Abstract
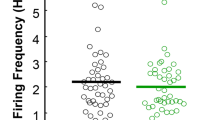
The role of the thalamus in schizophrenia has increasingly been studied in recent years. Deficits in the ventral thalamus have been described in only few postmortem and neuroimaging studies. We utilised our previously introduced neurodevelopmental animal model, the neonatal excitotoxic lesion of the ventral thalamus of Sprague–Dawley rats (Wolf et al., Pharmacopsychiatry 43:99–109, 22). At postnatal day (PD7), male pubs received bilateral thalamic infusions with ibotenic acid (IBA) or artificial cerebrospinal fluid (control). In adulthood, social interaction of two animals not familiar to each other was studied by a computerised video tracking system. This study displays clear lesion effects on social interaction of adult male rats. The significant reduction of total contact time and the significant increase in distance between the animals in the IBA group compared to controls can be interpreted as social withdrawal modelling a negative symptom of schizophrenia. The significant increase of total distance travelled in the IBA group can be hypothesised as agitation modelling a positive symptom of schizophrenia. Using a triple concept of social interaction, the percentage of no social interaction (Non-SI%) was significantly larger, and inversely, the percentage of passive social interaction (SI-passive%) was significantly smaller in the IBA group when compared to controls. In conclusion, on the background of findings in schizophrenic patients, the effects of neonatal ventral thalamic IBA lesions in adult male rats support the hypothesis of face and construct validity as animal model of schizophrenia.


Similar content being viewed by others
References
Kovelman JA, Scheibel AB (1984) A neurohistological correlate of schizophrenia. Biol Psychiatry 19:1601–1621
Jakob H, Beckmann H (1986) Prenatal developmental disturbances in the limbic allocortex in schizophrenics. J Neural Transm 65:303–326
Bogerts B (1993) Recent advances in the neuropathology of schizophrenia. Schizophr Bull 19:431–445
Lawrie SM, Abukmeil SS (1998) Brain abnormality in schizophrenia. A systematic and quantitative review of volumetric magnetic resonance imaging studies. Br J Psychiatry 172:110–120
Nelson MD, Saykin AJ, Flashman LA, Riordan HJ (1998) Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: a meta-analytic study. Arch Gen Psychiatry 55:433–440
Wright IC, Rabe-Hesketh S, Woodruff PW, David AS, Murray RM, Bullmore ET (2000) Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry 157:16–25
Weinberger DR (1987) Implications of normal brain development for the pathogenesis of schizophrenia. Arch Gen Psychiatry 44:660–669
Bloom FE (1993) Advancing a neurodevelopmental origin for schizophrenia. Arch Gen Psychiatry 50:224–227
Waddington JL (1993) Schizophrenia: developmental neuroscience and pathobiology. Lancet 341:531–536
Murray RM (1994) Neurodevelopmental schizophrenia: the rediscovery of dementia praecox. Br J Psychiatry Suppl 25:6–12
Lipska BK, Jaskiw GE, Weinberger DR (1993) Postpubertal emergence of hyperresponsiveness to stress and to amphetamine after neonatal excitotoxic hippocampal damage: a potential animal model of schizophrenia. Neuropsychopharmacology 9:67–75
Fiore M, Talamini L, Angelucci F, Koch T, Aloe L, Korf J (1999) Prenatal methylazoxymethanol acetate alters behavior and brain NGF levels in young rats: a possible correlation with the development of schizophrenia-like deficits. Neuropharmacology 38:857–869
Schmadel S, Schwabe K, Koch M (2004) Effects of neonatal excitotoxic lesions of the entorhinal cortex on cognitive functions in the adult rat. Neuroscience 128:365–374
Sumiyoshi T, Tsunoda M, Uehara T, Tanaka K, Itoh H, Sumiyoshi C, Kurachi M (2004) Enhanced locomotor activity in rats with excitotoxic lesions of the entorhinal cortex, a neurodevelopmental animal model of schizophrenia: behavioral and in vivo microdialysis studies. Neurosci Lett 364:124–129
Harich S, Koch M, Schwabe K (2008) Effects of repeated dizocilpine treatment on adult rat behavior after neonatal lesions of the entorhinal cortex. Prog Neuropsychopharmacol Biol Psychiatry 32:816–827
Lipska BK, Al-Amin HA, Weinberger DR (1998) Excitotoxic lesions of the rat medial prefrontal cortex. Effects on abnormal behaviors associated with neonatal hippocampal damage. Neuropsychopharmacology 19:451–464
Schneider M, Koch M (2005) Deficient social and play behavior in juvenile and adult rats after neonatal cortical lesion: effects of chronic pubertal cannabinoid treatment. Neuropsychopharmacology 30:944–957
Daenen EW, Van Der Heyden JA, Kruse CG, Wolterink G, Van Ree JM (2001) Adaptation and habituation to an open field and responses to various stressful events in animals with neonatal lesions in the amygdala or ventral hippocampus. Brain Res 918:153–165
Bouwmeester H, Wolterink G, Van Ree JM (2002) Neonatal development of projections from the basolateral amygdala to prefrontal, striatal, and thalamic structures in the rat. J Comp Neurol 442:239–249
Daenen EW, Wolterink G, Van Der Heyden JA, Kruse CG, Van Ree JM (2003) Neonatal lesions in the amygdala or ventral hippocampus disrupt prepulse inhibition of the acoustic startle response; implications for an animal model of neurodevelopmental disorders like schizophrenia. Eur Neuropsychopharmacol 13:187–197
Lipska BK, Luu S, Halim ND, Weinberger DR (2003) Behavioral effects of neonatal and adult excitotoxic lesions of the mediodorsal thalamus in the adult rat. Behav Brain Res 141:105–111
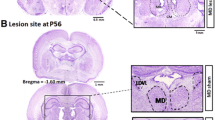
Wolf R, Matzke K, Paelchen K, Dobrowolny H, Bogerts B, Schwegler H (2010) Reduction of prepulse inhibition (PPI) after neonatal excitotoxic lesion of the ventral thalamus in pubertal and adult rats. Pharmacopsychiatry 43:99–109
Lipska BK, Swerdlow NR, Geyer MA, Jaskiw GE, Braff DL, Weinberger DR (1995) Neonatal excitotoxic hippocampal damage in rats causes post-pubertal changes in prepulse inhibition of startle and its disruption by apomorphine. Psychopharmacology (Berl) 122:35–43
Sams-Dodd F, Lipska BK, Weinberger DR (1997) Neonatal lesions of the rat ventral hippocampus result in hyperlocomotion and deficits in social behaviour in adulthood. Psychopharmacology (Berl) 132:303–310
Jones EG (1997) Cortical development and thalamic pathology in schizophrenia. Schizophr Bull 23:483–501
Weinberger DR (1997) On localizing schizophrenic neuropathology. Schizophr Bull 23:537–540
Andreasen NC (1997) The role of the thalamus in schizophrenia. Can J Psychiatry 42:27–33
Heckers S (1997) Neuropathology of schizophrenia: cortex, thalamus, basal ganglia, and neurotransmitter-specific projection systems. Schizophr Bull 23:403–421
Byne W, Hazlett EA, Buchsbaum MS, Kemether E (2009) The thalamus and schizophrenia: current status of research. Acta Neuropathol 117:347–368
Cronenwett WJ, Csernansky J (2010) Thalamic pathology in schizophrenia. Curr Top Behav Neurosci 4:509–528
Konick LC, Friedman L (2001) Meta-analysis of thalamic size in schizophrenia. Biol Psychiatry 49:28–38
Clinton SM, Meador-Woodruff JH (2004) Thalamic dysfunction in schizophrenia: neurochemical, neuropathological, and in vivo imaging abnormalities. Schizophr Res 69:237–253
Sim K, Cullen T, Ongur D, Heckers S (2006) Testing models of thalamic dysfunction in schizophrenia using neuroimaging. J Neural Transm 113:907–928
Lisman J (2012) Excitation, inhibition, local oscillations, or large-scale loops: what causes the symptoms of schizophrenia? Curr Opin Neurobiol 22:537–544
Pakkenberg B (1990) Pronounced reduction of total neuron number in mediodorsal thalamic nucleus and nucleus accumbens in schizophrenics. Arch Gen Psychiatry 47:1023–1028
Danos P, Baumann B, Bernstein HG, Franz M, Stauch R, Northoff G, Krell D, Falkai P, Bogerts B (1998) Schizophrenia and anteroventral thalamic nucleus: selective decrease of parvalbumin-immunoreactive thalamocortical projection neurons. Psychiatry Res 82:1–10
Byne W, Buchsbaum MS, Mattiace LA, Hazlett EA, Kemether E, Elhakem SL, Purohit DP, Haroutunian V, Jones L (2002) Postmortem assessment of thalamic nuclear volumes in subjects with schizophrenia. Am J Psychiatry 159:59–65
Andreasen NC, Ehrhardt JC, Swayze VW, Alliger RJ, Yuh WT, Cohen G, Ziebell S (1990) Magnetic resonance imaging of the brain in schizophrenia. The pathophysiologic significance of structural abnormalities. Arch Gen Psychiatry 47:35–44
Buchsbaum MS, Someya T, Teng CY, Abel L, Chin S, Najafi A, Haier RJ, Wu J, Bunney WE Jr (1996) PET and MRI of the thalamus in never-medicated patients with schizophrenia. Am J Psychiatry 153:191–199
Brickman AM, Buchsbaum MS, Shihabuddin L, Byne W, Newmark RE, Brand J, Ahmed S, Mitelman SA, Hazlett EA (2004) Thalamus size and outcome in schizophrenia. Schizophr Res 71:473–484
Harms MP, Wang L, Mamah D, Barch DM, Thompson PA, Csernansky JG (2007) Thalamic shape abnormalities in individuals with schizophrenia and their nonpsychotic siblings. J Neurosci 27:13835–13842
Smith MJ, Wang L, Cronenwett W, Mamah D, Barch DM, Csernansky JG (2011) Thalamic morphology in schizophrenia and schizoaffective disorder. J Psychiatr Res 45:378–385
Crow TJ (1980) Molecular pathology of schizophrenia: more than one disease process? Br Med J 280:66–68
Andreasen NC, Olsen S (1982) Negative v positive schizophrenia. Definition and validation. Arch Gen Psychiatry 39:789–794
File SE, Hyde JR (1978) Can social interaction be used to measure anxiety? Br J Pharmacol 62:19–24
Meyerson BJ, Hoglund AU (1981) Exploratory and socio-sexual behaviour in the male laboratory rat: a methodological approach for the investigation of drug action. Acta Pharmacol Toxicol (Copenh) 48:168–180
Guy AP, Gardner CR (1985) Pharmacological characterisation of a modified social interaction model of anxiety in the rat. NeuropsychoBiology 13:194–200
Corbett R, Hartman H, Kerman LL, Woods AT, Strupczewski JT, Helsley GC, Conway PC, Dunn RW (1993) Effects of atypical antipsychotic agents on social behavior in rodents. Pharmacol Biochem Behav 45:9–17
File SE, Seth P (2003) A review of 25 years of the social interaction test. Eur J Pharmacol 463:35–53
Wolf, Dobrowolny, Bogerts (2002) Search for an animal model of schizophrenia: effects of neonatal temporolimbic lesions on social interaction in adult rats. Pharmacopsychiatry 35:11
Wolf R, Dobrowolny H, Bogerts B (2004) Computergestützte Messung der sozialen Interaktion nach neonataler Ibotensäure-Läsion des temporolimbischen System der Ratte - ein Tiermodell der Schizophrenie? Nervenarzt 75(Suppl 2):S73
Panther P, Nullmeier S, Dobrowolny H, Schwegler H, Wolf R (2012) CPB-K mice a mouse model of schizophrenia? Differences in dopaminergic, serotonergic and behavioral markers compared to BALB/cJ mice. Behav Brain Res 230:215–228
Zadeh LA (1965) Fuzzy sets. Inf Control 8:338–353
Klir GJ, Yuan B (1995) Fuzzy sets and fuzzy logic: theory and applications. Prentice Hall, New Jersey
Pellegrino LJ, Pellegrino AS, Cushman AJ (1979) A stereotaxic atlas of the rat brain. Plenum, New York
Ellenbroek BA, Cools AR (1990) Animal models with construct validity for schizophrenia. Behav Pharmacol 1:469–490
Lipska BK, Weinberger DR (1995) Genetic variation in vulnerability to the behavioral effects of neonatal hippocampal damage in rats. Proc Natl Acad Sci USA 92:8906–8910
Ouhaz Z, Ba-M’hamed S, Mitchell AS, Elidrissi A, Bennis M (2015) Behavioral and cognitive changes after early postnatal lesions of the rat mediodorsal thalamus. Behav Brain Res 292:219–232
Geyer, Moghaddam B (2002) Animal models relevant to schizophrenia disorders. In: Davis KL, Charney DS, Coyle JT, Nemeroff C (eds) Neuropharmacology: the fifth generation of progress. Lippincott Williams and Wilkins, Philadelphia, pp 689–701
Arguello PA, Gogos JA (2006) Modeling madness in mice: one piece at a time. Neuron 52:179–196
Adriano F, Spoletini I, Caltagirone C, Spalletta G (2010) Updated meta-analyses reveal thalamus volume reduction in patients with first episode and chronic schizophrenia. Schizophr Res 123:1–14
Meador-Woodruff JH, Clinton SM, Beneyto M, McCullumsmith RE (2003) Molecular abnormalities of the glutamate synapse in the thalamus in schizophrenia. Ann N Y Acad Sci 1003:75–93
Ferrarelli F, Tononi G (2011) The thalamic reticular nucleus and schizophrenia. Schizophr Bull 37:306–315
Oke AF, Adams RN (1987) Elevated thalamic dopamine: possible link to sensory dysfunctions in schizophrenia. Schizophr Bull 13:589–604
Oke AF, Putz C, Adams RN, Bird ED (1992) Neuroleptic treatment is an unlikely cause of elevated dopamine in thalamus of schizophrenic subjects. Psychiatry Res 45:203–208
Simon H, Le MM, Galey D, Cardo B (1976) Silver impregnation of dopaminergic systems after radiofrequency and 6-OHDA lesions of the rat ventral brain. Brain Res 115:215–231
Young KA, Randall PK, Wilcox RE (1995) Startle and sensorimotor correlates of ventral thalamic dopamine and GABA in rodents. Neuroreport 6:2495–2499
Simpson EH, Winiger V, Biezonski DK, Haq I, Kandel ER, Kellendonk C (2014) Selective overexpression of dopamine D3 receptors in the striatum disrupts motivation but not cognition. Biol Psychiatry 76:823–831
Clinton SM, Haroutunian V, Davis KL, Meador-Woodruff JH (2003) Altered transcript expression of NMDA receptor-associated postsynaptic proteins in the thalamus of subjects with schizophrenia. Am J Psychiatry 160:1100–1109
Clinton SM, Haroutunian V, Meador-Woodruff JH (2006) Up-regulation of NMDA receptor subunit and post-synaptic density protein expression in the thalamus of elderly patients with schizophrenia. J Neurochem 98:1114–1125
Wiedholz LM, Owens WA, Horton RE, Feyder M, Karlsson R-M, Hefner K, Sprengel R, Celikel T, Daws LC, Holmes A (2014) Mice lacking the AMPA GluR1 receptor exhibit striatal hyperdopaminergia and ‘schizophrenia-related’ behaviors. Mol Psychiatry 13:631–640
Inta D, Miriam A, Vogt MA, Elkin H, Weber T, Juan M, Lima-Ojeda JM, Schneider M, Luoni A, Riva MA, Gertz K, Hellmann-Regen J, Kronenberg G, Meyer-Lindenberg A, Sprengel R, Gass P (2014) Phenotype of mice with inducible ablation of GluA1 AMPA receptors during late adolescence: relevance for mental disorders. Hippocampus 24:424–435
Wolf R, Dobrowolny H, Matzke K, Paelchen K, Bogerts B, Schwegler H (2006) Prepulse inhibition is different in two inbred mouse strains (CPB-K and BALB/cJ) with different hippocampal NMDA receptor densities. Behav Brain Res 166:78–84
DiChiara G, Morelli M, Porceddu ML, Gessa GL (1979) Role of thalamic gamma-aminobutyrate in motor functions: catalepsy and ipsiversive turning after intrathalamic muscimol. Neuroscience 4:1453–1465
Carlsson A, Lindqvist M (1963) Effect of chlorpromazine or haloperidol on formation of 3methoxytyramine and normetanephrine in mouse brain. Acta Pharmacol Toxicol (Copenh) 20:140–144
van Rossum JM (1966) The significance of dopamine-receptor blockade for the mechanism of action of neuroleptic drugs. Arch Int Pharmacodyn Ther 160:492–494
Olney JW, Farber NB (1995) Glutamate receptor dysfunction and schizophrenia. Arch Gen Psychiatry 52:998–1007
Olney JW, Newcomer JW, Farber NB (1999) NMDA receptor hypofunction model of schizophrenia. J Psychiatr Res 33:523–533
Tamminga C (1999) Glutamatergic aspects of schizophrenia. Br J Psychiatry Suppl 37:12–15
Carlsson A, Waters N, Carlsson ML (2000) Network interactions in schizophrenia—therapeutic implications. Brain Res Rev 31:342–349
Benes FM (2000) Emerging principles of altered neural circuitry in schizophrenia. Brain Res Rev 31:251–269
Lewis DA (2000) GABAergic local circuit neurons and prefrontal cortical dysfunction inschizophrenia. Brain Res Rev 31:270–276
Sharp FR, Tomitaka M, Bernaudin M, Tomitaka S (2001) Psychosis: pathological activation of limbic thalamocortical circuits by psychomimetics and schizophrenia? Trends Neurosci 24:330–334
Acknowledgements
This work was supported by the Federal Ministry of Education and Research of Germany (BMBF). We would like to thank Mrs. K. Paelchen and Mr. K. Matzke for excellent technical assistance. We declare that the experiments comply with the current laws of Germany.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Rights and permissions
About this article
Cite this article
Wolf, R., Dobrowolny, H., Nullmeier, S. et al. Effects of neonatal excitotoxic lesions in ventral thalamus on social interaction in the rat. Eur Arch Psychiatry Clin Neurosci 268, 461–470 (2018). https://doi.org/10.1007/s00406-017-0781-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00406-017-0781-2