Abstract
Purpose
To investigate the novel molecular mechanisms of the antioxidant and anti-inflammatory properties of S-allylmercaptocysteine (SAMC) based on a transcriptomic study in a nonalcoholic steatohepatitis (NASH) rat model
Methods
NASH was induced in Sprague–Dawley rats by feeding with a high fat diet (HFD) for 12 weeks. 200 mg/kg SAMC was fed by oral gavage for 4 weeks from 9 to 12 week.
Results
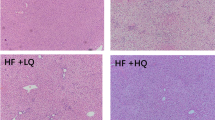
SAMC co-administration attenuated HFD-induced liver injury, including the increased serum ALT, hepatic oxidative stress and inflammation. Transcriptomic analysis revealed that SAMC dramatically induced the XRE- and ARE-driven drug metabolising enzymes (DMEs) including Akr7a3, Akr1b8, and Nqo1. The nuclear translocation of the upstream regulator of xenobiotics metabolism, AHR, and regulator of antioxidant responses, NRF2, were significantly increased by SAMC treatment. Furthermore, SAMC counteracted the effects of HFD on NF-κB/IκB and NLRP3/6 pathways with decreasing protein levels of ASC, cleaved caspase-1, IL-18, and IL-1β. These results were further verified in another mice NASH model induced by an MCD diet with SAMC co-administration.
Conclusion
We propose that SAMC triggers AHR/NRF2-mediated antioxidant responses which may further suppress the NLRP3/6 inflammasome pathway and NF-κB activation, contributing to the improvement of NASH.




Similar content being viewed by others
Data availability
Not applicable.
Abbreviations
- ARNT:
-
Ah receptor nuclear translocator
- AKRs:
-
Aldo-reductases
- ARE:
-
Antioxidant response element
- AHR:
-
Aryl-hydrocarbon receptor
- DMEs:
-
Drug metabolising enzymes
- FFA:
-
Free fatty acid
- H&E:
-
Haematoxylin and eosin
- HFD:
-
High fat diet
- MDA:
-
Malondialdehyde
- MCD:
-
Methionine-chlorine deficient
- NAS:
-
NAFLD activity scoring
- NAFLD:
-
Nonalcoholic fatty liver disease
- NASH:
-
Nonalcoholic steatohepatitis
- NRF2:
-
Nuclear factor (erythroid-derived 2)-like 2
- NLRP3:
-
NOD-like receptor protein 3
- NLRP6:
-
NOD-like receptor protein 6
- SAMC:
-
S-allylmercaptocysteine
- ALT:
-
Serum alanine aminotransferase
- XRE:
-
Xenobiotic response element
References
Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M (2016) Global epidemiology of nonalcoholic fatty liver disease-Meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 64(1):73–84. https://doi.org/10.1002/hep.28431
Chalasani N, Younossi Z, Lavine JE, Diehl AM, Brunt EM, Cusi K, Charlton M, Sanyal AJ (2012) The diagnosis and management of non-alcoholic fatty liver disease: practice guideline by the American Gastroenterological Association, American Association for the Study of Liver Diseases, and American College of Gastroenterology. Hepatology 142(7):1592–1609. https://doi.org/10.1053/j.gastro.2012.04.001
Abd El-Kader SM, El-Den Ashmawy EM (2015) Non-alcoholic fatty liver disease: The diagnosis and management. World J Hepatol 7(6):846–858. https://doi.org/10.4254/wjh.v7.i6.846
Shaker M, Tabbaa A, Albeldawi M, Alkhouri N (2014) Liver transplantation for nonalcoholic fatty liver disease: new challenges and new opportunities. World J Gastroenterol 20(18):5320–5330. https://doi.org/10.3748/wjg.v20.i18.5320
Romero-Gomez M, Zelber-Sagi S, Trenell M (2017) Treatment of NAFLD with diet, physical activity and exercise. J Hepatol 67(4):829–846. https://doi.org/10.1016/j.jhep.2017.05.016
Tanaka N, Kimura T, Fujimori N, Nagaya T, Komatsu M, Tanaka E (2019) Current status, problems, and perspectives of non-alcoholic fatty liver disease research. World J Gastroenterol 25(2):163–177. https://doi.org/10.3748/wjg.v25.i2.163
Oseini AM, Sanyal AJ (2017) Therapies in non-alcoholic steatohepatitis (NASH). Liver Int 37(Suppl 1):97–103. https://doi.org/10.1111/liv.13302
Perumpail BJ, Li AA, Iqbal U, Sallam S, Shah ND, Kwong W, Cholankeril G, Kim D, Ahmed A (2018) Potential therapeutic benefits of herbs and supplements in patients with NAFLD. Diseases. https://doi.org/10.3390/diseases6030080
Xiao J, Fai So K, Liong EC, Tipoe GL (2013) Recent advances in the herbal treatment of non-alcoholic Fatty liver disease. J Tradit Complement Med 3(2):88–94. https://doi.org/10.4103/2225-4110.110411
Xiao J, Xing F, Liu Y, Lv Y, Wang X, Ling MT, Gao H, Ouyang S, Yang M, Zhu J, Xia Y, So KF, Tipoe GL (2018) Garlic-derived compound S-allylmercaptocysteine inhibits hepatocarcinogenesis through targeting LRP6/Wnt pathway. Acta Pharm Sinica B 8(4):575–586. https://doi.org/10.1016/j.apsb.2017.10.003
Zhu X, Jiang X, Li A, Zhao Z, Li S (2017) S-Allylmercaptocysteine attenuates cisplatin-induced nephrotoxicity through suppression of apoptosis, oxidative stress, and inflammation. Nutrients. https://doi.org/10.3390/nu9020166
Xiao J, Liong EC, Ling MT, Ching YP, Fung ML, Tipoe GL (2012) S-allylmercaptocysteine reduces carbon tetrachloride-induced hepatic oxidative stress and necroinflammation via nuclear factor kappa B-dependent pathways in mice. Eur J Nutr 51(3):323–333. https://doi.org/10.1007/s00394-011-0217-0
Xu D, Xu M, Jeong S, Qian Y, Wu H, Xia Q, Kong X (2018) The Role of Nrf2 in liver disease: novel molecular mechanisms and therapeutic approaches. Front Pharmacol 9:1428. https://doi.org/10.3389/fphar.2018.01428
Li S, Yang G, Zhu X, Cheng L, Sun Y, Zhao Z (2017) Combination of rapamycin and garlic-derived S-allylmercaptocysteine induces colon cancer cell apoptosis and suppresses tumor growth in xenograft nude mice through autophagy/p62/Nrf2 pathway. Oncol Rep 38(3):1637–1644. https://doi.org/10.3892/or.2017.5849
Iranshahy M, Iranshahi M, Abtahi SR, Karimi G (2018) The role of nuclear factor erythroid 2-related factor 2 in hepatoprotective activity of natural products: a review. Food Chem Toxicol 120:261–276. https://doi.org/10.1016/j.fct.2018.07.024
Li J, Sapper TN, Mah E, Rudraiah S, Schill KE, Chitchumroonchokchai C, Moller MV, McDonald JD, Rohrer PR, Manautou JE, Bruno RS (2016) Green tea extract provides extensive Nrf2-independent protection against lipid accumulation and NFkappaB pro- inflammatory responses during nonalcoholic steatohepatitis in mice fed a high-fat diet. Mol Nutr Food Res 60(4):858–870. https://doi.org/10.1002/mnfr.201500814
Rushmore TH, Kong AN (2002) Pharmacogenomics, regulation and signaling pathways of phase I and II drug metabolizing enzymes. Curr Drug Metab 3(5):481–490
Kuramoto N, Baba K, Gion K, Sugiyama C, Taniura H, Yoneda Y (2003) Xenobiotic response element binding enriched in both nuclear and microsomal fractions of rat cerebellum. J Neurochem 85(1):264–273
Schroder K, Tschopp J (2010) The inflammasomes. Cell 140(6):821–832. https://doi.org/10.1016/j.cell.2010.01.040
Szabo G, Petrasek J (2015) Inflammasome activation and function in liver disease. Nat Rev Gastroenterol Hepatol 12(7):387–400. https://doi.org/10.1038/nrgastro.2015.94
Mridha AR, Wree A, Robertson AAB, Yeh MM, Johnson CD, Van Rooyen DM, Haczeyni F, Teoh NC, Savard C, Ioannou GN, Masters SL, Schroder K, Cooper MA, Feldstein AE, Farrell GC (2017) NLRP3 inflammasome blockade reduces liver inflammation and fibrosis in experimental NASH in mice. J Hepatol 66(5):1037–1046. https://doi.org/10.1016/j.jhep.2017.01.022
Hennig P, Garstkiewicz M, Grossi S, Di Filippo M, French LE, Beer HD (2018) The Crosstalk between Nrf2 and Inflammasomes. Int J Mol Sci. https://doi.org/10.3390/ijms19020562
Suzuki T, Yamamoto M (2015) Molecular basis of the Keap1-Nrf2 system. Free Radical Biol Med 88(Pt B):93–100. https://doi.org/10.1016/j.freeradbiomed.2015.06.006
Tipoe CTH, Liong EC, Leung TM, Lau TYH, Fung ML, Nanji AA (2009) Voluntary oral feeding of rats not requiring a very high fat diet is a clinically relevant animal model of non-alcoholic fatty liver disease (NAFLD). Histol Histopathol 24:1161–1169
Xiao J, Guo R, Fung ML, Liong EC, Chang RC, Ching YP, Tipoe GL (2013) Garlic-derived S-allylmercaptocysteine ameliorates nonalcoholic fatty liver disease in a rat model through inhibition of apoptosis and enhancing autophagy. Evid-Based Compl Altern Med 2013:642920. https://doi.org/10.1155/2013/642920
Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, Ferrell LD, Liu YC, Torbenson MS, Unalp-Arida A, Yeh M, McCullough AJ, Sanyal AJ (2005) Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology 41(6):1313–1321. https://doi.org/10.1002/hep.20701
Jones AS, Johnson MS, Nagy TR (2009) Validation of quantitative magnetic resonance for the determination of body composition of mice. Int J Body Compos Res 7(2):67–72
Xiao J, Ching YP, Liong EC, Nanji AA, Fung ML, Tipoe GL (2013) Garlic-derived S-allylmercaptocysteine is a hepato-protective agent in non-alcoholic fatty liver disease in vivo animal model. Eur J Nutr 52(1):179–191. https://doi.org/10.1007/s00394-012-0301-0
Aleksunes CDKALM (2010) Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev 62(1):1–96. https://doi.org/10.1124/pr.109.002014
Miao W, Hu L, Scrivens PJ, Batist G (2005) Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: direct cross-talk between phase I and II drug-metabolizing enzymes. J Biol Chem 280(21):20340–20348. https://doi.org/10.1074/jbc.M412081200
Itoh K, Chiba T, Takahashi S, Ishii T, Igarashi K, Katoh Y, Oyake T, Hayashi N, Satoh K, Hatayama I, Yamamoto M, Nabeshima Y (1997) An Nrf2/small Maf heterodimer mediates the induction of phase II detoxifying enzyme genes through antioxidant response elements. Biochem Biophys Res Commun 236(2):313–322
Yeager RL, Reisman SA, Aleksunes LM, Klaassen CD (2009) Introducing the "TCDD-inducible AhR-Nrf2 gene battery". Toxicol Sci 111(2):238–246. https://doi.org/10.1093/toxsci/kfp115
Buzzetti E, Pinzani M, Tsochatzis EA (2016) The multiple-hit pathogenesis of non-alcoholic fatty liver disease (NAFLD). Metabolism 65(8):1038–1048. https://doi.org/10.1016/j.metabol.2015.12.012
Kobayashi EH, Suzuki T, Funayama R, Nagashima T, Hayashi M, Sekine H, Tanaka N, Moriguchi T, Motohashi H, Nakayama K, Yamamoto M (2016) Nrf2 suppresses macrophage inflammatory response by blocking proinflammatory cytokine transcription. Nat Commun 7:11624. https://doi.org/10.1038/ncomms11624
Dinallo V, Marafini I, Di Fusco D, Di Grazia A, Laudisi F, Dwairi R, Paoluzi OA, Monteleone G, Monteleone I (2019) Protective effects of aryl hydrocarbon receptor signaling in celiac disease mucosa and in poly I:C-induced small intestinal atrophy mouse model. Front Immunol 10:91. https://doi.org/10.3389/fimmu.2019.00091
Henao-Mejia J, Elinav E, Jin C, Hao L, Mehal WZ, Strowig T, Thaiss CA, Kau AL, Eisenbarth SC, Jurczak MJ, Camporez JP, Shulman GI, Gordon JI, Hoffman HM, Flavell RA (2012) Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482(7384):179–185. https://doi.org/10.1038/nature10809
Shirin H, Pinto JT, Kawabata Y, Soh JW, Delohery T, Moss SF, Murty V, Rivlin RS, Holt PR, Weinstein IB (2001) Antiproliferative effects of S-allylmercaptocysteine on colon cancer cells when tested alone or in combination with sulindac sulfide. Can Res 61(2):725–731
Pinto JT, Krasnikov BF, Cooper AJ (2006) Redox-sensitive proteins are potential targets of garlic-derived mercaptocysteine derivatives. J Nutr 136(3 Suppl):835s–841s. https://doi.org/10.1093/jn/136.3.835S
Iuchi Y, Kaneko T, Matsuki S, Ishii T, Ikeda Y, Uchida K, Fujii J (2004) Carbonyl stress and detoxification ability in the male genital tract and testis of rats. Histochem Cell Biol 121(2):123–130. https://doi.org/10.1007/s00418-003-0607-3
Penning TM (2017) Aldo-keto reductase regulation by the Nrf2 system: implications for stress response, chemotherapy drug resistance, and carcinogenesis. Chem Res Toxicol 30(1):162–176. https://doi.org/10.1021/acs.chemrestox.6b00319
Ahmed MM, Wang T, Luo Y, Ye S, Wu Q, Guo Z, Roebuck BD, Sutter TR, Yang JY (2011) Aldo-keto reductase-7A protects liver cells and tissues from acetaminophen-induced oxidative stress and hepatotoxicity. Hepatology 54(4):1322–1332. https://doi.org/10.1002/hep.24493
Srivastava S, Harter TM, Chandra A, Bhatnagar A, Srivastava SK, Petrash JM (1998) Kinetic studies of FR-1, a growth factor-inducible aldo-keto reductase. Biochemistry 37(37):12909–12917. https://doi.org/10.1021/bi9804333
Pastel E, Pointud JC, Volat F, Martinez A, Lefrancois-Martinez AM (2012) Aldo-keto reductases 1B in endocrinology and metabolism. Front Pharmacol 3:148. https://doi.org/10.3389/fphar.2012.00148
Berg P, Pongratz I (2002) Two parallel pathways mediate cytoplasmic localization of the dioxin (aryl hydrocarbon) receptor. J Biol Chem 277(35):32310–32319. https://doi.org/10.1074/jbc.M203351200
Kovac S, Angelova PR, Holmstrom KM, Zhang Y, Dinkova-Kostova AT (1850) Abramov AY (2015) Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochem Biophys Acta 4:794–801. https://doi.org/10.1016/j.bbagen.2014.11.021
Liu X, Zhang X, Ding Y, Zhou W, Tao L, Lu P, Wang Y, Hu R (2017) Nuclear factor E2-related factor-2 negatively regulates NLRP3 inflammasome activity by inhibiting reactive oxygen species-induced NLRP3 priming. Antioxid Redox Signal 26(1):28–43. https://doi.org/10.1089/ars.2015.6615
Dinkova-Kostova AT, Talalay P (2000) Persuasive evidence that quinone reductase type 1 (DT diaphorase) protects cells against the toxicity of electrophiles and reactive forms of oxygen. Free Radical Biol Med 29(3–4):231–240
Bataille AM, Manautou JE (2012) Nrf2: a potential target for new therapeutics in liver disease. Clin Pharmacol Ther 92(3):340–348. https://doi.org/10.1038/clpt.2012.110
Ma Q, Kinneer K, Bi Y, Chan JY, Kan YW (2004) Induction of murine NAD(P)H:quinone oxidoreductase by 2,3,7,8-tetrachlorodibenzo-p-dioxin requires the CNC (cap 'n' collar) basic leucine zipper transcription factor Nrf2 (nuclear factor erythroid 2-related factor 2): cross-interaction between AhR (aryl hydrocarbon receptor) and Nrf2 signal transduction. Biochem J 377(Pt 1):205–213. https://doi.org/10.1042/bj20031123
Merrell MD, Cherrington NJ (2011) Drug metabolism alterations in nonalcoholic fatty liver disease. Drug Metab Rev 43(3):317–334. https://doi.org/10.3109/03602532.2011.577781
Qiu L, Lin J, Ying M, Chen W, Yang J, Deng T, Chen J, Shi D, Yang JY (2013) Aldose reductase is involved in the development of murine diet-induced nonalcoholic steatohepatitis. PLoS ONE 8(9):e73591–e73591. https://doi.org/10.1371/journal.pone.0073591
Pan CW, Pan ZZ, Hu JJ, Chen WL, Zhou GY, Lin W, Jin LX, Xu CL (2016) Mangiferin alleviates lipopolysaccharide and D-galactosamine-induced acute liver injury by activating the Nrf2 pathway and inhibiting NLRP3 inflammasome activation. Eur J Pharmacol 770:85–91. https://doi.org/10.1016/j.ejphar.2015.12.006
Leung WS, Yang ML, Lee SS, Kuo CW, Ho YC, Huang-Liu R, Lin HW, Kuan YH (2017) Protective effect of zerumbone reduces lipopolysaccharide-induced acute lung injury via antioxidative enzymes and Nrf2/HO-1 pathway. Int Immunopharmacol 46:194–200. https://doi.org/10.1016/j.intimp.2017.03.008
Wang K, Lv Q, Miao YM, Qiao SM, Dai Y, Wei ZF (2018) Cardamonin, a natural flavone, alleviates inflammatory bowel disease by the inhibition of NLRP3 inflammasome activation via an AhR/Nrf2/NQO1 pathway. Biochem Pharmacol 155:494–509. https://doi.org/10.1016/j.bcp.2018.07.039
Wang YC, Liu QX, Zheng Q, Liu T, Xu XE, Liu XH, Gao W, Bai XJ, Li ZF (2019) Dihydromyricetin alleviates sepsis-induced acute lung injury through inhibiting NLRP3 inflammasome-dependent pyroptosis in mice model. Inflammation. https://doi.org/10.1007/s10753-019-00990-7
Funding
This research was funded by Seed Fund for Basic Research of University Research Committee, grant number 20161159263.
Author information
Authors and Affiliations
Contributions
QY: investigation, data curation, and writing—original draft preparation. YYL, Z-YX, and ECL: investigation and data curation. JX and GLT: conceptualization, methodology, supervision, resources, and writing—reviewing.
Corresponding authors
Ethics declarations
Conflict of interest
We do not have any professional relationships with companies or manufacturers who will benefit from the results of the present study. We declare that the results of the study are presented clearly, honestly, and without fabrication, falsification, or inappropriate data manipulation.
Ethical approval
The manuscript does not contain clinical studies or patient data. The entire animal experiment procedures were approved by the Committee of Animal Use for Research and Teaching at The University of Hong Kong (CULATR No. 4618-18), which is fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care International (AAALAC International).
Consent to participate
Not applicable.
Consent for publication
We confirm that the manuscript has been read and approved by all named authors, and that there are no other persons who satisfied the criteria for authorship but are not listed. We further confirm that the order of authors listed in the manuscript has been approved by all of us.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yu, Q., Lee, Yy., Xia, Zy. et al. S-allylmercaptocysteine improves nonalcoholic steatohepatitis by enhancing AHR/NRF2-mediated drug metabolising enzymes and reducing NF-κB/IκBα and NLRP3/6-mediated inflammation. Eur J Nutr 60, 961–973 (2021). https://doi.org/10.1007/s00394-020-02305-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02305-1