate E, Bhimani M, Franks-Skiba K, Cooke R (1995) Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: implications for fatigue. J Physiol 486:689–694
Rassier DE, MacIntosh BR (2000) Coexistence of potentiation and fatigue in skeletal muscle. Braz J Med Biol Res 33:499–508
Geijl KD, Hvid LG, Willis SJ, Andersson E, Holmberg HC, Jensen R, Frandsen U, Hansen J, Plomgaard P, Ørtenblad N (2015) Repeated high-intensity exercise modulates Ca2+ sensitivity of human skeletal muscle fibers. Scand J Med Sci Sports 26:488–497
Laouris Y, Bevan L, Reinking RM, Stuart DG (2004) Associations between force and fatigue in fast-twitch motor units of a cat hindlimb muscle. Can J Physiol Pharmacol 82:577–588
Cheng AJ, Willis SJ, Zinner C, Chaillou T, Ivarsson N, Ortenblad N, Lanner JT, Holmberg HC, Westerblad H (2017) Post-exercise recovery of contractile function and endurance in humans and mice is accerated by heating and by cooling skeletal muscle. J Physiol 595:7413–7426
Chin ER, Allen DG (1997) Effects of reduced muscle glycogen concentration on force, Ca 2+ release and contractile protein function in intact mouse skeletal muscle. J Physiol 498:17–29
Fryer MW, Owen VJ, Lamb GD, Stephenson DG (1995) Effects of creatine phosphate and P i on Ca 2+ movements and tension development in rat skinned skeletal muscle fibres. J Physiol 482:123–140
Allen DG, Lannergren J, Westerblad H (1995) Muscle cell function during prolonged activity: cellular mechanisms of fatigue. Exp Physiol 80:497–527
Cheng AJ, Bruton JD, Lanner JT, Westerblad H (2015) Antioxidant treatments do not improve force recovery after fatiguing stimulation of mouse skeletal muscle fibres. J Physiol 593:457–472
Cheng AJ, Yamada T, Rassier DE, Andersson DC, Westerblad H, Lanner JT (2016) Reactive oxygen/nitrogen species and contractile function in skeletal muscle during fatigue and recovery. J Physiol 594:5149–5160
Celichowski J, Bichler E (2002) The time course of the last contractions during incompletely fused tetani of motor units in rat skeletal muscle. Acta NeurobiolExp (Wars) 62:7–17
Westerblad H, Lännergren J, Allen DG (1997) Slowed relaxation in fatigued skeletal muscle fibres of Xenopus and mouse. J Gen Physiol 109:385–399
Sakellariou GK, Vasilaki A, Palomero J, Kayani A, Zibrik L, McArdle A, Jackson MJ (2013) Studies of mitochondrial and nonmitochondrial sources implicate nicotinamide adenine dinucleotide phosphate oxidase(s) in the increased skeletal muscle superoxide generation that occurs during contractile activity. Antioxid Redox Signal 18:603–621
Chin ER, Allen DG (1996) The role of elevations in intracellular [Ca 2+] in the development of low frequency fatigue in mouse single muscle fibres. J Physiol 491:813–824
Allen DG, Lamb GD, Westerblad H (2008) Impaired calcium release during fatigue. J Appl Physiol 104:296–305
Cheng AJ, Willis SJ, Zinner C, Chaillou T, Ivarsson N, Ørtenblad N, Lanner JT, Holmberg H, and Westerblad H. Post-exercise recovery of contractile function and endurance in humans and mice is accelerated by heating and slowed by cooling skeletal muscle. J Physiol Accepted Article; doi: 101113/JP274870
Chin ER, Allen DG (1997) Effects of reduced muscle glycogen concentration on force, Ca2+ release and contractile protein function in intact mouse skeletal muscle. J Physiol 498:17–29
Helander I, Westerblad H, Katz A (2002) Effects of glucose on contractile function, [Ca2+]i, and glycogen in isolated mouse skeletal muscle. Am J Phys 282:C1306–C1312
Funding
This project was supported financially with grants from the Natural Sciences and Engineering Research Council of Canada and the Swedish Research Council for Sports Science.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design of the study, data collection, analysis and interpretation. All authors contributed to writing the manuscript. All experiments were conducted at the Cellular Muscle Function Laboratory in the Department of Physiology and Pharmacology at Karolinska Institutet, Stockholm, Sweden.
Corresponding author
Ethics declarations
Ethical approval
All procedures were approved by the University of Calgary Animal Care Committee and by the Stockholm North Ethical Committee on Animal Experiments at Karolinska Institutet.
Competing interests
None.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Glass, L.D., Cheng, A.J. & MacIntosh, B.R. Role of Ca2+ in changing active force during intermittent submaximal stimulation in intact, single mouse muscle fibers. Pflugers Arch - Eur J Physiol 470, 1243–1254 (2018). https://doi.org/10.1007/s00424-018-2143-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00424-018-2143-y