Abstract
Although nesting ecology is well studied in several crocodilian species, it is not known how nest attendance influences physiology and body condition of nesting females. In this study, we describe body condition and serum biochemical values of nesting female, non-nesting female and male spectacled caiman (Caiman crocodilus) and black caiman (Melanosuchus niger) in two areas of Central Amazonia. We also evaluated the effect of nest age and nest distance to water on body condition and blood parameters of nesting females. Body condition and plasmatic concentrations of glucose, triglycerides, lactate and uric acid of nesting females were significantly different from those of non-nesting females and males in C. crocodilus, but not in M. niger. Our study also demonstrated that nest age and distance to water had a negative effect on female body condition in C. crocodilus, but not in M. niger. Female C. crocodilus attending older nests or nests built further away from permanent water bodies tended to have lower body condition. Our results demonstrate that the nesting strategy of C. crocodilus has a metabolic cost associated with nest attendance for nesting females, which appear to depend on accumulated energetic reserves during nest attendance. In contrast, nest attendance had little effect on the physiology of female M. niger.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Body condition analysis is a non-destructive and non-invasive method that has proven valuable in ecological fields of many invertebrate (Moya-Laraño et al. 2008) and vertebrate species (Kitaysky et al. 1999; Stevenson and Woods 2006; Mazzotti et al. 2009). Body condition of crocodilians (Zweig et al. 2014) has been used in studies of the effects of environmental variables (Fujisaki et al. 2009; Mazzotti et al. 2012), diet (Santos et al. 1996; Delany et al. 1999), growth (Saafeld et al. 2008) and haematological and parasitological parameters (Padilla-Paz 2008). However, there is no available information on how nest attendance may influence physiology and body condition of nesting female crocodilians.
At least three of the four crocodilian species that occur in the Amazon basin construct their mound nests in várzea (seasonal flooded forest) habitats during the annual dry season, when the water levels are at their lowest values (Thorbjarnarson et al. 2000; Villamarín et al. 2011). When gravid females migrate to nesting areas, males and non-nesting females remain in the main water canals (Thorbjarnarson 1994; Ayarzagϋena and Castroviejo 2008). The spectacled caiman (Caiman crocodilus) is the species with the most generalist nesting strategy; some nests are built along the margins of lakes and canals, while others are deep inside the forest, sometimes at distances of hundreds of metres from a permanent water body (Da Silveira et al. 2010; Villamarín et al. 2011). Nesting female C. crocodilus usually remain hidden in vegetation near the nest, frequently under leaf litter or in the debris of fallen trees, where they attend the nest in an energy-conserving (they endure periods of food deprivation while attending nests; Barão-Nóbrega et al. 2016) lethargic state during the entire incubation period (Staton and Dixon 1977; Marioni et al. 2007). In contrast, nesting females of the sympatric black caiman (Melanosuchus niger) construct their nests close to the water, usually near lakes isolated from the main water canal during the dry season (Da Silveira et al. 1997; Villamarín et al. 2011). Female M. niger remain near their nests during the egg incubation period, usually in the water (where they have access to food resources), and often defend them aggressively (more energy demanding than just attending) against potential predators (Thorbjarnarson et al. 2000).
Body and physiological condition of nesting females (and thus nest attendance behaviour) are likely to depend on how long they have been attending the nest and how difficult it is for them to reach water, which depends on how far the nest is into the forest. As nesting C. crocodilus often uses terrestrial habitats far from water, it is important to consider the influence of dehydration on body condition analysis (Lane 1996; Coppo et al. 2006). The American alligator (Alligator mississippiensis) loses water due to evaporation at a rate that is directly related to temperature and inversely related to body size (Ross 1989). Without access to water, young crocodilians may lose up to 20% of their body weight per day (Coppo et al. 2006).
Although the effects of nest attendance on the nutritional and physiological condition of nesting female caimans have not been assessed previously, they can be evaluated by measuring the plasma concentrations of certain metabolites, such as glucose, lactate, and plasmatic triglycerides, which are the main source of metabolic energy in crocodilians (Black et al. 1963). For example, C. yacare, which endures periods of food deprivation due to not having immediate access to water in the dry season, exhibited significant reduction in plasmatic glucose and triglycerides (Campbell et al. 2008). Thus, measuring these metabolites provides a better understanding of the mobilization of energy by nesting females during nest attendance and to what extent are they relying on their accumulated reserves (Robin et al. 1988; Campbell et al. 2008). Similarly, attending nests further from water may result in higher dehydration rates in nesting females, especially in C. crocodilus. Dehydration can be evaluated by measuring plasmatic concentrations of total proteins (which if in low concentration provide insight into whether protein catabolization is being used as an additional energy source; Campbell et al. 2008) and uric acid, which is the main means of nitrogen excretion in dehydrated crocodilians (Khalil and Haggag 1958; Coulson and Hernandez 1959).
The female’s physiological state while attending the nest may influence her response to stress factors, such as nest predation attempts or capture, immobilization and post-capture handling procedures (Lance et al. 2000; Franklin et al. 2003). Therefore, monitoring and conservation programs that involve nest surveys and/or capture of attending females (e.g. Campos et al. 2008) should take into account the probable physiological condition of the female.
This study reports on the influence of nest attendance on body condition and physiological state of female caimans, and how these are affected by nesting strategy, by comparing two species (C. crocodilus and M. niger) in Central Amazonia. We hypothesized that (1) nesting females would exhibit lower body condition than non-nesting individuals in C. crocodilus, but not in M. niger; (2) caimans attending nests would have different serum biochemical values than those not attending nests in C. crocodilus, but not in M. niger; (3) body condition would reflect the results observed in the blood biochemistry analysis; (4) nest distance from standing water or time in attendance at the nest would have a negative effect on body condition and serum biochemical values of nesting females.
Materials and methods
Study areas
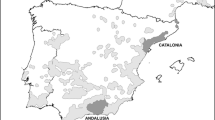
Our study was conducted in two sites in Central Amazonia (Fig. 1). These areas enclose várzea floodplains and are highly dynamic hydrological systems influenced by annual flooding (Wittmann et al. 2004; Junk et al. 2010). The main part of our study was carried out in the Piagaçu-Purus sustainable development reserve (PP-SDR), which is located between the Purus and Amazonas Rivers, approximately 350 km southwest of Manaus, Amazonas State, Brazil. PP-SDR encompasses about 834,245 ha. Várzea covers about half of the reserve, and includes lakes and canals covered by floating vegetation, as well as forest (Haugaasen and Peres 2006). The second part of the study was conducted in Mamirauá sustainable development reserve (MSDR), located between the Solimões (Amazon) and Japurá Rivers, approximately 250 km northwest of PP-SDR. MSDR encompasses an area of 1,124,000 ha, and is entirely composed of várzea floodplains (Mamirauá 1995).
Although species-specific nesting strategies are the same in both study areas (Villamarín et al. 2011), there is a predominance of C. crocodilus nests in PP-SDR, and M. niger in MSDR. The heterogeneous spatial distribution of these two crocodilians has been suggested to be the result of past hunting pressure (e.g. Magnusson 1985). Nonetheless, it is unlikely that the nesting strategy of C. crocodilus and M. niger (the focus of this study) in either area is shaped by past hunting pressure (Villamarín et al. 2011). In Central Amazonia, M. niger and C. crocodilus also show spatial segregation, ecological differences in habitat selection and resource use (Da Silveira et al. 1997; Magnusson 1985; Marioni et al. 2008).
Searching for nests
Nest surveys were carried out in 2012 from October to November in MSDR and between November and December in PP-SDR. In both sites, this period coincides with the annual dry season, when water levels are at their lowest and caimans are nesting. For both species, nesting starts in late September with the latest nests being built around mid-November (Villamarín et al. 2011). In PP-SDR, nests were located by walking the margins of water bodies and their adjacent areas of várzea forest, up to a distance of 100 m into the forest (Marioni et al. 2007). In MSDR, nest surveys were carried out up to 20 m landward of the water’s edge, which is where most M. niger nests are located (Villamarín et al. 2011).
We surveyed 14 water bodies in PP-SDR and found 118 nests of C. crocodilus and 4 nests of M. niger. We were only able to locate the attending females in 49 nests of C. crocodilus. In MSDR, we located 45 M. niger and 6 C. crocodilus nests in 8 water bodies, but were only able to locate 15 attending females of M. niger and 3 females of C. crocodilus. Because of the low sample size for C. crocodilus nesting females in MSDR, we did not include them in the analysis.
Straight-line distance from the nest to the forest edge in the direction of the nearest water body was measured with a hip-chain® (Forestry Suppliers, Inc, Jackson, Mississippi, USA) for each nest. Distance to water beyond the forest edge was not used to indicate distance from water as this varied during incubation. Coordinates of nests were registered with a Garmin® 78 S GPS recorder.
One egg from each C. crocodilus nest was opened on the same day the respective female was captured and the curled length (Crawshaw 1987; Campos et al. 2008) of the embryo measured with Vernier callipers (±0.05 mm). Embryo length was used as an index of nest age in analyses (Campos et al. 2008). As there is no information available on the relationship between embryo length and incubation time for C. crocodilus in Central Amazonia, our estimate of nest age was based on the relationship described for Caiman yacare in the Brazilian Pantanal, (Crawshaw 1987). Nest age of M. niger was not estimated, as embryo length could not be measured.
Female capture
Nesting females of both species were captured near the nest and physically restrained using a pole-snare (Ketch-All Animal Restraining Pole), ropes and tapes. We measured snout–vent length (SVL) and body mass, and marked the caiman by removal of a combination of three vertical tail scutes (Da Silveira et al. 1997). Females not attending nests (non-nesting) were located by their eyeshine when illuminated with a spotlight during nocturnal surveys, conducted from an aluminium boat with a 15 HP motor in canals with permanent water flow, locally called paranãs (Ayarzagüena 1983; Thorbjarnarson 1994). In PP-SDR, most females with SVL >60 cm are mature (Souza et al. 2010). Adult males of similar size (60 ≤ SVL ≤ 90) were also captured to provide an additional comparison with nesting and non-nesting females of C. crocodilus. In MSDR, all individuals of M. niger encountered in the canals were captured. Males and non-nesting females of both species were captured using a pole with a break-away noose, immobilized, measured, weighed and marked as described for nesting females (Da Silveira et al. 1997). Sex was identified by direct examination of the cloaca (Ziegler and Olbort 2007). All captured caimans were released within a maximum period of 30 min after capture.
Blood samples
Blood samples were collected within a maximum period of 15 min after capture (Guillette et al. 1997), immediately after physical immobilization and before the measuring and weighting procedures described above. Approximately 5 mL of blood was withdrawn from the supravertebral sinus (Sykes and Klaphake 2008) into a sterile syringe using a 22G × 1″ needle (0.7 × 25 mm). For larger individuals of M. niger, a 21G × 1″ needle (0.8 × 38 mm) was used. After collection, blood was transferred to tubes containing lithium heparin (BD Vacutainer™, São Paulo, Brazil) and kept in ice until processed.
All blood samples were processed immediately upon our arrival at our base camps in PP-SDR and MSDR. Initial processing was performed in improvised laboratories at both sites. Blood was centrifuged at 6400 rpm for five min. Plasma was transferred to a cryogenic tube (2 mL) and immediately frozen in liquid nitrogen. The time between blood collection and plasma separation ranged from one to twelve hours (mean ± SD = 5 ± 3 h). Plasma samples were then transported to Manaus and stored at −85 °C in an ultrafreezer (Sanyo Corp., New York, USA) until further analysis.
Body condition analysis
To determine whether Fulton’s K body condition index, which assumes isometric growth (Green 2001), could be used for this study, we calculated the regression slope (b) of the natural log of length on the natural log of mass with our data and tested whether it was significantly different from 3 (Zar 1999). In C. crocodilus, the regression slope (b) for all 92 caimans captured during our study was significantly different from three (b = 2.950; p < 0.05). However, as the regression slope (b) is very close to three, and the slope (b) of the 43 caimans not affected by the costs associated with reproduction alone (non-nesting females and adult males) was not significantly different from 3 (b = 3.003; p = 0.17), we used Fulton’s K as the body condition index for all individuals of this species. When the slope (b) value is close to three, the calculations for Fulton’s K and relative condition K b (LeCren 1951) are almost equal (Mazzotti et al. 2012). Furthermore, it was already demonstrated for Alligator mississippiensis that Fulton’s K is capable of showing differences in body condition either using the isometric growth value (b = 3) or the real value (K b ), even when slope (b) is significantly different from 3 (Zweig 2003). Thereby, caiman body condition in C. crocodilus was estimated according to the following equation: K = (body mass/SVLb) × 10n, where b = 3 and n = 5 (Green 2001; Nash et al. 2006; Zweig et al. 2014). As the slope (b) was significantly different from three (b = 2.93; p < 0.05) in M. niger, and exhibited the same value in both situations (with and without nesting females), body condition was evaluated by the relative condition index (K b ), according to the following equation: K b = (body mass/SVL2.93) × 105 (LeCren 1951).
Biochemical analysis
Biochemical analysis was performed in a Cobas Mira Plus automated system (Roche®, Basel, Switzerland). Plasmatic glucose, triglycerides, lactate, uric acid and protein (total) concentrations were determined using specific commercial kits of colorimetric reaction (Glicose liquiform, Triglicérides liquiform, Lactato liquiform, Ácido Úrico liquiform and Proteínas Totais). All reagents were purchased from the same supplier (LabTest™, Lagoa Santa, Minas Gerais, Brazil) and procedures were performed according to the manufacturer’s instructions. All metabolites were measured in duplicate. Lactate concentration could only be measured in C. crocodilus.
Statistical analysis
All statistical analysis and graphics was performed in Systat 8.0 statistical software (Systat 8.0, SPSS Inc., Chicago). Analysis of variance (ANOVA) followed by a Tukey’s post hoc test (p < 0.05) was used to evaluate whether there were significant differences in body condition between nesting and non-nesting individuals of C. crocodilus. In M. niger, SVL was added as a covariate in the analysis (ANCOVA) to account for differences in size between nesting and non-nesting individuals (F = 21.39; p < 0.001). Differences in blood biochemistry parameters between nesting females, non-nesting females and males were assessed by multivariate analysis of covariance (MANCOVA). Plasmatic concentrations of glucose, triglycerides, lactate, uric acid and total proteins were square-root transformed to meet the normality assumption of this test (Zar 1999). As there were significant differences in blood collection time (time between the beginning of capture effort and blood withdrawal) between captures near nests and far from nests for C. crocodilus (Mann–Whitney U = 87.0; p < 0.001), time to blood removal was included in the analysis as a covariate for that species. In M. niger, there was no significant difference in blood collection time between nesting and non-nesting individuals (Mann–Whitney U = 127.0; p = 0.200), due to the small sample size in all four analysed blood parameters (glucose and triglycerides: n < 10; total proteins and uric acid: n < 5) in non-nesting black caimans, we included males and non-nesting females in a single group, and total proteins and uric acid were analysed separately from glucose and triglycerides.
Multiple regression analysis was used to examine if body condition influenced serum biochemical values, with nesting and non-nesting individuals being analysed separately, and to examine the influence of nest variables (age and distance) on nesting female body condition and blood parameters. This multivariate analysis was followed by linear regression when we suspected the effect on a single blood parameter. Uric acid and total protein concentrations were not included in the analysis for non-nesting female and male M. niger because the number of samples was less than five.
Results
Nest surveys
Nest distance to forest edge ranged from 14 to 100 m (mean ± s.d. = 55 ± 31 m) in C. crocodilus and from zero to 8 (mean ± s.d. = 1.3 ± 2 m) in M. niger. Curled embryo length ranged from 46.1 to 239.0 mm (mean ± s.d. = 163.7 ± 51.5 mm) for C. crocodilus, which corresponds to a nest age variation between 12 and 65 days (mean ± s.d. = 44 ± 14 days).
Body condition analysis
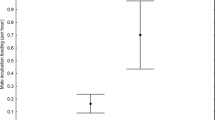
There was no significant differences in SVL between nesting and non-nesting C. crocodilus (F = 2.84; p = 0.070; Table 1). Fulton’s “K” body condition of nesting females was significantly lower than non-nesting females and adult males (F = 18.63; p < 0.001). However, no significant difference was observed in body condition between non-nesting females and adult males (p = 0.150; Fig. 2a). In M. niger, no significant difference (F = 0.57; p = 0.569) was observed in K b body condition between nesting and non-nesting individuals of M. niger (Fig. 2b), and there was also no effect of SVL (F = 0.19; p = 0.665).
Biochemistry analysis
Descriptive information from the blood plasma biochemistry analyses for C. crocodilus and M. niger are listed in Table 1. In C. crocodilus, MANCOVA indicated that animal group (nesting female, non-nesting female or adult male) affected four of five blood plasma biochemistry parameters (F = 11.84; p < 0.001; Fig. 3), but there was no effect of blood plasma collection time in any group (F = 1.07; p = 0.384). Plasmatic concentrations of glucose (F = 27.28; p < 0.001), triglycerides (F = 32.78; p < 0.001), lactate (F = 18.18; p < 0.001) and uric acid (F = 11.26; p < 0.001) differed between nesting and non-nesting individuals, but there was no significant difference between non-nesting females and adult males (glucose: p = 0.720; triglycerides: p = 0.970; lactate: p = 0.840; uric acid: p = 0.970). No significant difference was observed for plasmatic total protein concentration (F = 0.69; p = 0.506) between nesting and non-nesting individuals of C. crocodilus.
Plasmatic concentrations of glucose (a), triglycerides (b), lactate (c) and uric acid (d) of females captured near the nest (nesting females), females captured away from nests (non-nesting females) and adult males of Caiman crocodilus in PP-SDR. Each point represents a caiman. In all four blood parameters, significant difference (p < 0.05) was observed between nesting and non-nesting caimans, but not between non-nesting females and males
For M. niger, MANCOVA indicated no significant difference between nesting and non-nesting caimans (F = 1.07; p = 0.356; Fig. 4) in plasmatic concentrations of glucose (F = 1.18; p = 0.288) and triglycerides (F = 0.70; p = 0.411), but plasmatic concentration of glucose had a significant relationship with SVL (F = 5.63; p = 0.025). Plasmatic concentrations of uric acid and total proteins were not affected (F = 1.30; p = 0.032) by animal group (nesting or non-nesting caiman). SVL did not affect uric acid concentration (F = 0.14; p = 0.719), but influenced total protein concentration (F = 5.86; p = 0.027).
Regression analysis
Multiple regression analysis indicated that body condition did not affect (F = 2.18; p = 0.083) the plasmatic concentrations of glucose (F = 0.52; p = 0.472), lactate (F = 1.20; p = 0.280), uric acid (F = 0.60; p = 0.444) or total proteins (F = 2.71; p = 0.110) in nesting female C. crocodilus. However, there was a significant positive relationship (F = 6.93; p = 0.013) between body condition and plasmatic concentration of triglycerides. Linear regression indicated that body condition explained about 13% of variability in plasma triglyceride concentration in nesting female of C. crocodilus (r 2 = 0.13; 5.07; p = 0.03). In non-nesting C. crocodilus, multiple regression analysis indicated no relationship between body condition and the five blood plasma parameters evaluated (F = 0.46; p = 0.802). In M. niger, body condition had no significant effect on plasmatic concentrations of glucose and triglycerides in either nesting (F = 1.09; p = 0.368) or non-nesting caimans (F = 1.61; p = 0.244). Uric acid and total protein concentrations were also not significantly affected by body condition in nesting (n = 7; F = 0.32; p = 0.741) or non-nesting M. niger (n = 5; F = 1.63; p = 0.291).
Body condition of C. crocodilus was negatively affected (r 2 = 0.33; F = 5.58; p = 0.003) by nest distance to forest edge and nest age, and the relationships were described by the following equation: body condition = 2.734 − 0.010 × distance – 0.013 × age + 0.0002 × (distance × age). No significant effect (F = 1.86; p = 0.200) of nest distance to forest edge on female body condition was found for M. niger.
Effect of nest distance to forest edge was not significant for four of five serum biochemical values (F = 0.99; p = 0.441), and no significant effect of nest age was observed for any of the blood plasma parameters (F = 1.67; p = 0.176) in nesting female C. crocodilus. Nest distance explained about 18% of the variance in plasmatic glucose concentration in nesting female C. crocodilus (r 2 = 0.18; F = 7.67; p = 0.008). Low probability values in the linear regression of nest age on plasmatic concentrations of triglycerides, uric acid and total proteins in nesting female C. crocodilus indicated possible relationships for triglycerides (r 2 = 0.08; F = 2.98; p = 0.09), uric acid (r 2 = 0.08; F = 2.89; p = 0.09) and total proteins (r 2 = 0.09; F = 3.51; p = 0.07). In M. niger, no significant relationship (F = 0.52; p = 0.608) was found between nest distance to forest edge and plasmatic concentrations of glucose (F = 0.33; p = 0.574) or triglycerides (F = 0.67; p = 0.428). No effect of nest distance from forest edge on concentrations of uric acid and total proteins (F = 0.62; p = 0.596) was also observed in nesting female M. niger.
Discussion
This study provides evidence that nest attendance has a metabolic cost for nesting females in Caiman crocodilus, but not in Melanosuchus niger. Body condition analysis and biochemical data indicated differences between the two species of caiman, which exhibit distinct nesting strategies even though they reproduce in the same region in the same season.
Body condition of an individual is directly influenced by its capacity to acquire resources required to support the metabolic costs (Jakob et al. 1996). The presence of factors, such as competition, food shortage or parental care, which may have a negative influence on individual aptitude to acquire resources, often results in a significant reduction of energetic reserves (Mrosovksy and Sherry 1980). In PP-SDR, nest attendance had a major influence on the ability of C. crocodilus to acquire resources, because body condition of 80% of females attending nests is lower than the mean body condition observed in females not attending nests. The lowest body condition value observed in non-nesting females was higher than those of 50% of nesting females.
For M. niger in MSDR, there was no apparent difference in body condition between nesting and non-nesting caimans, suggesting that nesting female M. niger do not lose body condition during nest attendance. Furthermore, and similar to what has been reported in other crocodilian species (Saafeld et al. 2008; Cedeño-Vasquez et al. 2011), there was no difference in body condition between males and non-nesting females in either locality. This similarity in body condition between males and females could be due to the fact that both sexes have access to the same resources, since males and non-nesting females usually inhabit in the same locations during the nesting season (Ayarzagüena 1983; Thorbjarnarson 1994). Several authors have suggested that variation in body condition in individuals of the same species between distinct geographical regions is influenced by habitat and food availability (Rootes et al. 1991; Saafeld et al. 2008), or conspecific density (Da Silveira et al. 1997).
The lower levels of plasmatic glucose, triglycerides and lactate in nesting female C. crocodilus than those of non-nesting individuals of the same species indicate that nest attendance during egg incubation has a direct impact on the energy reserves of the females, which affects their physiological status. Plasmatic triglyceride levels was the blood parameter that most differed between nesting and non-nesting C. crocodilus. Nearly 80% of non-nesting females and 70% of adult males exhibited higher triglyceride blood concentrations than the maximum value observed in nesting females.
Lower blood plasma concentrations of triglycerides in nesting female C. crocodilus suggest a metabolic cost associated with reproduction, which seems directly influenced by the species’ nesting strategy. Females remain near their nests in forests, during incubation period (Marioni et al. 2007; Ayarzagϋena and Castroviejo 2008), which ranges from 60 to 70 days (estimated for Caiman crocodilus yacare in the Pantanal; Cintra 1988) and alter their dietary composition and feed less often than non-nesting females (Barão-Nóbrega et al. 2016). Even though M. niger nesting females also remain near their nests during the incubation period, which ranges from 80 to 90 days (Villamarín et al. 2011), they are rarely seen on land, and usually remain in water near their nests (Thorbjarnarson et al. 2000), where they can remain hydrated and probably feed. Low concentrations of plasma triglycerides (between 0.1 and 0.2 mmol/L) have been documented for captive broad-snouted caiman (Caiman latirostris) that were fed with a low-fat diet (Zayas et al. 2011). There is also an association between low concentrations of plasmatic triglycerides and prolonged fasting periods in Alligator mississippiensis (Black et al. 1963) and C. yacare (Campbell et al. 2008).
None of the nesting female C. crocodilus exhibited plasmatic glucose concentrations higher than the mean value observed for non-nesting females and adult males (5.6 mmol/L) of the same species, whereas nesting and non-nesting M. niger exhibited similar values. Furthermore, about 85% of nesting females of C. crocodilus had a lower glucose concentration than the range (3.3 to 5.6 mmol/L) registered for the great majority of reptiles (Sykes and Klaphake 2008). In contrast, only 13% of nesting female M. niger exhibited a lower glucose value than 3.3 mmol/L. Nevertheless, it should be noted that comparison and interpretation of glucose concentrations might be limited when the fasting period is not known (Zayas et al. 2011). Dietary analysis of the stomach contents of the same group of nesting females of C. crocodilus demonstrated that their feeding frequency was significantly lower than non-nesting females (Barão-Nóbrega et al. 2016). Therefore, higher glucose concentrations in non-nesting individuals of C. crocodilus and both nesting and non-nesting individuals of M. niger could result from shorter fasting periods, since higher glucose values tend to occur when food has been ingested more recently (Lane 1996; Zayas et al. 2011).
As crocodilians exhibit low metabolism (Coulson and Herbert 1981) and it has been demonstrated in captivity that metabolic rates differ between animals recently fed and animals submitted to prolonged fasting periods (Gatten 1980), struggle associated with capture in non-nesting individuals of C. crocodilus, reflected in blood levels of lactate (Franklin et al. 2003; Hartzler et al. 2006), might have contributed to an increase in glucose concentrations (Coulson and Hernandez 1979; Lance et al. 1993). However, as our results demonstrated that longer capture efforts (i.e. more stressful to the animal) did not influence the biochemical parameters analysed, lower plasmatic lactate concentrations in nesting females of C. crocodilus indicate that nest attendance has another metabolic cost, making them less aggressive to stress factors, such as capture.
Although physiological consequences of dehydration in crocodilian species are still poorly understood (Ross 1989; Coppo et al. 2006), their effects are likely to be considerable. Nesting female C. crocodilus exhibited higher dehydration levels than adult males and non-nesting females of the same species. Ninety percent and 57% of the uric acid concentrations in nesting females were superior to the mean and maximum values observed for non-nesting individuals, respectively. Uric acid blood concentrations of nesting female C. crocodilus were also superior to those of nesting female M. niger in MSDR (where no difference between nesting and non-nesting individuals was observed) and to reference values recorded for other crocodilian species (Huchzermeyer 2003). Evidence of dehydration associated with nest attendance has also been reported in birds (Ankney and Afton 1988).
The positive association between body condition and plasma concentration of triglycerides in nesting female C. crocodilus indicates that they are using lipids as their primary source of metabolic energy (Campbell et al. 2008), and so depend on their accumulated lipid reserves to maintain their metabolic requirements during nest attendance, as has been suggested for the marine turtles Chelonia mydas and Dermochelys coriacea (Paladino et al. 1996; Hamann et al. 2002). During periods of low food intake, the main source of metabolic energy in A. mississippiensis (Black et al. 1963) and C. yacare (Campbell et al. 2008) originates from degradation of lipids rather than carbohydrates, such as glucose. On the other hand, in M. niger, the absence of correlation between triglycerides and body condition suggests that females of this species do not depend only on their lipid reserves while attending their nests during the incubation period, which could be directly related to their easier access to food resources (Campbell et al. 2008).
Differences observed in body condition and blood parameters between nesting and non-nesting individuals in C. crocodilus are likely to result from differences in feeding frequency and dietary composition (Campbell et al. 2008; Barão-Nóbrega et al. 2016), variation in prey availability (Taylor 1979; Delany and Abercrombie 1986) or foraging behaviour (Platt and Brantley 1991). In A. mississippiensis, a diet dominated by fish is correlated with a higher body condition (Delany et al. 1999) and nutritional status is directly related to biomass consumed (Chabreck 1971). Feeding frequency and occurrence of fish in the diet of nesting female C. crocodilus is very low (Barão-Nóbrega et al. 2016). Even though there is no available information regarding the diet of nesting female M. niger, it has been suggested that fish availability in lakes, where M. niger nesting females remain while attending their nests, could be higher than in the canals, due to lower water depth and currents (Da Silveira et al. 1997; Da Silveira and Magnusson 1999). Therefore, the absence of differences in body condition and serum biochemical values between nesting and non-nesting individuals of M. niger could be related to eating frequency and diet composition.
Although the reproductive biology and nesting ecology of C. crocodilus (Staton and Dixon 1977; Gorzula 1978; Thorbjarnarson 1994; Campos et al. 2008), and to a lesser extent M. niger (Thorbjarnarson et al. 2000; Villamarín and Suaréz 2007; Villamarín et al. 2011) have been studied, general knowledge on what influences body condition and internal physiology of females during the nesting season is still incipient. Our study demonstrates that nest location in the forest and duration of nest attendance both have negative impacts on female body condition in C. crocodilus. Female C. crocodilus attending nests in later stages of the incubation period or further into the forest have lower body condition than those attending nests closer to water or at earlier stages of the incubation period. Loss of body mass, and consequently body condition, during the incubation period has been reported also for nest-attending female birds (Mrosovsky and Sherry 1980; Ankney and Afton 1988; Kitaysky et al. 1999) and lizards (Huang 2007).
Similar to what has been observed in bird species (Owens and Bennet 1994; Carrillo and Aparicio 2001), nesting female C. crocodilus are exposed to greater depredation risk during nest attendance. In some regions of Central Amazonia, nesting female C. crocodilus and their eggs represent an important food source for jaguar, Panthera onca (Da Silveira et al. 2010). Despite the apparent negative impact on female body condition, locating their nests further inside the forest might be a strategy of C. crocodilus to decrease nest and attending-female detection by predators. Behavioural decisions with a cost/benefit associated with hatching success have been reported in A. sinensis in captivity (Zhang et al. 2015), birds (Cresswell 1997) and pythons (DeNardo et al. 2012). In MSDR, high predation rates were observed on eggs in nests of C. crocodilus and M. niger located close (up to 20 m) to the forest edge (72% of nests attacked in C. crocodilus and 50% in M. niger; Da Silveira et al. 2010). Freshwater crocodile (Crocodylus johnstoni) hole nests are more easily detected by predators when located close to the forest edge (Somaweera et al. 2011). In PP-SDR and Piranha-SDR (same type of habitat as our study sites, and located 175 km northeast of PP-SDR), where 80% of C. crocodilus nests are built inside the forest, at distances greater than 20 m from forest edge, the rate of attacks by predators on C. crocodilus nests seems to be lower (about 20%) than in MSDR (Barão-Nóbrega et al. 2014). However, comparing predation rates on eggs in crocodilian nests is not simple, because predation patterns may vary with predator and nest density, availability of alternative food resources for the predator and with the amount of maternal care given (Staton and Dixon 1977).
Besides the negative effect on body condition, both nest distance and age significantly influenced blood parameters of nesting female C. crocodilus, but not in M. niger. As blood glucose regulates the consumption rate of an organism (Kaneko 2008), the positive correlation between glucose and nest distance in C. crocodilus suggests that building nests further away from water demands higher energy expenditure by females. Time in attendance at nests likely has negative effects on plasmatic triglycerides, uric acid and total proteins concentrations. Even though these relations were not statistical significant at p < 0.05, the probability values were low, and we consider that they are likely to be biologically significant. Considerable reduction in lipid reserves during incubation is expected because lipids represent the major source of metabolic energy in crocodilians (Black et al. 1963), a pattern also described in birds that exhibit intensive parental care (Ankney and Afton 1988; Robin et al. 1988). Male and female imperator penguins (Aptenodytes forsteri) consume nearly 80% of their lipid reserves, which represents a loss of 20–40% in body mass, during the 3-month fasting period associated with reproduction (Robin et al. 1988).
As uric acid is one of the main by-products of protein catabolism in reptiles (Frye 1991), lower body condition associated with higher concentrations of uric acid in nesting female C. crocodilus suggests dehydration and/or some utilization of proteins as an additional energy source, especially because there seems to be a negative relationship (though not statistically significant) between nest age and total protein concentration (i.e. nesting female C. crocodilus seem to exhibit lower total protein concentrations in later stages of the incubation period). Nesting female Anas clypeata (Northern Shoveler) loose 15% of their body mass during the nesting season due to utilization of proteins as an additional energetic source (Ankney and Afton 1988).
Although C. crocodilus and M. niger share the same nesting seasons and build their nests in the same regions in Central Amazonian floodplains, they exhibit distinct nesting strategies. The nesting strategy of C. crocodilus seems to impose a metabolic cost associated with nest attendance because non-nesting individuals exhibited higher body condition and healthier serum biochemical values than those of nesting females. While nesting female C. crocodilus tend to remain hidden in the nearby vegetation, some in a lethargic state (Marioni et al. 2007), nesting female M. niger usually remain in water and aggressively defend their nests (Thorbjarnarson et al. 2000). Differences in parental care provided by nesting females of these species could be related to resource availability, as has been documented for nesting birds (Martin 1992; Dearborn 2001). Furthermore, nest attendance may be influenced by body condition (Dearborn 2001; Huang 2007) and the female’s accumulated energy reserves, since it has been suggested that nest defence behaviour and the period of nest attendance (i.e. how much time the female remains near the nest) may vary among nesting females in crocodilians (Kushlan and Kushlan 1980; Ayarzagüena 1983; Zhang et al. 2015).
Other aspects not addressed in this study such as female body condition prior to nesting (Brandt et al. 2016) and reproductive allometry also need to be taken into account. Associations between female body size and clutch characteristics have been reported in several crocodilians (Thorbjarnarson 1996), including C. crocodilus and C. yacare (Campos et al. 2008). In C. latirostris, it has been reported that energetic investment on reproduction may be higher in smaller females, than in larger females (Verdade 2001). Additionally, even though we have not observed evidence of nest-site selection (nest distance to standing water) within the forest in relation to female size (i.e. social hierarchy) in PP-SDR, it has been reported that it might influence nest-site selection in different habitats in C. latirostris (Montini et al. 2006). While there are still many questions to be answered, the values of indices of body condition and estimates of blood parameters for C. crocodilus and M. niger during the nesting season we registered provide reference values (Table 1) for future studies.
Change history
13 November 2017
Although nesting ecology is well studied in several crocodilian species, it is not known how nest attendance influences physiology and body condition of nesting females. In this study, we describe body condition and serum biochemical values of nesting female, non-nesting female and male spectacled caiman (Caiman crocodilus) and black caiman (Melanosuchus niger) in two areas of Central Amazonia. We also evaluated the effect of nest age and nest distance to water on body condition and blood parameters of nesting females. Body condition and plasmatic concentrations of glucose, triglycerides, lactate and uric acid of nesting females were significantly different from those of non-nesting females and males in C. crocodilus, but not in M. niger. Our study also demonstrated that nest age and distance to water had a negative effect on female body condition in C. crocodilus, but not in M. niger. Female C. crocodilus attending older nests or nests built further away from permanent water bodies tended to have lower body condition. Our results demonstrate that the nesting strategy of C. crocodilus has a metabolic cost associated with nest attendance for nesting females, which appear to depend on accumulated energetic reserves during nest attendance. In contrast, nest attendance had little effect on the physiology of female M. niger.
References
Ankney CD, Afton AD (1988) Bioenergetics of breeding northern shovelers: diet, nutrient reserves, clutch size, and incubation. Condor 90:459–472. doi:10.2307/1368574
Ayarzagüena J (1983) Ecología del caiman de anteojos o baba (Caiman crocodilus L.) en los Llanos de Apure (Venezuela). Graficas Rublan, Venezuela
Ayarzagϋena J, Castroviejo J (2008) Las babas (Caiman crocodilus L.) de la estación Biológica el Frio (estado de Apure), Llanos de Orinoco, Venezuela. In: Ayarzagϋena J, Castroviejo J, Velasco A (ed) Contribución al conocimiento del género Caimán de Suramérica. pp. Publ, Asoc. Amigos de Donãna, Sevilha, pp 183–294
Barão-Nóbrega JAL, Marioni B, Villamarín F, Soares AMVM, Magnusson WE, Da Silveira R (2014) Researcher disturbance has minimal impact on natural predation of caiman nests in Central Amazonia. J Herpetol 48:338–342. doi:10.1670/13-081
Barão-Nóbrega JAL, Marioni B, Dutra-Araújo D, Botero-Arias R, Nogueira AJA, Magnusson WE, Da Silveira R (2016) Nest attendance influences the diet of nesting female spectacled caiman (Caiman crocodilus) in Piagaçu-Purus sustainable development Reserve, Central Amazonia, Brazil. Herpetol J 26:65–71.
Black AL, Simesen MG, Bartley JC (1963) Glucose and acetate metabolism in Alligator mississippiensis. Comp Biochem Phys 8.4:299–310. doi:10.1016/0010-406X(63)90164-6
Brandt LB, Beauchamp JS, Jeffery BM, Cherkiss MS, Mazzotti FJ (2016) Fluctuating water depths affect American alligator (Alligator mississippiensis) body condition in the Everglades, Florida, USA. Ecol Indic 67:441–450. doi:10.1016/j.ecolind.2016.03.003
Campbell HA, Micheli MA, Abe A (2008) A seasonal dependent change in the distribution and physiological condition of Caiman crocodilus yacare in the Paraguay River Basin. Wildl Res 35:150–157. doi:10.1071/WR07169
Campos Z, Magnusson WE, Sanaiotti T, Coutinho M (2008) Reproductive trade-offs in Caiman crocodilus crocodilus and Caiman crocodilus yacare: implications for size-related management quotas. Herpetol J 18:91–96.
Carrillo J, Aparicio JA (2001) Nest defence behaviour of the eurasian kestrel (Falco tinnunculus) against human predators. Ethology 107:865–875. doi:10.1046/j.1439-0310.2001.00718.x
Cedeño-Vásquez JR, González-Avila F, Castro-Pérez JM (2011) Condición corporal del cocodrilo del pântano (Crocodylus moreletii) en el Río Hondo, Quintana Roo, México. Quehacer Científico en Chiapas 11:19–26
Chabreck RH (1971) The foods and feeding habits of alligators from fresh and saline environments in Louisiana. Proc S A Game Fish Comm 25:117–124
Cintra R (1988) Nesting ecology of the Paraguayan caiman (Caiman yacare) in the Brazilian Pantanal. J Herpetol 22:219–222. doi:10.2307/1564000
Coppo JA, Mussart NB, Barboza NM, Fioranelli SA, Koza GA, Prado WS (2006) Physiological variations of serum electrolytes (Na, K, Ca, P, Mg, Cu) in farm-housed Caiman latirostris and Caiman yacare (Crocodylia: Alligatoridae). Analecta Veterinaria 26:9–15
Coulson RA, Herbert JD (1981) Relationship between metabolic rate and various physiological and biochemical parameters. A comparison of alligator, man and shrew. Comp Biochem Phys A 69:1–13. doi:10.1016/0300-9629(81)90632-0
Coulson RA, Hernandez T (1959) Source and function of urinary ammonia in the alligator. Am J Physiol 197:873–879
Coulson RA, Hernandez T (1979) Factors controlling glycogen breakdown in the alligator. Comp Biochem Phys C 64:115–121. doi:10.1016/0306-4492(79)90035-2
Crawshaw PG (1987) Nesting ecology of the paraguayan caiman (Caiman yacare) in the Pantanal of the Mato Grosso, Brazil. Master’s Thesis, University of Florida.
Cresswell W (1997) Nest predation: the relative effects of nest characteristics, clutch size and parental behavior. Anim Behav 53:93–103. doi:10.1006/anbe.1996.0281
Da Silveira R, Magnusson WE (1999) Diet of spectacled caiman and black caiman in the Anavilhanas archipelago, Central Amazonia, Brazil. J Herpetol 33:181–192. doi:10.2307/1565713
Da Silveira R, Magnusson WE, Campos Z (1997) Monitoring the distribution, abundance and breeding areas of Caiman crocodilus crocodilus and Melanosuchus niger in the Anavilhanas Archipelago, Central Amazonia. Brazil. J Herpetol 31:514–520. doi:10.2307/1565603
Da Silveira R, Ramalho E, Thorbjarnarson JB, Magnusson WE (2010) Depredation by jaguars on caimans and importance of reptiles in the diet of Jaguar. J Herpetol 44:418–424. doi:10.1670/08-340.1
Dearborn DC (2001) Body condition and retaliation in the parental effort decisions of incubating great frigatebirds (Fregata minor). Behav Ecol 12:200–206. doi:10.1093/beheco/12.2.200
Delany MF, Abercrombie CL (1986) American alligator food habits in Northcentral Florida. J Wildl Manage 50:348–353. doi:10.2307/3801926
Delany MF, Linda SB, Moore CT (1999) Diet and condition of American alligators in 4 Florida lakes. P S A Fish Wildl Agencies 53:375–389
Denardo DF, Lourdais O, Stahlschmidt ZR (2012) Are females maternal manipulators, selfish mothers, or both? Insight from pythons. Herpetologica 68:299–307. doi:10.1655/HERPETOLOGICA-D-12-00023.1
Franklin CE, Davis BM, Peucker SKJ, Stephenson H, Mayer R, Whittier J, Lever J, Grigg GC (2003) Comparison of stress induced by manual restraint and immobilization in the estuarine crocodile, Crocodylus porosus. J Exp Zool 298:86–92. doi:10.1002/jez.a.10233
Frye FL (1991) Hematology applied to clinical reptile medicine. In: Frye FL (ed) Reptile care: an atlas of diseases and treatments. T. F. H. Publications, Inc., New Jersey, pp 209–262
Fujisaki I, Rice KG, Pearlstine LG, Mazzotti FJ (2009) Relationship between body condition of American alligators and water depth in the Everglades, Florida. Hydrobiologia 635:329–338. doi:10.1007/s10750-009-9925-3
Gatten RE (1980) Metabolic rates of fasting and recently fed spectacled caimans (Caiman crocodilus). Herpetologica 36:361–364
Gorzula SJ (1978) An ecological study of Caiman crocodilus crocodilus inhabiting savanna lagoons in the Venezuelan Guayana. Oecologia 35:21–34. doi:10.1007/BF00345539
Green AJ (2001) Mass/length residuals: Measures of body condition or generators of spurious results? Ecology 82:1473–1483. doi:10.1890/0012-9658(2001)082[1473:MLRMOB]2.0.CO;2
Guillette LJ, Woodward AR, Crain DA, Masson GR, Palmer BD, Cox MC, You-Xiang Q, Orlando EF (1997) The reproductive cycle of the female American alligator (Alligator mississippiensis). Gen Comp Endocr 108:87–101. doi:10.1006/gcen.1997.6953
Hamann M, Limpus CJ, Whittier JM (2002) Patterns of lipid storage and mobilization in the female green turtle (Chelonia mydas). Comp Biochem Phys B 172:485–493. doi:10.1007/s00360-002-0271-2
Hartzler LK, Munns SL, Bennett AF, Hicks JW (2006) Recovery from an activity-induced metabolic acidosis in the American alligator, Alligator mississippiensis. Comp Biochem Phys A 143:368–374. doi:10.1016/j.cbpa.2005.12.024
Haugaasen T, Peres CA (2006) Floristic, edaphic and structural characteristics of flooded and unflooded forests in the lower rio Purus region of central Amazonia, Brazil. Acta Amazonica 36:25–36. doi:10.1590/S0044-59672006000100005
Huang W (2007) Costs of egg caring in the skink, Mabuya longicaudata. Ecol Res 22:659–664. doi:10.1007/s11284-006-0068-y
Huchzermeyer FW (2003) Crocodiles, biology, husbandry and diseases. CABI Publishing, London
Jakob EM, Marshall SD, Uetz GW (1996) Estimating fitness: a comparison of body condition indices. Oikos 77:61–67. doi:10.2307/3545585
Junk WJ, Piedade MTF, Whittmann F, Schöngart J, Parolin P (2010) Amazon floodplain forests: ecophysiology, biodiversity and sustainable management. Ecological Studies. Springer, Heidelberg
Kaneko JJ (2008) Carbohydrate metabolism and its diseases. In: Kaneko JJ, Harvey JW, Bruss ML (eds) Clinical biochemistry of domestic animals, 6th edn. Academic Press, London, pp 45–80
Khalil F, Haggag G (1958) Nitrogenous excretion in crocodiles. J Exp Zool 35:552–555
Kitaysky AS, Wingfield JC, Piatt JF (1999) Dynamics of food availability, body condition, and physiological stress response in breeding black-legged kittiwakes. Funct Ecol 13:577–584. doi:10.1046/j.1365-2435.1999.00352.x
Kushlan JA, Kushlan MS (1980) Function of nest attendance in the american alligator. Herpetologica 36:27–32
Lance VA, Elsey RM, Coulson RA (1993) Biological activity of alligator, avian and mammalian insulin in juvenile alligators: Plasma glucose and amino acids. Gen Comp Endocr 89:267–275. doi:10.1006/gcen.1993.1032
Lance VA, Morici LA, Esley RM (2000) Physiology and endocrinology of stress in crocodilians. In Grigs GC, Seebacher F, Franklin CE (ed) Crocodilian biology and evolution. Surrey Beatty and Sons, Chipping Norton, pp. 327–340
Lane TJ (1996) Crocodilians. In: Saunders E (ed) Reptile medicine and surgery. Saunders, Philadelphia, pp 78–98
LeCren ED (1951) The length-weight relationship and seasonal cycle in gonad weight and condition in the perch (Perca fluviatilis). J Anim Ecol 20:201–219. doi:10.2307/1540
Magnusson WE (1985) Habitat selection, parasites and injuries in Amazonian crocodilians. Amazoniana 9:193–204
Mamirauá (1995) Mamirauá: plano de manejo. Brasília: Sociedade Civil Mamirauá, Conselho Nacional de Desenvolvimento Científico e Tecnológico, Ministério da Ciência e Tecnologia e Instituto de Proteção Ambiental do Estado do Amazonas. Manaus, Brazil. http://www.mamiraua.org.br/pt-br/downloads/plano-de-manejo/. Accessed 23 Mar 2016
Marioni B, Von Muhlen EM, Da Silveira R (2007) Nesting of Melanosuchus niger and Caiman crocodilus in the Piagaçu-Purus Sustainable Development Reserve, Central Amazonia, Brazil. Crocodile Specialist Group Newsl 26:8–9
Marioni B, Da Silveira R, Magnusson WE, Thorbjarnarson J (2008) Feeding behavior of two sympatric caiman species, Melanosuchus niger and Caiman crocodilus in the Brazilian Amazon. J Herpetol 42:768–772. doi:10.1670/07-306R1.1
Martin TE (1992) Interaction of nest predation and food limitation in reproductive strategies. Curr Ornithol 9:163–197. doi:10.1007/978-1-4757-9921-7_5
Mazzotti FJ, Best GR, Brandt LA, Cherkiss MS, Jeffery BM, Rice KG (2009) Alligator and crocodiles as indicators of restauration of Everglades ecosystems. Ecol Indic 9:137–149. doi:10.1016/j.ecolind.2008.06.008
Mazzotti FJ, Cherkiss MS, Brandt LA, Fujisaki I, Hart K, Jeffery B, McTurry ST, Platt, SG, Rainwater TR, Vinci J (2012) Body condition of Morelet’s crocodile (Crocodylus moreletti) from Northern Belize. J Herpetol 46:356–362. doi:10.1670/11-188
Montini JP, Piña CI, Larriera A, Siroski P, Verdade LM (2006) The relationship between nesting hábitat and hatching success in Caiman latirostris (Crocodylia, Alligatoridae). Phyllomedusa 5:91–96. doi:10.11606/issn.2316-9079.v5i2p91-96
Moya-Laraño J, Marcías-Ordóñez R, Blanckenhorn WU, Férnandez-Montraveta C (2008) Analyzing body condition: mass, volume or density? J Anim Ecol 77:1099–1108. doi:10.1111/j.1365-2656.2008.01433.x
Mrosovsky N, Sherry DF (1980) Animal anorexias. Science 207:837–842. doi:10.1126/science.6928327
Nash RDM, Valencia AH, Geffen AJ (2006) The origin of Fulton’s condition factor—setting the record straight. Fisheries 31:236–238
Owens IPF, Bennett PM (1994) Mortality costs of parental care and sexual dimorphism in birds. Proc R Soc Lond B Biol 257:1–8. doi:10.1098/rspb.1994.0086
Padilla-Paz SE (2008) Hematología, índice corporal y lesiones externas del cocodrilo de pantano (Crocodylus moreletii) en los humedales del norte del Estado de Campeche, México. Master’s Thesis, El Colegio de la Frontera Sur
Paladino FV, Spotila JR, O’Connor MP, Gatten RE (1996) Respiratory physiology of adult leatherback turtles (Dermochelys coriacea) while nesting on land. Chelonian Conserv Bio 2:223–229
Platt SG, Brantley CG (1991) Observations of foraging behavior in captive juvenile Alligator mississippiensis. Herpetol Rev 22:83–84
Robin J, Frain M, Sardet C, Groscolas R, Maho YL (1988) Protein and lipid utilization during long-term fasting in emperor penguins. Am Phys Soc 254:61–68
Rootes WL, Chabreck RH, Wright VL, Brown BW, Hess TJ (1991) Growth rates of American Alligators in estuarine and palustrine wetlands in Louisiana. Estuaries 14:489–494. doi:10.2307/1352272
Ross JP (1989) Crocodiles and alligators. Merehurst Press, United Kingdom
Saafeld DT, Webb KK, Conway WC, Calkins GE, Duguay JP (2008) Growth and condition of American alligators (Alligator mississippiensis) in an inland wetland of East Texas. South Nat 7:541–550. doi:10.1656/1528-7092-7.3.541
Santos SA, Nogueira MS, Pinheiro MS, Campos Z, Magnusson WE, Mourão GM (1996) Diets of Caiman crocodilus yacare from different habitats in the Brazilian Pantanal. Herpetol J 6:111–117
Somaweera R, Webb JK, Shine R (2011) It’s a dog-eat-croc world: ding predation on the nests of freshwater crocodiles in tropical Australia. Ecol Res 26:957–967. doi:10.1007/s11284-011-0853-0
Souza MM, Silva SEM, Araújo ML, Barcellos JFM, Mendonça W, Marioni B, Da Silveira R (2010) Reproductive biology of Caiman crocodilus at Piagaçu-Purus Sustainable Development Reserve, Central Amazonia. Proceedings of the 20th Working Meeting of the IUCN-SSC Crocodile Specialist Group. IUCN: Gland, Switzerland, 230–230.
Staton MA, Dixon JR (1977) Breeding biology of the spectacle caiman, Caiman crocodilus crocodilus in the Venezuelan Llanos. Wildl Res Rep (US Fish Wildl Serv) 5:1–21
Stevenson RD, Woods WA Jr (2006) Condition indices for conservation: new uses for evolving tools. Integr Comp Biol 46:1169–1190. doi:10.1093/icb/icl052
Sykes IVJM, Klaphake E (2008) Reptile hematology. Vet Clin N Am 11:481–500. doi:10.1016/j.cvex.2008.03.005
Taylor JA (1979) The foods and feeding habits of subadult Crocodylus porosus Schneider in northern Australia. Aust Wildl Res 6:347–359. doi:10.1071/WR9790347
Thorbjarnarson JB (1994) Reproductive ecology of the spectacled caiman (Caiman crocodilus) in the Venezuelan Llanos. Copeia 1994:907–919. doi:10.2307/1446713
Thorbjarnarson JB (1996) Reproductive characteristics of the order Crocodilia. Herpetologica 52:8–24
Thorbjarnarson JB, Da Silveira R, Marigo LC (2000) Secrets of the flooded forest. Where do Amazonia’s top aquatic predators nest? Scientists find the hidden nurseries of black caiman. Nat Hist 109:70–79
Verdade LM (2001) Allometry of reproduction in broad-snouted caiman (Caiman latirostris). Braz J Biol 61:431–435. doi:10.1590/S1519-69842001000300012
Villamarín F, Suaréz E (2007) Nesting of Black caiman (Melanosuchus niger) in Northeastern Ecuador. J Herpetol 41:164–167. doi:10.1670/0022-1511(2007)41[164:NOTBCM]2.0.CO;2
Villamarín F, Marioni B, Botero-Arias R, Thorbjarnarson JB Magnusson WE (2011) Conservation and management implications of nest-site selection of the sympatric crocodilians Melanosuchus niger and Caiman crocodilus in Central Amazonia, Brazil. Biol Conserv 144:913–919. doi:10.1016/j.biocon.2010.12.012
Wittmann F, Junk WJ, Piedade MTF (2004) The várzea forests in Amazonia: flooding and the highly dynamic geomorphology interact with natural forest succession. For Ecol Manag 196:199–212. doi:10.1016/j.foreco.2004.02.060
Zar JH (1999) Biostatistical analysis. Prentice-Hall, New Jersey
Zayas MA, Rodríguez HA, Galoppo GH, Stoker C, Durando M, Luque EH, Muñoz-de-Toro M (2011) Hematology and blood biochemistry of young broad-snouted caimans (Caiman latirostris). J Herpetol 45:516–524. doi:10.1670/10-158.1
Zhang F, Messenger K, Wang Y (2015) Relationship between nest defense behaviours and reproductive benefits in Chinese alligators. Amphibia-Reptilia 36:141–147. doi:10.1163/15685381-00002990
Ziegler T, Olbort S (2007) Genital structures and sex identification in crocodiles. Crocodile Specialist Group Newsl 26:16–17
Zweig CL (2003) Body condition index analysis for the American alligator (Alligator mississippiensis). Master’s thesis, University of Florida
Zweig CL, Rice KG, Percival F, Mazzotti FJ (2014) Body condition factor analysis for the American alligator (Alligator mississippiensis). Herpetol Rev 45:216–219
Acknowledgements
This work is part of the Master’s thesis of J.A.L.B.N. at University of Aveiro, Portugal. Instituto Piagaçu (IPI), Instituto de Desenvolvimento Sustentável Mamirauá (IDSM), Instituto Nacional de Pesquisas da Amazónia (INPA) and Universidade Federal do Amazonas (UFAM) provided logistic support. Federal (SISBIO/ ICMBio Nº 24054-1; SISBIO/ICMBio No. 22178-2) and Amazonas State–SDS/CEUC (N° 022/2009) environmental authorities provided the research license in PP-SDR and MSDR. Data collection in MSDR was associated with the activities of the field course: training in research on caimans of the Caiman Research in Conservation and Management Program–IDSM (7th edition). E.S.L., J.L.M. and W.E.M. are research fellowship recipients from Conselho Nacional de Desenvolvimento Cientifico e Tecnológico (CNPq, Brazil). We thank Mariana Martins D.V.M. and Priscilla M. Rodrigues Pacioullo D.V.M for their assistance in collection and processing the blood samples. We also thank the three anonymous reviewers for their commentaries, which improved the quality of this manuscript. Finally, a special thanks to M. J. O. Bastos, Chiquinho, Ned “Jacuraru”, “João Jacaré” Carvalho and H. Pinto for their support in the field. Without their assistance and vast knowledge on caiman nesting, this study would not have been possible.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Data availability
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
Additional information
Communicated by G. Heldmaier.
The original version of this article was revised due to a retrospective Open Access.
A correction to this article is available online at https://doi.org/10.1007/s00360-017-1133-2.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Barão-Nóbrega, J.A.L., Marioni, B., Botero-Arias, R. et al. The metabolic cost of nesting: body condition and blood parameters of Caiman crocodilus and Melanosuchus niger in Central Amazonia. J Comp Physiol B 188, 127–140 (2018). https://doi.org/10.1007/s00360-017-1103-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00360-017-1103-8