Abstract
Purpose
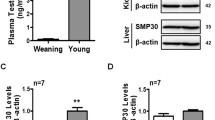
The effect of an AA deficiency on catecholamine biosynthesis in adult mice in vivo is unknown. Therefore, we quantified catecholamine and the expression of catecholamine synthetic enzymes in the adrenal glands of senescence marker protein-30 (SMP30)/gluconolactonase (GNL) knockout (KO) mice placed in an AA-deficient state.
Methods
At 30 days of age, mice were divided into the following 4 groups: AA (−) SMP30/GNL KO, AA (+) SMP30/GNL KO, AA (−) wild type (WT), and AA (+) WT. The AA (+) groups were given water containing 1.5 g/L AA, whereas the AA (−) groups received water without AA until the experiment ended. In addition, all mice were fed an AA-depleted diet. Catecholamine levels were measured by a liquid chromatographic method. Tyrosine hydroxylase, dopa decarboxylase, dopamine β-hydroxylase, and phenylethanolamine N-methyltransferase mRNA expression levels were measured with the quantitative real-time polymerase chain reaction (qPCR). Tyrosine hydroxylase and dopamine β-hydroxylase protein levels were quantified by Western blot analysis.
Results
In the adrenals of AA (−) SMP30/GNL KO mice, noradrenaline and adrenaline levels decreased significantly compared to other three groups of mice, although there were no significant differences in dopamine β-hydroxylase or phenylethanolamine N-methyltransferase mRNA content. Moreover, there was no significant difference in their dopamine β-hydroxylase protein levels. On the other hand, AA depletion did not affect dopamine levels in adrenal glands of mice.
Conclusion
An AA deficiency decreases the noradrenaline and adrenaline levels in adrenal glands of mice in vivo.






Similar content being viewed by others
Abbreviations
- AA:
-
Ascorbic acid
- AD:
-
Adrenaline
- ANOVA:
-
Analysis of variance
- BH3 · radical:
-
Trihydrobiopterin radical
- BH4 :
-
Tetrahydrobiopterin
- cDNA:
-
Complementary DNA
- Cu+ :
-
Cuprous ion
- Ct:
-
Threshold cycle
- DA:
-
Dopamine
- DBH:
-
Dopamine β-hydroxylase
- DDC:
-
Dopa decarboxylase
- ECD:
-
Electrochemical detector
- EDTA:
-
Ethylenediaminetetraacetic acid
- GAPDH:
-
Glyceraldehyde 3-phosphate dehydrogenase
- GNL:
-
Gluconolactonase
- HPLC:
-
High-performance liquid chromatography
- KO:
-
Knockout
- NA:
-
Noradrenaline
- PNMT:
-
Phenylethanolamine N-methyltransferase
- qPCR:
-
Quantitative real-time polymerase chain reaction
- RGN:
-
Regucalcin
- SMP30:
-
Senescence marker protein-30
- SVCT:
-
Sodium-dependent vitamin C transporter
- TH:
-
Tyrosine hydroxylase
- WT:
-
Wild type
References
Kawashima T, Ohkubo K, Fukuzumi S (2010) Radical scavenging reactivity of catecholamine neurotransmitters and the inhibition effect for DNA cleavage. J Phys Chem B 114:675–680. doi:10.1021/jp909314t
Axelrod J (1974) Neurotransmitters. Sci Am 230:59–71
Ziegler MG, Elayan H, Milic M, Sun P, Gharaibeh M (2012) Epinephrine and the metabolic syndrome. Curr Hypertens Rep 14:1–7. doi:10.1007/s11906-011-0243-6
Nagatsu T, Stjarne L (1998) Catecholamine synthesis and release. Overview. Adv Pharmacol 42:1–14
Fitzpatrick PF (1989) The metal requirement of rat tyrosine hydroxylase. Biochem Biophys Res Commun 161:211–215
Frantom PA, Seravalli J, Ragsdale SW, Fitzpatrick PF (2006) Reduction and oxidation of the active site iron in tyrosine hydroxylase: kinetics and specificity. Biochemistry 45:2372–2379. doi:10.1021/bi052283j
Haavik J, Martinez A, Olafsdottir S, Mallet J, Flatmark T (1992) The incorporation of divalent metal ions into recombinant human tyrosine hydroxylase apoenzymes studied by intrinsic fluorescence and 1H-NMR spectroscopy. Eur J Biochem 210:23–31
Seitz G, Gebhardt S, Beck JF, Bohm W, Lode HN, Niethammer D, Bruchelt G (1998) Ascorbic acid stimulates DOPA synthesis and tyrosine hydroxylase gene expression in the human neuroblastoma cell line SK-N-SH. Neurosci Lett 244:33–36
Ramsey AJ, Hillas PJ, Fitzpatrick PF (1996) Characterization of the active site iron in tyrosine hydroxylase. Redox states of the iron. J Biol Chem 271:24395–24400
Kuzkaya N, Weissmann N, Harrison DG, Dikalov S (2003) Interactions of peroxynitrite, tetrahydrobiopterin, ascorbic acid, and thiols: implications for uncoupling endothelial nitric-oxide synthase. J Biol Chem 278:22546–22554. doi:10.1074/jbc.M302227200
Haavik J, Blau N, Thony B (2008) Mutations in human monoamine-related neurotransmitter pathway genes. Hum Mutat 29:891–902. doi:10.1002/humu.20700
Fortin D, Coulon JF, Roberge AG (1993) Comparative study of biochemical parameters and kinetic properties of dopamine-β-hydroxylase activity from cat and rat adrenals. Comp Biochem Physiol B 104:567–575
Evans JP, Ahn K, Klinman JP (2003) Evidence that dioxygen and substrate activation are tightly coupled in dopamine β-monooxygenase. Implications for the reactive oxygen species. J Biol Chem 278:49691–49698. doi:10.1074/jbc.M300797200
Huyghe BG, Klinman JP (1991) Activity of membranous dopamine β-monooxygenase within chromaffin granule ghosts. Interaction with ascorbate. J Biol Chem 266:11544–11550
Alexandre B, Humberto F, Learmonth DA, Patrício S (2009) Dopamine β-monooxygenase: mechanism, substrates and inhibitors. Curr Enzyme Inhib 5:27–43
Koh DS, Hille B (1997) Modulation by neurotransmitters of catecholamine secretion from sympathetic ganglion neurons detected by amperometry. Proc Natl Acad Sci USA 94:1506–1511
Horio F, Ozaki K, Yoshida A, Makino S, Hayashi Y (1985) Requirement for ascorbic acid in a rat mutant unable to synthesize ascorbic acid. J Nutr 115:1630–1640
Lane DJ, Lawen A (2009) Ascorbate and plasma membrane electron transport-enzymes vs efflux. Free Radic Biol Med 47:485–495
Rose RC, Bode AM (1993) Biology of free radical scavengers: an evaluation of ascorbate. FASEB J 7:1135–1142
Nakashima Y, Suzue R, Sanada H, Kawada S (1972) Effect of ascorbic acid on tyrosine hydroxylase activity in vivo. Arch Biochem Biophys 152:515–520
Kawai K, Ito H, Kubota H, Takemori K, Makino S, Horio F (2003) Changes in catecholamine metabolism by ascorbic acid deficiency in spontaneously hypertensive rats unable to synthesize ascorbic acid. Life Sci 72:1717–1732
Nakashima Y, Suzue R, Sanada H, Kawada S (1970) Effect of ascorbic acid on hydroxylase activity. I. Stimulation of tyrosine hydroxylase and tryptophan-5-hydroxylase activities by ascorbic acid. J Vitaminol 16:276–280
Bornstein SR, Yoshida-Hiroi M, Sotiriou S, Levine M, Hartwig HG, Nussbaum RL, Eisenhofer G (2003) Impaired adrenal catecholamine system function in mice with deficiency of the ascorbic acid transporter (SVCT2). FASEB J 17:1928–1930. doi:10.1096/fj.02-1167fje
Savini I, Rossi A, Pierro C, Avigliano L, Catani MV (2008) SVCT1 and SVCT2: key proteins for vitamin C uptake. Amino Acids 34:347–355. doi:10.1007/s00726-007-0555-7
Fujita T, Uchida K, Maruyama N (1992) Purification of senescence marker protein-30 (SMP30) and its androgen-independent decrease with age in the rat liver. Biochim Biophys Acta 1116:122–128
Yamaguchi M, Yamamoto T (1978) Purification of calcium binding substance from soluble fraction of normal rat liver. Chem Pharm Bull 26:1915–1918
Kondo Y, Inai Y, Sato Y, Handa S, Kubo S, Shimokado K, Goto S, Nishikimi M, Maruyama N, Ishigami A (2006) Senescence marker protein 30 functions as gluconolactonase in L-ascorbic acid biosynthesis, and its knockout mice are prone to scurvy. Proc Natl Acad Sci USA 103:5723–5728
Ishigami A, Fujita T, Handa S, Shirasawa T, Koseki H, Kitamura T, Enomoto N, Sato N, Shimosawa T, Maruyama N (2002) Senescence marker protein-30 knockout mouse liver is highly susceptible to tumor necrosis factor-α- and Fas-mediated apoptosis. Am J Pathol 161:1273–1281
Sato Y, Kajiyama S, Amano A, Kondo Y, Sasaki T, Handa S, Takahashi R, Fukui M, Hasegawa G, Nakamura N, Fujinawa H, Mori T, Ohta M, Obayashi H, Maruyama N, Ishigami A (2008) Hydrogen-rich pure water prevents superoxide formation in brain slices of vitamin C-depleted SMP30/GNL knockout mice. Biochem Biophys Res Commun 375:346–350
Kondo Y, Sasaki T, Sato Y, Amano A, Aizawa S, Iwama M, Handa S, Shimada N, Fukuda M, Akita M, Lee J, Jeong KS, Maruyama N, Ishigami A (2008) Vitamin C depletion increases superoxide generation in brains of SMP30/GNL knockout mice. Biochem Biophys Res Commun 377:291–296
Ishikawa Y, Hashizume K, Kishimoto S, Tezuka Y, Nishigori H, Yamamoto N, Kondo Y, Maruyama N, Ishigami A, Kurosaka D (2012) Effect of vitamin C depletion on UVR-B induced cataract in SMP30/GNL knockout mice. Exp Eye Res 94:85–89. doi:10.1016/j.exer.2011.11.010
Koike K, Kondo Y, Sekiya M, Sato Y, Tobino K, Iwakami SI, Goto S, Takahashi K, Maruyama N, Seyama K, Ishigami A (2010) Complete lack of vitamin C intake generates pulmonary emphysema in senescence marker protein-30 knockout mice. Am J Physiol Lung Cell Mol Physiol 298:L784–L792. doi:00256.2009
Arai KY, Sato Y, Kondo Y, Kudo C, Tsuchiya H, Nomura Y, Ishigami A, Nishiyama T (2009) Effects of vitamin C deficiency on the skin of the senescence marker protein-30 (SMP30) knockout mouse. Biochem Biophys Res Commun 385:478–483
Sato Y, Arai KY, Nishiyama T, Nomura Y, Kishimoto Y, Aizawa S, Maruyama N, Ishigami A (2012) Ascorbic acid deficiency leads to epidermal atrophy and UVB-induced skin pigmentation in SMP30/GNL knockout hairless mice. J Invest Dermatol 132:2112–2115. doi:10.1038/jid.2012.105
Amano A, Aigaki T, Maruyama N, Ishigami A (2010) Ascorbic acid depletion enhances expression of the sodium-dependent vitamin C transporters, SVCT1 and SVCT2, and uptake of ascorbic acid in livers of SMP30/GNL knockout mice. Arch Biochem Biophys 496:38–44
Furusawa H, Sato Y, Tanaka Y, Inai Y, Amano A, Iwama M, Kondo Y, Handa S, Murata A, Nishikimi M, Goto S, Maruyama N, Takahashi R, Ishigami A (2008) Vitamin C is not essential for carnitine biosynthesis in vivo: verification in vitamin C-depleted senescence marker protein-30/gluconolactonase knockout mice. Biol Pharm Bull 31:1673–1679
Iwama M, Shimokado K, Maruyama N, Ishigami A (2011) Time course of vitamin C distribution and absorption after oral administration in SMP30/GNL knockout mice. Nutrition 27:471–478. doi:10.1016/j.nut.2010.04.010
Sato Y, Uchiki T, Iwama M, Kishimoto Y, Takahashi R, Ishigami A (2010) Determination of dehydroascorbic acid in mouse tissues and plasma by using tris(2-carboxyethyl)phosphine hydrochloride as reductant in metaphosphoric acid/ethylenediaminetetraacetic acid solution. Biol Pharm Bull 33:364–369
Tsunoda M (2006) Recent advances in methods for the analysis of catecholamines and their metabolites. Anal Bioanal Chem 386:506–514. doi:10.1007/s00216-006-0675-z
Chomczynski P, Sacchi N (1987) Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem 162:156–159. doi:10.1006/abio.1987.99990003-2697(87)90021-2
Aalinkeel R, Hu Z, Nair BB, Sykes DE, Reynolds JL, Mahajan SD, Schwartz SA (2010) Genomic analysis highlights the role of the JAK-STAT Signaling in the anti-proliferative effects of dietary flavonoid-‘Ashwagandha’ in prostate cancer cells. Evid Based Complement Alternat Med 7:177–187
Zhu W, Qin W, Atasoy U, Sauter ER (2009) Circulating microRNAs in breast cancer and healthy subjects. BMC Res Notes 2:89
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Towbin H, Staehelin T, Gordon J (1979) Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA 76:4350–4354
Tsuchimochi H, Nakamoto T, Matsukawa K (2010) Centrally evoked increase in adrenal sympathetic outflow elicits immediate secretion of adrenaline in anaesthetized rats. Exp Physiol 95:93–106
Markoglou N, Wainer IW (2001) Synthesis and characterization of immobilized dopamine β-hydroxylase in membrane-bound and solubilized formats. J Biochem Biophys Methods 48:61–75
Nagatsu T, Levitt M, Udenfriend S (1964) Tyrosine hydroxylase. The initial step in norepinephrine biosynthesis. J Biol Chem 239:2910–2917
Levine M, Morita K, Heldman E, Pollard HB (1985) Ascorbic acid regulation of norepinephrine biosynthesis in isolated chromaffin granules from bovine adrenal medulla. J Biol Chem 260:15598–15603
Sears ML (1969) Vitamin C as a requirement for the storage of norepinephrine by the iris. Biochem Pharmacol 18:253–256
Acknowledgments
The authors thank Ms. Phyllis Minick for the excellent English editorial assistance. Vitamin C powder was kindly provided by DSM Nutrition Japan. This study is supported by Japan Society for the Promotion of Science (JSPS) KAKENHI Grant Number 24380073 (A.I.) and 23590441 (N.M.) and by JSPS Research Fellowship for Young Scientists Grant Number 00234778 (A.A.).
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Amano, A., Tsunoda, M., Aigaki, T. et al. Effect of ascorbic acid deficiency on catecholamine synthesis in adrenal glands of SMP30/GNL knockout mice. Eur J Nutr 53, 177–185 (2014). https://doi.org/10.1007/s00394-013-0515-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00394-013-0515-9