Abstract
Purpose
Functional and structural changes in cardiovascular and renal systems resulting from obesity and metabolic syndrome represent a severe risk to human health. Lifestyle interventions such as combining healthy diet with adequate physical exercise protocols are good strategies to manage these pathologies. In this research, the effects of lentil protein hydrolysate administration, combined or not with a mixed training protocol, on insulin resistance, cardiovascular and renal functionality were studied in the obese Zucker rat experimental model.
Methods
Thirty-two rats (16 lean and 16 obese subdivided in sedentary and trained animals) were administered lentil protein hydrolysate, whereas another 32 subdivided in the same experimental design were administered placebo. The experiment lasted for 8 weeks. At the end of the experimental period, insulin resistance and different parameters of cardiovascular and renal functionality were measured.
Results
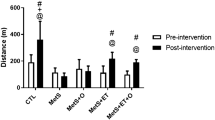
The individual or combined interventions with lentil protein hydrolysate and mixed training protocol were efficient at counteracting some of the metabolic, cardiovascular and renal alterations characterizing the obese Zucker rat. Specifically, lentil protein hydrolysate decreased hyperphagia, amplitude of QRS complex, plasma ACE and selectin E expression in aorta, while increasing urinary volume and pH. Exercise showed beneficial actions on HOMA-IR, QRS amplitude, QTc interval, urinary volume, kidney weight and Mn-SOD activity. Interestingly, most of the mentioned benefits of exercise were more consistent when protein hydrolysate was also administered.
Conclusion
The interesting synergies between the two interventions assessed qualify them as alternative therapeutic strategies to treat cardiovascular and kidney diseases associated to the metabolic syndrome.



Similar content being viewed by others
References
Grundy SM (2016) Metabolic syndrome update. Trends Cardiovasc Med 26:364–373. https://doi.org/10.1016/j.tcm.2015.10.004
Tune JD, Goodwill AG, Sassoon DJ, Mather KJ (2017) Cardiovascular consequences of metabolic syndrome. Transl Res 183:57–70. https://doi.org/10.1016/j.trsl.2017.01.001
Zhang X, Lerman L (2017) The metabolic syndrome and chronic kidney disease. Transl Res 183:14–25. https://doi.org/10.1016/j.trsl.2016.12.004
Saklayen MG (2018) The global epidemic of the metabolic syndrome. Curr Hypertens Rep 20(2):12. https://doi.org/10.1007/s11906-018-0812-z
Casacchia T, Rocca C, Granieri MC, Beretta G, Amelio D et al (2019) Leopoldia comosa prevents metabolic disorders in rats with high-fat diet-induced obesity. Eur J Nutr 58(3):965–979
Casacchia T, Occhiuzzia MA, Grandea F, Rizzuti B, Granieri MC, Rocca C et al (2019) A pilot study on the nutraceutical properties of the citrus hybrid Tacle® as a dietary source of polyphenols for supplementation in metabolic disorders. J Funct Foods 52:370–381
Brown L, Poudyal H, Panchal SK (2015) Functional foods as potential therapeutic options for metabolic syndrome. Obes Rev 16(11):914–941. https://doi.org/10.1111/obr.12313
Konstantinidi M, Koutelidakis AE (2019) Functional foods and bioactive compounds: a review of its possible role on weight management and obesity's metabolic consequences. Medicines (Basel) 6(3):E94. https://doi.org/10.3390/medicines6030094
Kapravelou G, Martínez R, Nebot E, López-Jurado M, Aranda P, Arrebola F et al (2017) The combined intervention with germinated Vigna radiata and aerobic interval training protocol is an effective strategy for the treatment of non-alcoholic fatty liver disease (NAFLD) and other alterations related to the metabolic syndrome in Zucker rats. Nutrients 9(7):E774. https://doi.org/10.3390/nu9070774
Martínez R, Kapravelou G, Donaire A, Lopez-Chaves C, Arrebola F, Galisteo M et al (2018) Effects of a combined intervention with a lentil protein hydrolysate and a mixed training protocol on the lipid metabolism and hepatic markers of NAFLD in Zucker rats. Food Funct 9:830–850. https://doi.org/10.1039/c7fo01790a
Urbano G, Porres JM, Frías J, Vidal-Valverde C (2007) Nutritional value. Lentil: an ancient crop for modern times. Springer, Dordetch, pp 47–93
Martínez R, López-Jurado M, Wanden-Berghe C, Sanz-Valero J, Porres JM, Kapravelou G (2016) Beneficial effects of legumes on parameters of the metabolic syndrome: a systematic review of trials in animal models. Br J Nutr 116(3):402–424. https://doi.org/10.1017/S0007114516001963
Becerra-Tomás N, Díaz-López A, Rosique-Esteban N, Ros E, Buil-Cosiales P, Corella D et al (2018) Legume consumption is inversely associated with type 2 diabetes incidence in adults: a prospective assessment from the PREDIMED study. Clin Nutr 37(3):906–913. https://doi.org/10.1016/j.clnu.2017.03.015
Kapravelou G, Martínez R, Andrade AM, López Chaves C, López-Jurado M, Aranda P et al (2015) Improvement of the antioxidant and hypolipidaemic effects of cowpea flours (Vigna unguiculata) by fermentation: results of in vitro and in vivo experiments. J Sci Food Agric 95:1207–1216. https://doi.org/10.1002/jsfa.6809
Xuan CL, Yao FR, Guo LR, Liu Q, Chang SK, Liu KX, Sun CW (2013) Comparison of extracts from cooked and raw lentil in antagonizing angiotensin II-induced hypertension and cardiac hypertrophy. Eur Rev Med Pharmacol Sci 17(19):2644–2653
Bautista-Expósito S, Martínez-Villaluenga C, Dueñas M, Silván JM, Frias J, Peñas E (2018) Combination of pH-controlled fermentation in mild conditions and enzymatic hydrolysis by Savinase as a cost-effective approach to improve metabolic health-promoting properties of lentils. J Funct Foods 48:9–18
Tabatabaei-Malazy O, Larijani B, Abdollahi M (2015) Targeting metabolic disorders by natural products. J Diabetes Metab Disord 14:57. https://doi.org/10.1186/s40200-015-0184-8
World Health Organization (2019) Global strategy on diet, physical activity and health. https://www.who.int/dietphysicalactivity/factsheet_adults/en/. Accessed 16 July 2019
Kapravelou G, Martínez R, Andrade AM, Nebot E, Camiletti-Moirón D, Aparicio VA et al (2015) Aerobic interval exercise improves parameters of nonalcoholic fatty liver disease (NAFLD) and other alterations of metabolic syndrome in obese Zucker rats. Appl Physiol Nutr Metab 40(12):1242–1252. https://doi.org/10.1139/apnm-2015-0141
Shen Y, Xu X, Yue K, Xu G (2015) Effect of different exercise protocols on metabolic profiles and fatty acid metabolism in skeletal muscle in high-fat diet-fed rats. Obesity 23(5):1000–1006. https://doi.org/10.1002/oby.21056
Rivera L, Morón R, Zarzuelo A, Galisteo M (2009) Long-term resveratrol administration reduces metabolic disturbances and lowers blood pressure in obese Zucker rats. Biochem Pharmacol 77(6):1053–1063. https://doi.org/10.1016/j.bcp.2008.11.027
Martínez R, Kapravelou G, López-Chaves C, Cáceres E, Coll-Risco I, Sánchez-González C et al (2019) Aerobic interval exercise improves renal functionality and affects mineral metabolism in obese Zucker rats. Am J Physiol Renal Physiol 316(1):F90–F100. https://doi.org/10.1152/ajprenal.00356.2018
Van Hoose L, Sawers Y, Loganathan R, Vacek JL, Stehno-Bittel L, Novikova L et al (2010) Electrocardiographic changes with the onset of diabetes and the impact of aerobic exercise training in the Zucker Diabetic Fatty (ZDF) rat. Cardiovasc Diabetol 9:56. https://doi.org/10.1186/1475-2840-9-56
Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes (2010) Official Journal of the European Union L276/33-79
Bautista-Expósito S, Peñas E, Dueñas M, Silván JM, Frias J, Martínez-Villaluenga C (2018) Individual contributions of Savinase and Lactobacillus plantarum to lentil functionalization during alkaline pH-controlled fermentation. Food Chem 257:341–349. https://doi.org/10.1016/j.foodchem.2018.03.044
Coll-Risco I, Aparicio VA, Nebot E, Camiletti-Moirón D, Martínez R, Kapravelou G et al (2016) Effects of interval aerobic training combined with strength exercise on body composition, glycaemic and lipid profile and aerobic capacity of obese rats. J Sports Sci 34:1452–1460. https://doi.org/10.1080/02640414.2015.1119296
Wisløff U, Helgerud J, Kemi OJ, Ellingsen Ø (2001) Intensity-controlled treadmill running in rats: V02max and cardiac hypertrophy. Am J Physiol Heart Circ Physiol 280:H1301–H1310. https://doi.org/10.1152/ajpheart.2001.280.3.H1301
Clemente VJC, Martín S, Porres J, Fuentes S, Ramírez PA (2011) Efecto de la suplementación de vitamina en el rendimiento de una prueba incremental de consumo máximo de oxígeno en ratas Wistar. Arch Med Dep XXVIII:168–173
Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28(7):412–419. https://doi.org/10.1007/BF00280883
Cohen G, Kim M, Ogwu V (1996) A modified catalase assay suitable for a plate reader and for the analysis of brain cell cultures. J Neurosci Methods 67:53–56
Lawrence RA, Sunde RA, Schwartz GL, Hoekstra WG (1974) Glutathione peroxidase activity in rat lens and other tissues in relation to dietary selenium intake. Exp Eye Res 18:563–569
Ukeda H, Maeda S, Ishii T, Sawamura M (1997) Spectrophotometric assay for superoxide dismutase based on tetrazolium Salt 3′-{1-[(Phenylamino)-carbonyl]-3,4-tetrazolium}-bis(4-methoxy-6-nitro) benzenesulfonic acid hydrate reduction by xanthine–xanthine oxidase. Anal Biochem 251:206–209. https://doi.org/10.1006/abio.1997.2273
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Ohkawa H, Ohishi N, Yagi K (1979) Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem 95:351–358
Martínez-Villaluenga C, Frias J, Vidal-Valverde C (2008) Alpha-galactosides: antinutritional factors or functional ingredients? Crit Rev Food Sci Nutr 48(4):301–316. https://doi.org/10.1080/10408390701326243
Hutchinson C (2016) A review of iron studies in overweight and obese children and adolescents: a double burden in the young? Eur J Nutr 55(7):2179–2197. https://doi.org/10.1007/s00394-016-1155-7
Moore E, Bellomo R (2011) Erythropoietin (EPO) in acute kidney injury. Ann Intensive Care 1(1):3. https://doi.org/10.1186/2110-5820-1-3
Urbano G, López-Jurado M, Aranda P, Vidal-Valverde C, Tenorio E, Porres J (2000) The role of phytic acid in legumes: antinutrient or beneficial function? J Physiol Biochem 56(3):283–294
Sponholtz T, van den Heuvel ER, Xanthakis V, Vasan RS (2019) Association of variability in body mass index and metabolic health with cardiometabolic disease risk. J Am Heart Assoc 8(7):e010793. https://doi.org/10.1161/JAHA.118.010793
Chen YF, Shibu MA, Fan MJ, Chen MC, Viswanadha VP, Lin YL et al (2016) Purple rice anthocyanin extract protects cardiac function in STZ-induced diabetes rat hearts by inhibiting cardiac hypertrophy and fibrosis. J Nutr Biochem 31:98–105. https://doi.org/10.1016/j.jnutbio.2015.12.020
Vega RB, Konhilas JP, Kelly DP, Leinwand LA (2017) Molecular mechanisms underlying cardiac adaptation to exercise. Cell Metab 25(5):1012–1026. https://doi.org/10.1016/j.cmet.2017.04.025
Superko HR, Momary KM, Pendyala LK, Williams PT, Frohwein S, Garrett BC et al (2011) Firefighters, heart disease, and aspects of insulin resistance: the FEMA Firefighter Heart Disease Prevention study. J Occup Environ Med 53(7):758–764. https://doi.org/10.1097/JOM.0b013e31821f64c3
Guo X, Li Z, Guo L, Yu S, Yang H, Zheng L et al (2017) Effects of metabolically healthy and unhealthy obesity on prolongation of corrected QT interval. Am J Cardiol 119(8):1199–1204. https://doi.org/10.1016/j.amjcard.2016.12.033
Mutiso SK, Rono DK, Bukachi F (2014) Relationship between anthropometric measures and early electrocardiographic changes in obese rats. BMC Res Notes 7:931. https://doi.org/10.1186/1756-0500-7-931
Patel MS, Miranda-Nieves D, Cheng J, Haller CA, Chaikof EL (2017) Targeting P-selectin glycoprotein ligand-1/ P-selectin interactions as a novel therapy for metabolic syndrome. Transl Res 183:1–13. https://doi.org/10.1016/j.trsl.2016.11.007
Younes H, Alphonse JC, Behr SR, Demigné C, Rémésy C (1999) Role of fermentable carbohydrate supplements with a low-protein diet in the course of chronic renal failure: experimental bases. Am J Kidney Dis 33(4):633–646
Solbu MD, Norvik JV, Storhaug HM, Eriksen BO, Melsom T, Eggen AE et al (2016) The association between adiponectin, serum uric acid and urinary markers of renal damage in the general population: cross-sectional data from the Tromsø Study. Kidney Blood Press Res 41(5):623–634. https://doi.org/10.1159/000447931
Sabboh H, Besson C, Tressol JC, Coudray C, Horcajada MN, Coxam V et al (2006) Organic potassium salts or fibers effects on mineral balance and digestive fermentations in rats adapted to an acidogenic diet. Eur J Nutr 45(6):342–348
Acknowledgements
The authors want to acknowledge the Spanish Ministry of Science, Innovation and Universities and the European Union through projects AGL2013-43247-R, DEP2014-58296-R, RTC-2017-6540-1, RTI2018-100934-B-I00 and FEDER program, respectively. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. This work is part of the PhD Thesis of Janin Constantino, included in the Doctoral Program in Nutrition and Food Sciences (Universidad de Granada). The special contribution to the development of the experiments by the Experimental Animal Unit of CIC (Universidad de Granada) is also acknowledged.
Author information
Authors and Affiliations
Contributions
JMP, PA, ML-J, and RM contributed to the study conception and design. Material preparation, data collection and analysis were performed by JMP, JC, GK, CL-C, MG, and RM. The first draft of the manuscript was written by JMP, MG, RM, and ML-J and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
There are no conflicts of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Porres, J.M., Constantino, J., Kapravelou, G. et al. The combined treatment with lentil protein hydrolysate and a mixed training protocol is an efficient lifestyle intervention to manage cardiovascular and renal alterations in obese Zucker rats. Eur J Nutr 59, 3473–3490 (2020). https://doi.org/10.1007/s00394-020-02181-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-020-02181-9