Abstract
Purpose
Obesity leads to the clustering of cardiovascular (CV) risk factors and the metabolic syndrome (MetS) also in children and is often accompanied by non-alcoholic fatty liver disease. Quality of dietary fat, beyond the quantity, can influence CV risk profile and, in particular, omega-3 fatty acids (FA) have been proposed as beneficial in this setting. The aim of the study was to evaluate the associations of individual CV risk factors, characterizing the MetS, with erythrocyte membrane FA, markers of average intake, in a group of 70 overweight/obese children.
Methods
We conducted an observational study. Erythrocyte membrane FA were measured by gas chromatography. Spearman correlation coefficients (rS) were calculated to evaluate associations between FA and features of the MetS.
Results
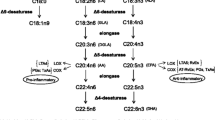
Mean content of Omega-3 FA was low (Omega-3 Index = 4.7 ± 0.8%). Not omega-3 FA but some omega-6 FA, especially arachidonic acid (AA), were inversely associated with several features of the MetS: AA resulted inversely correlated with waist circumference (rS = − 0.352), triglycerides (rS = − 0.379), fasting insulin (rS = − 0.337) and 24-h SBP (rS = − 0.313). Total amount of saturated FA (SFA) and specifically palmitic acid, correlated positively with waist circumference (rS = 0.354), triglycerides (rS = 0.400) and fasting insulin (rS = 0.287). Fatty Liver Index (FLI), a predictive score of steatosis based on GGT, triglycerides and anthropometric indexes, was positively correlated to palmitic acid (rS = 0.515) and inversely to AA (rS = − 0.472).
Conclusions
Our data suggest that omega-6 FA, and especially AA, could be protective toward CV risk factors featuring the MetS and also to indexes of hepatic steatosis in obese children, whereas SFA seems to exert opposite effects.


Similar content being viewed by others
Abbreviations
- AA:
-
Arachidonic acid
- ALA:
-
Alpha-linoleic acid
- ALT:
-
Alanine aminotransferase
- AST:
-
Aspartate aminotransferase
- BP:
-
Blood pressure
- CV:
-
Cardiovascular
- D5D:
-
Delta-5 desaturase
- D6D:
-
Delta-6 desaturase
- DBP:
-
Diastolic blood pressure
- DGLA:
-
Dihomo-gamma-linolenic acid
- DHA:
-
Docosahexaenoic acid
- EPA:
-
Eicosapentaenoic acid
- FA:
-
Fatty acid
- FLI:
-
Fatty Liver Index
- GGT:
-
Gamma-glutamylatransferase
- GLA:
-
Gamma-linolenic acid
- LA:
-
Linoleic acid
- MetS:
-
Metabolic syndrome
- NAFLD:
-
Non-alcoholic fatty liver disease
- O-DBP:
-
Office diastolic blood pressure
- O-SBP:
-
Office systolic blood pressure
- PA:
-
Palmitic acid
- RCT:
-
Randomized controlled trial
- SBP:
-
Systolic blood pressure
- SCD:
-
Delta-9 desaturase
- SFA:
-
Saturated fatty acid
- TRI:
-
Triglycerides
References
Steinberger J, Daniels SR, Eckel RH et al (2009) Progress and challenges in metabolic syndrome in children and adolescents: a scientific statement from the american heart association atherosclerosis, hypertension, and obesity in the young committee of the council on cardiovascular disease in the young. Circulation 119:628–647. https://doi.org/10.1161/CIRCULATIONAHA.108.191394
Nobili V, Carpino G, Alisi A et al (2014) Role of docosahexaenoic acid treatment in improving liver histology in pediatric nonalcoholic fatty liver disease. PLoS One 9:e88005. https://doi.org/10.1371/journal.pone.0088005
Musso G, Gambino R, Cassader M, Pagano G (2011) Meta-analysis: natural history of non-alcoholic fatty liver disease (NAFLD) and diagnostic accuracy of non-invasive tests for liver disease severity. Ann Med 43:617–649. https://doi.org/10.3109/07853890.2010.518623
Vessby B, Uusitupa M, Hermansen K et al (2001) Substituting dietary saturated for monounsaturated fat impairs insulin sensitivity in healthy men and women: the KANWU Study. Diabetologia 44:312–319
Chiu S, Williams PT, Krauss RM (2017) Effects of a very high saturated fat diet on LDL particles in adults with atherogenic dyslipidemia: a randomized controlled trial. PLoS One 12:e0170664. https://doi.org/10.1371/journal.pone.0170664
Mozaffarian D, Micha R, Wallace S (2010) Effects on coronary heart disease of increasing polyunsaturated fat in place of saturated fat: a systematic review and meta-analysis of randomized controlled trials. PLoS Med 7:e1000252. https://doi.org/10.1371/journal.pmed.1000252
de Souza RJ, Mente A, Maroleanu A et al (2015) Intake of saturated and trans unsaturated fatty acids and risk of all cause mortality, cardiovascular disease, and type 2 diabetes: systematic review and meta-analysis of observational studies. BMJ 351:h3978. https://doi.org/10.1136/bmj.h3978
Derosa G, Cicero AFG, D’Angelo A et al (2016) Effects of n-3 pufas on fasting plasma glucose and insulin resistance in patients with impaired fasting glucose or impaired glucose tolerance. Biofactors 42:316–322. https://doi.org/10.1002/biof.1277
Miller PE, Van Elswyk M, Alexander DD (2014) Long-chain omega-3 fatty acids eicosapentaenoic acid and docosahexaenoic acid and blood pressure: a meta-analysis of randomized controlled trials. Am J Hypertens 27:885–896. https://doi.org/10.1093/ajh/hpu024
Bonafini S, Antoniazzi F, Maffeis C et al (2015) Beneficial effects of ω-3 PUFA in children on cardiovascular risk factors during childhood and adolescence. Prostaglandins Other Lipid Mediat. https://doi.org/10.1016/j.prostaglandins.2015.03.006
Bonafini S, Fava C (2017) Omega-3 fatty acids and cytochrome P450-derived eicosanoids in cardiovascular diseases: which actions and interactions modulate hemodynamics? Prostaglandins Other Lipid Mediat 128–129:34–42. https://doi.org/10.1016/j.prostaglandins.2017.01.004
Zhang YY, Liu W, Zhao TY, Tian HM (2017) Efficacy of omega-3 polyunsaturated fatty acids supplementation in managing overweight and obesity: a meta-analysis of randomized clinical trials. J Nutr Health Aging 21:187–192. https://doi.org/10.1007/s12603-016-0755-5
Calder PC (2013) Omega-3 polyunsaturated fatty acids and inflammatory processes: nutrition or pharmacology? Br J Clin Pharmacol 75:645–662. https://doi.org/10.1111/j.1365-2125.2012.04374.x
Wolfram G, Bechthold A, Boeing H et al (2015) Evidence-based guideline of the german nutrition society: fat intake and prevention of selected nutrition-related diseases. Ann Nutr Metab 67:141–204. https://doi.org/10.1159/000437243
Marventano S, Kolacz P, Castellano S et al (2015) A review of recent evidence in human studies of n-3 and n-6 PUFA intake on cardiovascular disease, cancer, and depressive disorders: does the ratio really matter? Int J Food Sci Nutr 66:611–622. https://doi.org/10.3109/09637486.2015.1077790
Belury MA, Cole RM, Bailey BE et al (2016) Erythrocyte linoleic acid, but not oleic acid, is associated with improvements in body composition in men and women. Mol Nutr Food Res 60:1206–1212. https://doi.org/10.1002/mnfr.201500744
Wang DD, Li Y, Chiuve SE et al (2016) Association of specific dietary fats with total and cause-specific mortality. JAMA Intern Med 176:1134–1145. https://doi.org/10.1001/jamainternmed.2016.2417
Vanhala M, Saltevo J, Soininen P et al (2012) Serum omega-6 polyunsaturated fatty acids and the metabolic syndrome: a longitudinal population-based cohort study. Am J Epidemiol 176:253–260. https://doi.org/10.1093/aje/kwr504
Cole TJ, Bellizzi MC, Flegal KM, Dietz WH (2000) Establishing a standard definition for child overweight and obesity worldwide: international survey. BMJ 320:1240–1243
Tagetti A, Bonafini S, Zaffanello M et al (2017) Sleep-disordered breathing is associated with blood pressure and carotid arterial stiffness in obese children. J Hypertens 35:125–131. https://doi.org/10.1097/HJH.0000000000001123
Wühl E, Witte K, Soergel M et al (2002) Distribution of 24-h ambulatory blood pressure in children: normalized reference values and role of body dimensions. J Hypertens 20:1995–2007
National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents (2004) The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics 114:555–576
Maffeis C, Grezzani A, Pietrobelli A et al (2001) Does waist circumference predict fat gain in children? Int J Obes Relat Metab Disord 25:978–983. https://doi.org/10.1038/sj.ijo.0801641
Sharma AK, Metzger DL, Daymont C et al (2015) LMS tables for waist-circumference and waist-height ratio Z-scores in children aged 5–19 y in NHANES III: association with cardio-metabolic risks. Pediatr Res 78:1–7. https://doi.org/10.1038/pr.2015.160
Zimmet P, Alberti KGM, Kaufman F et al (2007) The metabolic syndrome in children and adolescents—an IDF consensus report. Pediatr Diabetes 8:299–306. https://doi.org/10.1111/j.1399-5448.2007.00271.x
Lurbe E, Agabiti-Rosei E, Cruickshank JK et al (2016) 2016 European Society of Hypertension guidelines for the management of high blood pressure in children and adolescents. J Hypertens 34:1887–1920. https://doi.org/10.1097/HJH.0000000000001039
Matthews DR, Hosker JP, Rudenski AS et al (1985) Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia 28:412–419
Bedogni G, Bellentani S, Miglioli L et al (2006) The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol 6:33. https://doi.org/10.1186/1471-230X-6-33
Harris WS, Von Schacky C (2004) The Omega-3 Index: a new risk factor for death from coronary heart disease? Prev Med (Baltim) 39:212–220. https://doi.org/10.1016/j.ypmed.2004.02.030
Alsharari ZD, Risérus U, Leander K et al (2017) Serum fatty acids, desaturase activities and abdominal obesity—a population-based study of 60-year old men and women. PLoS One 12:e0170684. https://doi.org/10.1371/journal.pone.0170684
Heuer T, Krems C, Moon K et al (2015) Food consumption of adults in Germany: results of the German National Nutrition Survey II based on diet history interviews. Br J Nutr 113:1603–1614. https://doi.org/10.1017/S0007114515000744
Ahrén B, Mari A, Fyfe CL et al (2009) Effects of conjugated linoleic acid plus n-3 polyunsaturated fatty acids on insulin secretion and estimated insulin sensitivity in men. Eur J Clin Nutr 63:778–786. https://doi.org/10.1038/ejcn.2008.45
Akinkuolie AO, Ngwa JS, Meigs JB, Djoussé L (2011) Omega-3 polyunsaturated fatty acid and insulin sensitivity: a meta-analysis of randomized controlled trials. Clin Nutr 30:702–707. https://doi.org/10.1016/j.clnu.2011.08.013
Harris WS (2010) The omega-3 index: clinical utility for therapeutic intervention. Curr Cardiol Rep 12:503–508. https://doi.org/10.1007/s11886-010-0141-6
Sun D, Cuevas AJ, Gotlinger K et al (2014) Soluble epoxide hydrolase-dependent regulation of myogenic response and blood pressure. Am J Physiol Heart Circ Physiol 306:H1146–H1153. https://doi.org/10.1152/ajpheart.00920.2013
Ubhayasekera SJKA., Staaf J, Forslund A et al (2013) Free fatty acid determination in plasma by GC-MS after conversion to Weinreb amides. Anal Bioanal Chem 405:1929–1935. https://doi.org/10.1007/s00216-012-6658-3
Sato Y, Fujimoto S, Mukai E et al (2014) Palmitate induces reactive oxygen species production and β-cell dysfunction by activating nicotinamide adenine dinucleotide phosphate oxidase through Src signaling. J Diabetes Investig 5:19–26. https://doi.org/10.1111/jdi.12124
Vasu S, McClenaghan NH, McCluskey JT, Flatt PR (2013) Effects of lipotoxicity on a novel insulin-secreting human pancreatic β-cell line, 1.1B4. Biol Chem. https://doi.org/10.1515/hsz-2013-0115
Bermudez B, Ortega-Gomez A, Varela LM et al (2014) Clustering effects on postprandial insulin secretion and sensitivity in response to meals with different fatty acid compositions. Food Funct 5:1374–1380. https://doi.org/10.1039/c4fo00067f
Staaf J, Ubhayasekera SJKA., Sargsyan E et al (2016) Initial hyperinsulinemia and subsequent β-cell dysfunction is associated with elevated palmitate levels. Pediatr Res 80:267–274. https://doi.org/10.1038/pr.2016.80
Aristizabal JC, Barona J, Gonzalez-Zapata LI et al (2016) Fatty acid content of plasma triglycerides may contribute to the heterogeneity in the relationship between abdominal obesity and the metabolic syndrome. Metab Syndr Relat Disord 14:311–317. https://doi.org/10.1089/met.2015.0168
Wang L, Manson JE, Forman JP et al (2010) Dietary fatty acids and the risk of hypertension in middle-aged and older women. Hypertension 56:598–604. https://doi.org/10.1161/HYPERTENSIONAHA.110.154187
Vafeiadou K, Weech M, Altowaijri H et al (2015) Replacement of saturated with unsaturated fats had no impact on vascular function but beneficial effects on lipid biomarkers, E-selectin, and blood pressure: results from the randomized, controlled Dietary Intervention and VAScular function (DIVAS) study. Am J Clin Nutr 102:40–48. https://doi.org/10.3945/ajcn.114.097089
Miura K, Stamler J, Nakagawa H et al (2008) Relationship of dietary linoleic acid to blood pressure: the international study of macro-micronutrients and blood pressure study. Hypertension 52:408–414. https://doi.org/10.1161/HYPERTENSIONAHA.108.112383
Böhm A, Halama A, Meile T et al (2014) Metabolic signatures of cultured human adipocytes from metabolically healthy versus unhealthy obese individuals. PLoS One 9:e93148. https://doi.org/10.1371/journal.pone.0093148
Aldámiz-Echevarría L, Prieto JA, Andrade F et al (2007) Arachidonic acid content in adipose tissue is associated with insulin resistance in healthy children. J Pediatr Gastroenterol Nutr 44:77–83. https://doi.org/10.1097/01.mpg.0000237931.53470.ba
Das UN (2013) Arachidonic acid and lipoxin A4 as possible endogenous anti-diabetic molecules. Prostaglandins Leukot Essent Fatty Acids 88:201–210. https://doi.org/10.1016/j.plefa.2012.11.009
Clifton PM, Nestel PJ (1998) Relationship between plasma insulin and erythrocyte fatty acid composition. Prostaglandins Leukot Essent Fatty Acids 59:191–194
Felton CV, Stevenson JC, Godsland IF (2004) Erythrocyte-derived measures of membrane lipid composition in healthy men: associations with arachidonic acid at low to moderate but not high insulin sensitivity. Metabolism 53:571–577
Arm JP, Boyce JA, Wang L et al (2013) Impact of botanical oils on polyunsaturated fatty acid metabolism and leukotriene generation in mild asthmatics. Lipids Health Dis 12:141. https://doi.org/10.1186/1476-511X-12-141
Imamura F, Sharp SJ, Koulman A et al (2017) A combination of plasma phospholipid fatty acids and its association with incidence of type 2 diabetes: the EPIC-InterAct case-cohort study. PLoS Med 14:e1002409. https://doi.org/10.1371/journal.pmed.1002409
Delgado GE, März W, Lorkowski S et al (2017) Omega-6 fatty acids: Opposing associations with risk—the Ludwigshafen Risk and Cardiovascular Health Study. J Clin Lipidol 11:1082–1090.e14. https://doi.org/10.1016/j.jacl.2017.05.003
Akter S, Kurotani K, Sato M et al (2017) High serum phospholipid dihomo-γ-linoleic acid concentration and low ∆5-desaturase activity are associated with increased risk of type 2 diabetes among japanese adults in the hitachi health study. J Nutr 147:1558–1566. https://doi.org/10.3945/jn.117.248997
Hua M-C, Su H-M, Yao T-C et al (2017) Alternation of plasma fatty acids composition and desaturase activities in children with liver steatosis. PLoS One 12:e0182277. https://doi.org/10.1371/journal.pone.0182277
Nilsen DWT, Aarsetoey H, Pönitz V et al (2017) The prognostic utility of dihomo-gamma-linolenic acid (DGLA) in patients with acute coronary heart disease. Int J Cardiol 249:12–17. https://doi.org/10.1016/j.ijcard.2017.09.202
Ouchi S, Miyazaki T, Shimada K et al (2017) Decreased circulating dihomo-gamma-linolenic acid levels are associated with total mortality in patients with acute cardiovascular disease and acute decompensated heart failure. Lipids Health Dis 16:150. https://doi.org/10.1186/s12944-017-0542-2
Daneshmand R, Kurl S, Tuomainen T-P, Virtanen JK (2017) Associations of estimated ∆-5-desaturase and ∆-6-desaturase activities with stroke risk factors and risk of stroke: the Kuopio Ischaemic Heart Disease Risk Factor Study. Br J Nutr 117:582–590. https://doi.org/10.1017/S000711451700054X
Warensjö E, Sundström J, Lind L, Vessby B (2006) Factor analysis of fatty acids in serum lipids as a measure of dietary fat quality in relation to the metabolic syndrome in men. Am J Clin Nutr 84:442–448
Vessby B, Gustafsson I-B, Tengblad S et al (2002) Desaturation and elongation of fatty acids and insulin action. Ann N Y Acad Sci 967:183–195
Maffeis C, Banzato C, Talamini G, Obesity Study Group of the Italian Society of Pediatric Endocrinology and Diabetology (2008) Waist-to-height ratio, a useful index to identify high metabolic risk in overweight children. J Pediatr 152:207–213. https://doi.org/10.1016/j.jpeds.2007.09.021
Maffeis C, Corciulo N, Livieri C et al (2003) Waist circumference as a predictor of cardiovascular and metabolic risk factors in obese girls. Eur J Clin Nutr 57:566–572. https://doi.org/10.1038/sj.ejcn.1601573
Reccia I, Kumar J, Akladios C et al (2017) Non-alcoholic fatty liver disease: a sign of systemic disease. Metabolism 72:94–108. https://doi.org/10.1016/j.metabol.2017.04.011
Jiang J, Zhang S, Liu Y et al (2014) EETs alleviate ox-LDL-induced inflammation by inhibiting LOX-1 receptor expression in rat pulmonary arterial endothelial cells. Eur J Pharmacol 727:43–51. https://doi.org/10.1016/j.ejphar.2014.01.045
Shahabi P, Siest G, Meyer UA, Visvikis-Siest S (2014) Human cytochrome P450 epoxygenases: variability in expression and role in inflammation-related disorders. Pharmacol Ther 144:134–161. https://doi.org/10.1016/j.pharmthera.2014.05.011
Arnold C, Konkel A, Fischer R, Schunck W-HH (2010) Cytochrome P450-dependent metabolism of omega-6 and omega-3 long-chain polyunsaturated fatty acids. PharmacolRep 62:536–547
Sergeant S, Rahbar E, Chilton FH (2016) Gamma-linolenic acid, Dihommo-gamma linolenic, eicosanoids and inflammatory processes. Eur J Pharmacol 785:77–86. https://doi.org/10.1016/j.ejphar.2016.04.020
Acknowledgements
Part of this work was performed in the LURM (Laboratorio Universitario di Ricerca Medica) Research Center, University of Verona.
Funding
The study is supported by a Grant of the Italian Ministry of Health (GR-2011-02349630) to CF in agreement with the ‘Regione Veneto’ and the ‘Azienda Ospedaliera Universitaria Integrata di Verona’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The author declares that they have no competing interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bonafini, S., Tagetti, A., Gaudino, R. et al. Individual fatty acids in erythrocyte membranes are associated with several features of the metabolic syndrome in obese children. Eur J Nutr 58, 731–742 (2019). https://doi.org/10.1007/s00394-018-1677-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-018-1677-2