Abstract
Background
In December 2019, unexplained cases of pneumonia emerged in Wuhan, China, which were found to be secondary to the novel coronavirus SARS-CoV-2. On March 11, 2020, the WHO declared the Coronavirus Disease 2019 (COVID-2019) outbreak, a pandemic.
Objective
To clarify the neurological complications of SARS-CoV-2 infection including the potential mechanisms and therapeutic options.
Methods
We conducted a systematic literature search from December 01, 2019 to May 14, 2020 using multiple combinations of keywords from PubMed and Ovid Medline databases according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines. We included articles with cases of COVID-19 where neurological involvement was evident.
Results
We were able to identify 82 cases of COVID-19 with neurological complications. The mean age was 62.3 years. 37.8% of the patients were women (n = 31). 48.8% of the patients (n = 40) had cerebrovascular insults, 28% (n = 23) had neuromuscular disorders, and 23% of the patients (n = 19) had encephalitis or encephalopathy.
Conclusions
Neurological manifestations of COVID-19 are not rare, especially large vessel stroke, Guillain–Barre syndrome, and meningoencephalitis. Moving forward, further studies are needed to clarify the prevalence of the neurological complications of SARS-CoV-2 infection, investigate their biological backgrounds, and test treatment options. Physicians should be cautious not to overlook other neurological diagnoses that can mimic COVID-19 during the pandemic.
Similar content being viewed by others
Introduction
Coronaviruses (CoV) are a family of enveloped, positive-sense, single-stranded RNA viruses that have been described for more than 50 years. Some strains are found to be zoonotic, whereas others may infect humans and transmit from human-to-human (HCoV) [1]. Multiple strains of Coronavirus are associated with human disease, causing mainly respiratory infections [2]. Of these, SARS-CoV-1, MERS-CoV, HCoV-OC43, and HCoV-229E are associated with neurological complications [3]. Large territory stroke, polyneuropathy, myopathy, seizures, and status epilepticus were reported in patients with SARS-CoV-1 outbreak in 2002/2003 [4, 5]. Intranasal inoculation of the SARS-CoV-1 virus to mice transgenic for the human ACE2 protein led to severe neuronal loss at the brainstem and specifically the respiratory center at the medulla. This was postulated to contribute to the respiratory failure seen in the most severe cases of coronavirus infections, in addition to the primary lung pathology [6]. The full spectrum of COVID-19 is not fully described yet. Some patients may have no clinical symptoms [7, 8], while others might suffer from severe disease and even die. The most commonly recognized symptoms are cough, fever, fatigue, shortness of breath, myalgia, anosmia, and sputum production. Other less-reported symptoms include diarrhea, hemoptysis, and nasal congestion [7, 9]. Growing evidence suggests that SARS-CoV-2 virus has neuroinvasive potential, like other coronaviruses. Hence, it was only a matter of time before neurological complications were reported, especially stroke, neuromuscular disorders, and meningoencephalitis. The goal of this review is to outline the broad spectrum of the neurological consequences of SARS-CoV-2 infection.
Methods
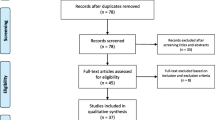
We conducted a systematic literature search from December 01, 2019 to May 14, 2020 from PubMed and Ovid Medline databases according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines [10]. The following search strategy was implemented and these keywords and their synonyms (in the all fields) were combined in each database as follows: (“COVID 19” OR “coronavirus”) AND (“brain” OR “CNS” OR “spinal cord” OR “nerve” OR “neurologic” OR “stroke” OR “cerebrovascular” OR “cerebral vein thrombosis” OR “sinus thrombosis” OR “Intracerebral hemorrhage” OR “hemorrhage” OR “myelitis” OR “GBS” OR “Guillain Barre syndrome” OR “neuropathy” OR “radiculopathy” OR “cranial neuropathy” OR “myopathy” OR “myositis” OR “rhabdomyolysis” OR “encephalitis” OR “encephalopathy” OR “meningitis” OR “meningoencephalitis” OR “seizure” OR “convulsion” OR “epilepsy”) [Fig. 1]. We included case series and case reports of COVID-19 with evident neurological symptoms or signs. After exclusion of duplicates, all articles were evaluated through title and abstract screening by three independent reviewers (M.G., Q.A., and G.M.). The same three reviewers performed an accurate reading of all full-text articles assessed for eligibility and performed a collection of data to minimize the risk of bias. In case of disagreement among the investigators regarding the inclusion and exclusion criteria, the senior investigator (G.M.) made the final decision. Articles were included if they met the following inclusion criteria: (i) described patients with neurological signs or symptoms attributed to COVID-19 (e.g., focal neurological deficit or impairment of consciousness); (ii) written in English language; and (iii) published in a peer-reviewed journal. The exclusion criteria were: (i) studies conducted in animals or in vitro models or basic science studies; (ii) patients age less than 18 years; and (iii) conference proceedings, pooled analysis, clinical trials, case control studies, case reports, or case series of anosmia or mental health problems in COVID-19, reviews, and books. We assessed the quality of the included studies using the JBI Critical Appraisal Tool, as shown in supplementary Tables 1 and 2.[11]
For each study, the following descriptive, microbiological, and clinical information was extracted: patient demographic data, SARS-CoV-2 testing from nasal swab and CSF, neurological symptoms and signs and their onset in relation to respiratory or gastrointestinal (GI) symptoms or anosmia or dysgeusia, any neurological investigations and CSF or any other relevant laboratory testing (such as CK, LDH, CRP, D-dimer, lupus anticoagulant, fibrinogen, ganglioside antibodies), neurological diagnosis, occurrence of respiratory failure (defined as need for intubation, abnormal PO2 in blood gas, or Glasgow Coma Scale score less than or equal 8), treatments administered for the neurological diagnosis, and final outcome. We studied the following outcomes: good, recovering, poor, and deceased. Good outcome was defined as discharge of the patient to home or a quarantine facility, or the use of the following descriptive terms in the study: “no morbidity”, “no worsening” or “discharged well” or “good recovery”. Recovering outcome was defined as discharge of the patient to a rehabilitation facility or use of the following descriptive terms: “began to improve”, “recovering”, or “stayed in the floor”. Poor outcome was defined as continuing deterioration of the patient’s clinical status, need for ICU admission, continued intubation, or use of the following descriptive terms: “poor” or “no improvement after a certain time of treatment”, at the time of submitting the manuscript. Deceased was defined as reported death within 30 days of COVID-19 diagnosis.
Results
Through the search strategy, we could identify 42 articles about neurological involvement by COVID-19. We were able to identify 82 cases of COVID-19 with neurological complications. The mean age was 62.3 years (22–91). 37.8% of the patients were women (n = 31) (Table 1). All but two patients had positive nasopharyngeal (NP) or oropharyngeal (OP) SARS-CoV-2 RT-PCR swabs. Only two patients had positive CSF SARS-CoV-2 RT-PCR, one of which showed negative NP swab testing. 48.8% of patients (n = 40) had cerebrovascular insults (CVIs), 28% (n = 23) had neuromuscular disorders (NMDs), and 23% (n = 19) developed CNS complications related to CNS infection or inflammation. 32.9% of patients (n = 27) were recovering, 18.3% (n = 15) had good outcomes, 25.6% (n = 21) had poor outcomes, and 18.3% (n = 15) died. In 23.2% of patients (n = 19), the neurologic syndrome was the initial presentation of COVID-19, four of which developed respiratory symptoms 2–8 days after the onset of neurologic syndrome. In two patients, the neurologic syndrome was preceded by 2–3 days of GI symptoms, while in two other patients, it was preceded only by anosmia and dysgeusia (Fig. 2).
Cerebrovascular complications
5% (n = 2) of the CVIs were due to cerebral vein thrombosis (CVT), 5% (n = 2) were intracerebral hemorrhages (ICH), 2.5% (n = 1) were aneurysmal subarachnoid hemorrhage and ICH, and 87.5% (n = 35) were ischemic stroke. Three out of 35 patients (9%) had cardioembolic stroke, five had small vessel disease stroke (14%), and 27 (77%) had large vessel occlusion (LVO) stroke. 80% (n = 28) of the ischemic stroke patients had elevated D-dimer levels, 57% (n = 20) had elevated C-reactive protein, 28.5% (n = 10) had elevated fibrinogen, and 14.3% (n = 5) were tested positive for lupus anticoagulant antibodies (Table 2). Out of the 27 LVO stroke patients, seven were under the age of 50, six underwent thrombectomy, four were treated with IV tPA, six with therapeutic low-molecular-weight heparin (LMWH), three with apixaban, four with dual antiplatelets (DAPs), and one with rivaroxaban and DAPs. Six of those patients died. One of the ischemic stroke patients developed hemorrhagic conversion after thrombectomy. The LVO stroke were distributed in the following territories: left middle cerebral artery territory (MCA) (n = 6), right MCA (n = 6), left internal carotid artery (ICA) (n = 2), right ICA (n = 2), left common carotid artery (n = 1), basilar artery (n = 1), left vertebral artery (n = 1), left posterior cerebral artery (PCA) (n = 1), right PCA (n = 1), bilateral multiple vascular territory infarcts (n = 2), and unspecified (n = 4). Stroke with LVO was the presenting manifestation of COVID-19 in eight patients, three of which were under the age of 45 (Table 3).
Neuromuscular complications
17.4% (n = 4) of the NMDs patients had rhabdomyolysis, 4.3% (n = 1) had polyneuritis cranialis, 4.3% (n = 1) had oculomotor nerve palsy, and 73.9% (n = 17) had Guillain–Barre syndrome (GBS). Seven out of the seventeen GBS cases had facial weakness, one of which manifested with isolated facial diplegia. In two cases, GBS was the first presentation of SARS-CoV-2 infection, while in 14 other patients, GBS developed 3–24 days after the onset of flu-like symptoms. One patient presented 2 weeks after anosmia and ageusia without respiratory or GI symptoms. Electrophysiologic studies showed evidence of acute motor and sensory axonal variants (AMSAN) in five patients, mixed axonal and demyelinating patterns in two patients, and demyelinating patterns in six patients. EMG was not performed in four patients, one of which was diagnosed with Miller Fisher syndrome and positive serum GD1b-IgG antibody. CSF studies revealed albuminocytologic dissociation in ten patients, were normal in three patients (protein < 45 mg/dL, WBCs 0–5 cells/μl). In one patient, CSF protein and WBCs were 54 mg/dL and 9 cells/μL respectively, and CSF was not performed in three patients. 14 out of 17 patients received IVIG, one patient received IVIG and plasmapheresis, one patient showed spontaneous recovery, and one patient received prednisone. Six out of the seventeen patients developed respiratory failure, one of which died (Table 4).
CNS infectious or inflammatory complications
We identified 19 cases with confirmed COVID-19 which were suspected to have encephalitis, based on the presence of one of the following criteria: (a) meningeal signs, (b) altered mental status, focal neurological signs, or seizures, without better alternative explanation, or (c) suggestive MRI findings. 68.4% (n = 13) were ultimately diagnosed as meningoencephalitis, 5.3% (n = 1) as rhombencephalitis, 5.3% (n = 1) as acute necrotizing hemorrhagic encephalopathy, 5.3% (n = 1) as encephalopathy, 10.5% (n = 2) presented with status epilepticus, one of which was focal, and 5.3% (n = 1) had CNS demyelinating lesions. In the 13 meningoencephalitis cases, only four CSF samples revealed lymphocytic pleocytosis, two of which had positive CSF SARS-CoV-2 RT-PCR. Nine patients developed respiratory failure, six received plasmapheresis, and one died. One patient showed postmortem evidence of the presence of viral particles in the neurons and capillary endothelial cells in the frontal lobe (Table 5).
Discussion
Our systematic review indicates that SARS-CoV-2 infection is not solely a respiratory illness, as neurological complications are not rare. Ischemic and hemorrhagic stroke, Guillain–Barre syndrome, and its variants, encephalitis, and seizure have all been observed, which emphasizes the importance of neurological surveillance. In an analysis of 214 cases of COVID-19 in Wuhan, China, 78 (36.4%) had neurological complications. Patients with severe infection were more likely to have neurological manifestations like alteration in sensorium and muscle weakness (45.5% Vs 30.2% in non-severe). The manifestations involved both the central and peripheral nervous system. The severity of these manifestations ranged from acute cerebrovascular disease and impaired consciousness to dizziness and headache [60].
Cerebrovascular complications
In our review, ischemic stroke was the most common neurological manifestation, occurring in 42.7% of the subjects, with LVO representing 77% of the ischemic stroke. According to a series of 388 COVID-19 patients from Italy, thromboembolic events occurred in 21% of the patients, including venous thromboembolism, ischemic stroke, and acute coronary syndrome [61]. The exact mechanism of the hypercoagulable state is not well understood. D-dimers might play a major prothrombotic role in COVID-19 patients. In this review, 80% of the ischemic stroke patients had elevated D-dimer levels, which are independently associated with poor outcome [62]. Severe COVID-19 respiratory infection often leads to sepsis induced hypercoagulability, evident by increased intravascular platelet activation, increased fibrinogen, and mild prolongation of PT and aPTT [63]. Indeed, a study in Wuhan, China, showed that 71.4% of patients who died of COVID-19 had disseminated intravascular coagulation (DIC) [62] Moreover, SARS-CoV-2 virus is known to bind angiotensin-converting enzyme 2 (ACE2) on endothelial cells which promotes a proinflammatory and vasoconstrictive state of endothelial dysfunction leading to end organ damage, including stroke. ACE2 recombinant therapy, therefore, may be a promising targeted therapy for COVID-19-related stroke [64]. Transient production of antiphospholipid antibodies may also play a role. In a study by Harzallah et al., 25 out of 56 patients with confirmed or suspected SARS-CoV-2 infection were positive for lupus anticoagulants, and five patients had either anticardiolipin or anti–β2-glycoprotein I antibodies [65]. Zhang et al. detected antiphospholipid antibodies in three COVID-19 patients; all of them had multiple cerebral infarcts [66]. In this review, five of the LVO stroke tested positive for lupus anticoagulant.
When presenting in the appropriate time window, thrombolytic treatment of COVID-19 patients with ischemic stroke is reasonable. The role of anticoagulation, like LMWH, in this clinical context is still unclear [64]. Harzallah et al. recommended early anticoagulation therapy for individuals with SARS-CoV-2 infection and positive lupus anticoagulant [65]. Previous investigations suggested that COVID-19 is associated with both platelet and clotting cascade activation [67]. Further clinical trials are necessary to determine the role of antiplatelets and/or anticoagulation for the treatment and prevention of thrombotic events in COVID-19, including milder cases.
Neuromuscular complications
Previously identified coronaviruses, including SARS-CoV-1 and MERS, were associated with GBS [68]. In our review, neuromuscular disorders are the second most commonly encountered neurological complication of SARS-CoV-2 infection (28%), especially GBS. The mechanisms of GBS related to SARS-CoV-2 are still incompletely understood. Both para- and post-infectious mechanisms were proposed [20, 26]. Two patients, in our review, did not experience preceding fever, respiratory, or GI symptoms and GBS was the initial presentation. This suggests a para-infectious process, as has been reported recently with Zika virus [69]. One possible immunological explanation is the cytokine release syndrome (CRS), caused by an exacerbated recruitment and activation of macrophages, neutrophils, and natural killer cells (NK) in response to SARS-CoV-2 infection. Cytokines involved in CRS include IL-1β, IL-1Ra, IL-6, IL-17, TNF-α, CCL2, and sIL2-Rα; a critical step in the process is binding of IL-6 to IL-6R (sIL-6R) causing JAK-STAT3 activation, and subsequent secretion of vascular endothelial growth factor (VEGF), monocyte chemoattractant protein–1 (MCP-1), IL-8, and more IL-6, as well as decreased E-cadherin expression [70, 71]. This cytokine storm can produce extensive tissue damage, including the peripheral nervous system [72], and appears to correlate with COVID-19 severity. Accordingly, several therapeutic options are under study, with the intent to stabilize the immune system in COVID-19 and either prevent or minimize the consequences of this storm, as reviewed by Diamanti et al. [73]
A second mechanism explaining GBS in COVID-19 may be production of antibodies against ganglioside components of the peripheral nerves, owing to molecular mimicry with surface antigens of the infectious pathogen. This mechanism explains GBS following Campylobacter jejuni infection [74], which is frequently associated with axonal findings on electrophysiology. Similar molecular mimicry phenomena may occur in COVID-19 [75], and in fact, five cases in our review were electrophysiologically characterized as AMSAN, yet the exact frequency of ganglioside antibodies remains unknown, because those antibodies were not tested in most cases that we reviewed. Furthermore, sporadic reports of other autoimmune complications in the context of SARS-CoV-2 infection, such as steroid responsive encephalitis [76], immune thrombocytopenic purpura [77], and autoimmune hemolytic anemia [78], suggest that SARS-CoV-2 infection may serve as a trigger for autoimmune disorders.
With the emergence of more cases of acute neuropathies temporally linked to SARS-CoV-2 infection, we should gain a better understanding of the underlying pathophysiology and potential therapeutic options of GBS related to COVID-19. Since these neuropathies are treatable and they pose increased morbidity and mortality, neurologists, intensivists, and internists working with COVID-19 patients must be vigilant of this association.
CNS infection by COVID-19
Coronavirus can be neuroinvasive and cause direct CNS infection; this was convincingly demonstrated by the detection of particles and/or RNA of SARS-CoV-1, a virus with 79% genetic homology to SARS-CoV-2 [79], in human autopsies [80, 81]. Likewise, two of the cases that we reviewed showed positive CSF SARS-CoV-2 PCR [13, 15], and one [37] documented the evidence of viral particles in the neurons and capillary endothelial cells of the frontal lobe in a postmortem examination. The authors concluded that there was an active viral entry across the brain microvasculature into the neurons, as there was blebbing of viral particles coming in and out of the endothelial membrane [37]. Mechanistically, SARS-CoV-2 virus may enter the CNS through hematogenous route or retrograde synaptic transmission. The ACE 2 protein, which functions as a receptor for SARS-CoV-2, is abundantly expressed in the endothelial cells, supporting glia and neurons, and might be the binding site facilitating hematogenous entry. The systemic hyper-inflammation increases the permeability of the blood–brain and blood-CSF barriers, which might facilitate CNS entry, as well [82, 83]. Retrograde synaptic transmission may occur via the olfactory nerve [84]. This possibility is supported by the fact that anosmia is a frequent early sign of COVID-19 [85]. It has been proposed that SARS-CoV-2 neurotropism may explain not only the common symptoms of encephalitis, but also the respiratory failure, by involvement of the medullary respiratory centers. This mechanism has been demonstrated in animal models, but not yet in humans [86].
It should be noted, however, that in most cases of suspected “meningoencephalitis”, the virus could not be detected in the CSF. There are several potential explanations for this negative result. One is that the encephalitis in COVID-19 is more often immune-mediated, resulting from post-infectious or para-infectious mechanisms, and cytokine dysregulation, as previously discussed in GBS, rather than a result of direct viral invasion [83]. The response of the encephalitic syndrome to plasmapheresis in five cases [48] supports this notion, as does the occurrence of acute necrotizing encephalopathy in one case [12]. Acute necrotizing encephalopathy was previously described following influenza and other viral infections and attributed to cytokine storm [87]. A second explanation is that the virus may cause endothelial injury and induce a thrombotic microangiopathy (TMA)-like state, which can lead to severe encephalopathy with no evidence of inflammation based on CSF studies. A third theory is that PCR testing in the CSF has suboptimal sensitivity for the detection of SARS-CoV-2. This limitation of PCR is well known to neurologists, as it occurs with several other neuroinvasive viruses, including West Nile Virus [88], and enterovirus-D68 causing acute flaccid myelitis [89]. Detection of intrathecal virus-specific antibodies and their ratio to serum antibodies, and the recently developed metagenomic sequencing technology [90, 91], may increase the sensitivity of viral detection in the CSF in those cases, and it would be interesting to explore the utility of those techniques in COVID-19 in the near future. Detailed investigations, including CSF studies, imaging, and, when possible, autopsy, are required to better elucidate those mechanisms.
Epileptic complications
Although two COVID-19 patients in our review presented with status epilepticus, one of them had an established history of epilepsy from another cause. Lu et al. studied 304 COVID-19 patients and concluded that none of these patients had acute symptomatic seizures or status epilepticus [92]. The available data are too limited to make conclusions about the association of COVID-19 with development of seizures. Nevertheless, patients with severe SARS-CoV-2 infection, especially those hospitalized in intensive care units, are at risk for subclinical seizures or nonconvulsive status epilepticus (NCSE), owing to polypharmacy, metabolic derangements, toxemia, hypoxic-anoxic brain injury, or less commonly stroke or encephalitis related to SARS-CoV-2 infection. Therefore, continuous video EEG monitoring may be warranted in selected cases, as delayed diagnosis and treatment of NCSE will increase morbidity and mortality [93].
Finally, Lovati et al. reported a case of HSV-1 encephalitis, where the diagnosis and treatment were delayed because of anchoring on COVID-19 and its neurological complications [94]. Despite all the reports of COVID-19 neurological complications, other pathologies are still more common. Ignoring this would result in significant delays in diagnosing and treating neurological patients.
A number of recently published systematic reviews on COVID-19 have addressed the same topic (Table 6). The novelty of our review lies in the breadth of coverage, allowing it to serve as a primer for neurologists by summarizing the most recent evidence and the most important and relevant practical points. However, our study has some limitations. First, most of the used evidence is based on single case reports or small series, which limits its generalizability. Second, some case reports did not complete or report the full work-up required to exclude alternative causes of the neurological syndrome presented. Third, many cases are reported from specific ethnic populations, and hence, several demographics, genetic, or microbiologic variables might preclude the applicability of the conclusions in different populations. Fourth, several stroke patients had multiple comorbidities and potentially causative vascular risk factors that we did not include in our analysis. Fifth, due to incomplete work-up of several of the ischemic stroke studies included in the review, we did not have enough data for accurate stroke mechanism classification per TOAST in several cases. Last but not least, because of the small number of subjects studied and the suboptimal quality of study design, it is not possible to reach firm conclusions about the causal effect of COVID-19 for some neurological disorders.
Conclusions
Neurological manifestations of COVID-19 are not rare, especially large vessel stroke, Guillain–Barre syndrome, and meningoencephalitis. They could be related to the direct cytopathic effect of the virus, the inflammatory response, hypercoagulable state, or complications of treatment and ICU stay. Moving forward, further studies are needed to clarify the prevalence of the neurological complications of COVID-19, investigate their biological background, and test treatment options. Physicians should be cautious not to overlook other neurological diagnoses that can mimic COVID-19 during the pandemic.
Data availability
All the data supporting our findings are contained within manuscript.
References
Paules CI, Marston HD, Fauci AS (2020) Coronavirus infections—more than just the common cold. JAMA 323(8):707–708
Perlman S (2020) Another decade, another coronavirus. N Engl J Med 382(8):760–762
Niu J et al (2020) Non-invasive bioluminescence imaging of HCoV-OC43 infection and therapy in the central nervous system of live mice. Antiviral Res 173:104646
Tsai LK, Hsieh ST, Chang YC (2005) Neurological manifestations in severe acute respiratory syndrome. Acta Neurol Taiwan 14(3):113–119
Lau KK et al (2004) Possible central nervous system infection by SARS coronavirus. Emerg Infect Dis 10(2):342–344
Netland J et al (2008) Severe acute respiratory syndrome coronavirus infection causes neuronal death in the absence of encephalitis in mice transgenic for human ACE2. J Virol 82(15):7264–7275
Guan WJ, Ni ZY, Hu Y et al (2020) Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med 382(18):1708–1720
Pérez CA (2020) Looking ahead: the risk of neurologic complications due to COVID-19. Neurol Clin Pract. https://doi.org/10.1212/CPJ.0000000000000836
Guan WJ, Liang WH, Zhao Y et al (2020) Comorbidity and its impact on 1590 patients With COVID-19 in China: a nationwide analysis. Eur Respir J 55(5):2000547
Moher D, Shamseer L, Clarke M et al (2015) Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 4(1):1
Moola S, Munn Z, Sears K et al (2015) Conducting systematic reviews of association (etiology): The Joanna Briggs Institute's Approach. Int J Evid Based Healthc. 13(3):163–169 (12–54 Tables 1)
Poyiadji N, Shahin G, Noujaim D et al (2020) COVID-19-associated acute hemorrhagic necrotizing encephalopathy: CT and MRI features. Radiology 201187
Moriguchi T, Harii N, Goto J et al (2020) A first case of Meningitis/Encephalitis associated with SARS-Coronavirus-2. Int J Infect Dis. 94:55–58
Duong L, Xu P, Liu A (2020) Meningoencephalitis without respiratory failure in a young female patient with COVID-19 infection in Downtown Los Angeles, early April 2020. Brain Behav Immun. https://doi.org/10.1016/j.bbi.2020.04.024
Huang Y, Jiang D, Huang J (2020) SARS-CoV-2 detected in cerebrospinal fluid by PCR in a case of COVID-19 encephalitis. Brain Behav Immun. https://doi.org/10.1016/j.bbi.2020.05.012
Ye M, Ren Y, Lv T (2020) Encephalitis as a clinical manifestation of COVID-19. Brain Behav Immun. https://doi.org/10.1016/j.bbi.2020.04.017
Yin R, Feng W, Wang T et al (2020) Concomitant neurological symptoms observed in a patient diagnosed with coronavirus disease 2019. J Med Virol. https://doi.org/10.1002/jmv.25888
Filatov A et al (2020) Neurological complications of coronavirus disease (covid-19): encephalopathy. Cureus 12(3):e7352
Wong PF, Craik S, Newman P et al (2020) Lessons of the month 1: a case of rhombencephalitis as a rare complication of acute COVID-19 infection. Clin Med (Lond) clinmed.2020-0182
Zhao H, Shen D, Zhou H et al (2020) Guillain-Barré syndrome associated with SARS-CoV-2 infection: causality or coincidence? Lancet Neurol 19(5):383–384
Sedaghat Z, Karimi N (2020) Guillain Barre syndrome associated with COVID-19 infection: a case report. J Clin Neurosci 76:233–235
Virani A, Rabold E, Hanson T et al (2020) Guillain-Barré Syndrome associated with SARS-CoV-2 infection. IDCases 20:e00771
El Otmani H, El Moutawakil B, Rafai MA et al (2020) Covid-19 and Guillain-Barré syndrome: more than a coincidence! Rev Neurol (Paris) 176(6):518–519
Alberti P, Beretta S, Piatti M et al (2020) Guillain-Barré syndrome related to COVID-19 infection. Neurol Neuroimmunol Neuroinflamm 7(4):e741
Camdessanche JP, Morel J, Pozzetto B et al (2020) COVID-19 may induce Guillain-Barré syndrome. Rev Neurol (Paris) 176(6):516–518
Padroni M, Mastrangelo V, Asioli GM et al (2020) Guillain–Barré syndrome following COVID-19: new infection, old complication? J Neurol 24:1–3
Scheidl E, Canseco DD, Hadji-Naumov A et al (2020) Guillain-Barre syndrome during SARS-CoV-2 pandemic: a case report and review of recent literature. J Peripher Nerv Syst. https://doi.org/10.1111/jns.12382
Ottaviani D, Boso F, Tranquillini E et al (2020) Early Guillain-Barré syndrome in coronavirus disease 2019 (COVID-19): a case report from an Italian COVID-hospital. Neurol Sci 41(6):1351–1354
Abdelnour L, Abdalla ME, Babiker S (2020) COVID 19 infection presenting as motor peripheral neuropathy. J Formos Med Assoc 119(6):1119–1120
Juliao Caamaño DS, Beato RA (2020) Facial diplegia, a possible atypical variant of Guillain-Barré Syndrome as a rare neurological complication of SARSCoV-2. J Clin Neurosci
Wei H, Yin H, Huang M et al (2020) The 2019 novel cornoavirus pneumonia with onset of oculomotor nerve palsy: a case study. J Neurol 25:1–4
Jin M, Tong Q (2020) Rhabdomyolysis as potential late complication associated with COVID-19. Emerg Infect Dis 26(7)
Suwanwongse K, Shabarek N (2020) Rhabdomyolysis as a presentation of 2019 novel coronavirus disease. Cureus 12(4):e7561
Vollono C, Rollo E, Romozzi M et al (2020) Focal status epilepticus as unique clinical feature of COVID-19: a case report. Seizure 21(78):109–112
Sohal S, Mossammat M (2020) COVID-19 presenting with seizures. IDCases 1:e00782
Zanin L, Saraceno G, Panciani PP et al (2020) SARS-CoV-2 can induce brain and spine demyelinating lesions. Acta Neurochir (Wien) 4:1–4
Paniz-Mondolfi A, Bryce C, Grimes Z et al (2020) Central nervous system involvement by severe acute respiratory syndrome Coronavirus -2 (SARS-CoV-2). J Med Virol 92(7):699–702
González-Pinto T, Luna-Rodríguez A, Moreno-Estébanez A et al (2020) Emergency room neurology in times of COVID-19: malignant ischemic stroke and SARS-COV2 infection. Eur J Neurol. https://doi.org/10.1111/ene.14286
Zhou B, She J, Wang Y et al (2020) A case of coronavirus disease 2019 with concomitant acute cerebral infarction and deep vein thrombosis. Front Neurol 22(11):296
Valderrama EV, Humbert K, Lord A et al (2020) Severe acute respiratory syndrome Coronavirus 2 infection and ischemic stroke. Stroke STROKEAHA120030153
Viguier A, Delamarre L, Duplantier J et al (2020) Acute ischemic stroke complicating common carotid artery thrombosis during a severe COVID-19 infection. J Neuroradiol. https://doi.org/10.1016/j.neurad.2020.04.003
Hughes C, Nichols T, Pike M et al (2020) Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med 7(5):001691
Sharifi-Razavi A, Karimi N, Rouhani N (2020) COVID-19 and intracerebral haemorrhage: causative or coincidental? New Microbes New Infect 27(35):100669
Toscano G, Palmerini F, Ravaglia S et al (2020) Guillain-Barré Syndrome associated with SARS-CoV-2. N Engl J Med NEJMc2009191
Gutiérrez-Ortiz C, Méndez A, Rodrigo-Rey S et al (2020) Miller Fisher Syndrome and polyneuritis cranialis in COVID-19. Neurology. https://doi.org/10.1212/WNL.0000000000009619
Chan KH, Farouji I, Abu Hanoud A et al (2020) Weakness and elevated creatinine kinase as the initial presentation of coronavirus disease 2019 (COVID-19). Am J Emerg Med. https://doi.org/10.1016/j.ajem.2020.05.015
Bernard-Valnet R, Pizzarotti B, Anichini A et al (2020) Two patients with acute meningo-encephalitis concomitant to SARS-CoV-2 infection. Eur J Neurol. https://doi.org/10.1111/ene.14298
Dogan L, Kaya D, Sarikaya T et al (2020) Plasmapheresis treatment in COVID-19-related autoimmune meningoencephalitis: case series. Brain Behav Immun. https://doi.org/10.1016/j.bbi.2020.05.022
Avula A, Nalleballe K, Narula N et al (2020) COVID-19 presenting as stroke. Brain Behav Immun. https://doi.org/10.1016/j.bbi.2020.04.077
Al Saiegh F, Ghosh R, Leibold A et al (2020) Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2020-323522
Beyrouti R, Adams ME, Benjamin L et al (2020) Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. https://doi.org/10.1136/jnnp-2020-323586
Oxley TJ, Mocco J, Majidi S et al (2020) Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. 382(20):e60
Tunç A, Ünlübaş Y, Alemdar M et al (2020) Coexistence of COVID-19 and acute ischemic stroke report of four cases. J Clin Neurosci. https://doi.org/10.1016/j.jocn.2020.05.018
Li Y, Wang M, Zhou Y, Chang J (2020) Acute cerebrovascular disease following COVID-19: a single center, retrospective, observational study. Available at SSRN: https://ssrn.com/abstract=3550025
Weitz JI, Fredenburgh JC, Eikelboom JW (2017) A test in context: D-Dimer. J Am Coll Cardiol. 70(19):2411–2420
Ranucci M, Ballotta A, Di Dedda U et al (2020) The procoagulant pattern of patients with COVID-19 acute respiratory distress syndrome. J Thromb Haemost
Clyne B, Olshaker JS (1999) The C-reactive protein. J Emerg Med Nov-Dec 17(6):1019–1025
Liu F, Li L, Xu M et al (2020) Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol 127:104370
Levy JH, Goodnough LT (2015) How I use fibrinogen replacement therapy in acquired bleeding. Blood 125:1387
Mao L, Jin H, Wang M et al (2020) Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in Wuhan, China. JAMA Neurol e201127
Lodigiani C, Iapichino G, Carenzo L et al (2020) Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res 23(191):9–14
Tang N, Li D, Wang X (2020) Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost 18(4):844–847
Violi F, Pastori D, Cangemi R et al (2020) Hypercoagulation and antithrombotic treatment in Coronavirus 2019: a new challenge. Thromb Haemost 120(6):949–956
Hess DC, Eldahshan W, Rutkowski E (2020) COVID-19-related stroke. Transl Stroke Res 7:1–4
Harzallah I, Debliquis A, Drénou B (2020) Lupus anticoagulant is frequent in patients with Covid-19. J Thromb Haemost. https://doi.org/10.1111/jth.14867
Zhang Y, Xiao M, Zhang S et al (2020) Coagulopathy and antiphospholipid antibodies in patients with Covid-19. N Engl J Med 382(17):e38
Connell NT, Battinelli EM, Connors JM (2020) Coagulopathy of COVID-19 and antiphospholipid antibodies. J Thromb Haemost. https://doi.org/10.1111/jth.14893
Chao CC et al (2003) Peripheral nerve disease in SARS: report of a case. Neurology 61(12):1820–1821
da Silva IRF, Frontera JA, Bispo de Filippis AM et al (2017) Neurologic complications associated with the zika virus in Brazilian adults. JAMA Neurol 74(10):1190–1198
Moore JB, June CH (2020) Cytokine release syndrome in severe COVID-19. Science 368(6490):473–474
Jamilloux Y, Henry T, Belot A et al (2020) Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine interventions. Autoimmun Rev 19(7):102567
Lyons-Weiler J (2020) Pathogenic priming likely contributes to serious and critical illness and mortality in COVID-19 via autoimmunity. J Transl Autoimmun 9(3):100051
Diamanti AP, Rosado MM, Pioli C et al (2020) Cytokine release syndrome in COVID-19 patients, a new scenario for an old concern: the fragile balance between infections and autoimmunity. Int J Mol Sci 21(9):E3330
Willison HJ, Jacobs BC, van Doorn PA (2016) Guillain–Barré Syndrome. Lancet 388(10045):717–727
Cappello F (2020) Is COVID-19 a proteiform disease inducing also molecular mimicry phenomena? Cell Stress Chaperones 25(3):381–382
Pilotto A, Odolini S, Masciocchi SS et al (2020) Steroid-responsive encephalitis in Covid-19 disease. Ann Neurol. https://doi.org/10.1002/ana.25783
Zulfiqar AA, Lorenzo-Villalba N, Hassler P et al (2020) Immune thrombocytopenic purpura in a patient with Covid-19. N Engl J Med 382(18):e43
Lazarian G, Quinquenel A, Bellal M et al (2020) Autoimmune haemolytic anaemia associated with COVID-19 infection. Br J Haematol. https://doi.org/10.1111/bjh.16794
Wang H, Li X, Li T et al (2020) The genetic sequence, origin, and diagnosis of SARS-CoV-2. Eur J Clin Microbiol Infect Dis 24:1–7
Gu J, Gong E, Zhang B et al (2005) Multiple organ infection and the pathogenesis of SARS. J Exp Med 202(3):415–424
Xu J, Zhong S, Liu J et al (2005) Detection of severe acute respiratory syndrome coronavirus in the brain: potential role of the chemokine mig in pathogenesis. Clin Infect Dis 41(8):1089–1096
McGonagle D, Sharif K, O'Regan A et al (2020) The role of cytokines including interleukin-6 in COVID-19 induced pneumonia and macrophage activation syndrome-like disease. Autoimmun Rev 3:102537
Qin C, Zhou L, Hu Z et al (2020) Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin Infect Dis. https://doi.org/10.1093/cid/ciaa248
Mao XY, Jin WL (2020) The COVID-19 pandemic: consideration for brain infection. Neuroscience 437:130–131
Gane SB, Kelly C, Hopkins C (2020) Isolated sudden onset anosmia in COVID-19 infection. A novel syndrome? Rhinology 58(3):299–301
Li YC, Bai WZ, Hashikawa T (2020) The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J Med Virol
Wu X, Wu W, Pan W et al (2015) Acute necrotizing encephalopathy: an underrecognized clinicoradiologic disorder. Mediators Inflamm 2015:792578
Briese T, Glass WG, Lipkin WI (2000) Detection of West Nile Virus sequences in cerebrospinal fluid. Lancet 355(9215):1614–1615
Chong PF, Kira R, Mori H et al (2018) Clinical features of acute Flaccid Myelitis temporally associated with an Enterovirus D68 outbreak: results of a nationwide survey of acute flaccid paralysis in Japan, August–December 2015. Clin Infect Dis 66(5):653–664
Schubert RD, Hawes IA, Ramachandran PS et al (2019) Pan-viral serology implicates enteroviruses in acute flaccid myelitis. Nat Med 25(11):1748–1752
Wilson MR, Sample HA, Zorn KC et al (2019) Clinical metagenomic sequencing for diagnosis of meningitis and encephalitis. N Engl J Med 380(24):2327–2340
Lu L, Xiong W, Liu D et al (2020) New onset acute symptomatic seizure and risk factors in coronavirus disease 2019: a retrospective multicenter study. Epilepsia. https://doi.org/10.1111/epi.16524
Gelisse P, Rossetti AO, Genton P et al (2020) How to carry out and interpret EEG recordings in COVID-19 patients in ICU? Clin Neurophysiol. https://doi.org/10.1016/j.clinph.2020.05.006
Lovati C, Osio M, Pantoni L (2020) Diagnosing herpes simplex-1 encephalitis at the time of COVID-19 pandemic. Neurol Sci
Whittaker A, Anson M, Harky A (2020) Neurological manifestations of COVID-19: a systematic review and current update. Acta Neurol Scand 142(1):14–22
Montalvan V, Lee J, Bueso T et al (2020) Neurological manifestations of COVID-19 and other coronavirus infections: a systematic review. Clin Neurol Neurosurg 194:105921
Leonardi M, Padovani A, McArthur JC (2020) Neurological manifestations associated with COVID-19: a review and a call for action. J Neurol 1–4
Asadi-Pooya A, Simani L (2020) Central nervous system manifestations of COVID-19: a systematic review. J Neurol Sci 413:116832
Munhoz R, Pedroso J, Nascimento F et al (2020) Neurological complications in patients with SARS-CoV-2 infection: a systematic review. Arq Neuropsiquiatr 78(5):290–300
Romoli M, Jelcic I, Bernard-Valnet R et al (2020) A systematic review of neurological manifestations of SARS-CoV-2 infection: the devil is hidden in the details. Eur J Neurol
Funding
No funding was obtained for this study.
Author information
Authors and Affiliations
Contributions
Dr. Ghannam planned the search strategy, made the inclusion and exclusion criteria, and built the key words for the systematic review. Dr. Ghannam, Dr. Alshaer and Dr. Manousakis participated in articles screening and assessing their eligibility to the study. Both Dr. Ghannam and Dr. Manousakis completed the final form of PRISMA flowchart of the selection of the studies for this review. Dr. Ghannam, Dr. Alshaer, Dr. Al-Chalabi, Dr. Zakarna and Dr. Robertson were responsible for drafting and editing the manuscript. Dr. Ghannam was responsible for making the tables and the figures. Dr. Manousakis participated in critical revision of the manuscript for intellectual content. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Ethical approval
There was no ethics committee approval as the data have been analyzed in a retrospective manner and have no effect on treatment of the patient.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ghannam, M., Alshaer, Q., Al-Chalabi, M. et al. Neurological involvement of coronavirus disease 2019: a systematic review. J Neurol 267, 3135–3153 (2020). https://doi.org/10.1007/s00415-020-09990-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-020-09990-2