Abstract
Glycyrrhiza glabra L. has become an endangered medicinal plant due to the unabated extraction of glycyrrhizin. Glycyrrhizin is a triterpenoid saponin that is a root centric secondary metabolite having numerous pharmacological properties, such as anti-inflammatory, immunomodulatory, antiallergic, antiulcer, and is found to be effective even against HIV. Harvesting of the roots for high value glycyrrhizin destroys the whole plant causing existential threat to the plant itself and consequent damage to biodiversity. The present study establishes that hairy root cultures of G. glabra, using an optimized elicitor, can dramatically enhance focused production of glycyrrhizin at a much faster pace year-round without causing destruction of the plant. Hairy root cultures of G. glabra were developed using the Agrobacterium rhizogenes A4 strain. The glycyrrhizin content was enhanced using different biotic and abiotic elicitors, for example, PEG (polyethylene glycol), CdCl2, cellulase, and mannan at different concentrations and durations. PEG at 1% concentration enhanced the yield of glycyrrhizin up to 5.4-fold after 24 h of exposure, whereas 200 µg mL−1 cellulase enhanced glycyrrhizin yield to 8.6-fold after 7 days of treatment. Mannan at 10 mg L−1 concentration enhanced the production of glycyrrhizin up to 7.8-fold after 10 days of stress. Among different antioxidant enzymes, SOD activity was significantly enhanced under drought, cellulase and mannan stress. This identification of elicitors can result in abundant supply of valuable glycyrrhizin to meet broad spectrum demand through commercial production without endangering G. glabra L.
Similar content being viewed by others
Introduction
Glycyrrhiza glabra L. (Fam. Fabaceae) commonly known as licorice or mulethi (Gantait et al. 2010) contains a significant amount of glycyrrhizin (2–8% dry weight) in its roots. Glycyrrhizin is an oleanane-type triterpenoid saponin (Seki et al. 2011) having numerous medicinal properties, such as immunomodulatory, anti-inflammatory, antiulcer, and antiallergic (Seki et al. 2008). It is also effective against HIV (Ito et al. 1987, 1988) and severe acute respiratory syndrome (SARS) like viruses (Nasrollahi et al. 2014). Glycyrrhizin is used globally as a flavoring agent, natural sweetener, and preservative as well. It is effectively used in the treatment of menstrual cramps, menopause symptoms, dropsy, fever, irritated urinary and respiratory passages, hypoglycemia, and influenza. Glycyrrhizin is also used as a diuretic, demulcent, emollient, antispasmodic, expectorant, mild laxative, and as a cough remedy (Alam et al. 2017). The overall demand of glycyrrhizin, which stood at US$ 1.70 bn in 2016, is anticipated to reach US$ 2.3 bn by 2025 (https://www.transparencymarketresearch.com/licorice-extracts-market.html).
Glycyrrhiza glabra is categorized as an endangered plant because the extraction of glycyrrhizin from the plant roots destroys the whole plant (Sawai and Saito 2011; Sharma and Thokchom 2014). Previously, a slow growth method for conservation of G. glabra germplasm was established and was being maintained in the host institute (Srivastava et al. 2013). As an alternative source, hairy root cultures of licorice were established which provide constant high production of glycyrrhizin while maintaining its stable nature for longer time periods (Tenea et al. 2008). Hairy roots synthesize bioactive compounds that are similar or even higher in quantity as compared to normal root cultures (Agostini et al. 2013). These roots are genetically modified roots and also cost effective due to the requirement of hormone-free medium for their growth and proliferation (Baíza et al. 1999; Lu et al. 2008; Hussain et al. 2012). Agrobacterium rhizogenes, a soil-borne bacterium, facilitates hairy root induction (Thwe et al. 2016). Many studies have been published on A. rhizogenes-mediated hairy root induction in G. glabra (Kovalenko et al. 2004; Mehrotra et al. 2008; Shirazi et al. 2012; Tenea et al. 2008) but so far enhancement of glycyrrhizin in hairy roots by using elicitors such as drought, cellulase and mannan treatments has not been reported yet.
Secondary metabolite production is affected by the variation in natural environment. Factors like drought, temperature, and microbial infections can cause variations in the content of bioactive compounds (Zhao et al. 2005). Strategic implementation of these factors under controlled conditions can also cause strong changes in secondary metabolite yield (Torkamani et al. 2014). Proper optimization of sucrose along with other culture conditions plays a vital role in determining the biomass and secondary metabolite contents in hairy root cultures of G. glabra (Wongwicha et al. 2011). Many reports are available in which elicitation of hairy root cultures has proven to be very effective for enhancement of the yield of secondary metabolites.
The two abiotic stresses, sonication and vacuum infiltration as well as biotic stresses, salicylic acid and jasmonic acid treatments were pre-meditated for enhancement of isoflavones in soybean (Theboral et al. 2014). Elicitors are molecules that mimic pathogen attack like conditions, resulting plant activation of their defense system for fighting those unfavorable conditions (Chandra et al. 2014). The role of elicitors in manipulating yield has also been reported in other plants. For example, the yield of azadirachtin was increased fivefold by the use of biotic elicitors (Claviceps purpurea) on Azadirachta indica hairy root cultures (Satdive et al. 2007). Ajmalicine and solasodine production reportedly increased up to 14.8- and 4.0-fold after NaCl treatment in hairy root cultures of Rauwolfia serpentina and Solanum khasianum, respectively (Srivastava et al. 2016).
Reactive oxygen species (ROS) play an important role in biotic and abiotic environmental stress conditions (Chen et al. 2017a). Treatment with elicitors induces oxidative stress. A correlation has also been reported between the expression of individual rol genes and production of reactive oxygen species (ROS) in plant cells (Bulgakov 2008). The antioxidant system is involved in combating ROS and is important in preventing losses caused by free radicals (Tohma and Gulçin 2010). To neutralize the effect of stresses plants use the antioxidative activities of their enzymes, like superoxide dismutase (SOD), ascorbate peroxidase (APX), and catalase (CAT) etc. (Hatami and Ghorbanpour 2014). This antioxidative enzyme activity results in other collateral responses of plants, such as enhancement of secondary metabolites (Moharrami et al. 2017). Keeping this in mind, the present study dealt with the effects of biotic and abiotic elicitors on the yield of glycyrrhizin and on the SOD activity in hairy roots of G. glabra.
Materials and Methods
Plant Material
The experimental material of elite G. glabra (rhizome) was obtained from the Indian Institute of Integrated Medicine, Jammu, under an inter-institutional project entitled “In vitro conservation of endangered plant species” two decades ago. Since then, it was being maintained in our lab as in vitro multiple shoots in MS medium (Murashige and Skoog 1962) with some modifications along with 1.0 mg L−1 IAA and 0.1 mg L−1 BAP as growth supplements. The cultures were maintained at 28 ± 2 °C temperature and 60 µmol photon m−2 s−1 light for 16 h day and 8 h dark period.
Induction and Proliferation of Hairy Root Cultures
Shoots of G. glabra were cultured on ½ MS medium with 0.1 mg L−1 IAA for the induction of in vitro roots. These roots were treated as control roots in all the comparative analysis with hairy roots. Leaves from in vitro-grown shoots at three different stages, that is, very young, 2–3 weeks-old and more than 4-weeks-old were used for transformation. Prior to 2 days of transformation, these leaves were excised and pre-cultured on ½ MS solid medium. A single colony from the A. rhizogenes A4 strain was picked up and cultured on liquid YMB medium at 28 °C for 16–18 h at 220 rpm. Bacterial cells (OD600 = 0.6) were harvested by centrifugation at 4000 rpm. For re-suspending the pellets, MS liquid medium with MES buffer and 100 µM acetosyringone was used. The medium was incubated at 28 °C at 100–150 rpm for at least 1 h. Precultured leaf sections were pierced with a needle and immersed in infection medium for 20–30 min with gentle hand shaking or on a rotary shaker at 70 rpm. Leaf sections immersed in sterile water were also placed under similar conditions to serve as control. Infected leaves were blotted dry on sterile filter paper and placed on co-cultivation medium containing 100 mM acetosyringone for 2–3 days in a dark room at 28 °C. After co-cultivation, leaves were washed with sterilized water containing cefotaxime (250 mg L−1), placed on ½ MS or full MS medium supplemented with cefotaxime (250 mg L−1) but without growth hormones. After emergence of putative hairy roots, approximately 3–4 cm long roots were cut and subcultured on separate medium. The concentration of antibiotic was decreased after every subculture and finally omitted prior to transfer in a liquid medium. Full grown hairy roots were transferred to 250 mL flask and placed on a rotary shaker under continuous agitation at 80 rpm and incubated at 25 ± 2 °C with a 16 h light/8 h dark period.
Extraction of Genomic DNA and Identification of Positive Lines
Genomic DNA was extracted from putative hairy root lines and control roots using a 5 prime DNA isolation kit according to the manufacturer’s instructions. PCR amplification was performed with the TL region specific primers forward-5′-AAGTTGAATGAGTATGACTGCC-3′ and reverse-5′-GGACGACATGGCACTCTGGGAG-3′. PCR amplification conditions were at 94 °C for 3 min followed by 36 cycles of 94 °C for 30 s, 58 °C for 30 s, 72 °C 45 s, and then 72 °C for 5 min.
Growth Kinetics and HPLC Analysis
Growth kinetics of hairy roots was examined with the use of 100 mg fresh inoculums of 3-week-old hairy roots. Biomass production was recorded every 10 days until 40 days. Glycyrrhizin was quantified from three selected positive lines through high performance liquid chromatography (HPLC) analysis. Roots were harvested and dried in an oven for 2–4 days. Dried roots were crushed to fine powder in a pestle mortar and extracted with 70% of methanol for 16–18 h at 40–45 °C. Sample preparation and HPLC analysis was performed following the protocol by Nasrollahi et al. (2014) with little modification. The glycyrrhizin standard was used at 1 mg L−1 concentration. The isocratic elution mode was used for determining glycyrrhizin content through the methanol–water–acetic acid (60:34:6) mobile phase with a flow rate of 1 mL min−1 (Nasrollahi et al. 2014). The sample injection volume was 5 µL at a wavelength of 254 nm. Analyses of all the samples were accomplished through a HPLC (HPLC, waters Pvt. Ltd. Milford USA) consisting of S 717 autosampler, µBondapak C18 column (4.0 mm X 25.0 mm), M 6000 pump, 996 PDA detector and millennium software.
RNA Isolation and qRT-PCR
Total RNA was extracted from 30-day-old hairy roots along with control roots with the use of the Qiagen RNeasy Plant Mini Kit (Qiagen, MD, USA) according to the manufacturer’s instructions. DNAse I (Ambion) treatment was given to RNA and 5 µg total RNA was used for synthesis of first strand cDNA by using the Revert Aid First Strand cDNA synthesis Kit (Fermentas, Life Sciences, ON, Canada). qRT-PCR was done in the Step One Plus Real-Time PCR System (Applied Biosystems 7500). Expression profile of important genes of glycyrrhizin biosynthesis such as 3-hydroxy-3-methylglutaryl-CoA reductase (HMGR), farnesyl pyrophosphate synthase (FPPS), squalene synthase (SQS), b-amyrin synthase (bAS), and CYP88D6 were investigated through qRT-PCR. Three independent biological replicates of one hairy root line along with three technical replicates were analyzed by qRT-PCR analysis. Actin was used as a reference gene to normalize cDNA. Primers are listed in Table 1. The relative gene expression was determined through the comparative CT method.
Preparation of Abiotic and Biotic Elicitors
PEG 6000 (for drought stress) and CdCl2 (for heavy metal stress) were used as abiotic elicitors whereas cellulase and mannan were used as biotic elicitors. PEG was used in four different concentrations, that is, 1%, 5%, 10%, and 15% for three different durations 24 h, 48 h, and 72 h. CdCl2 was used at 0.1 mM, 0.5 mM, 1.0 mM and 1.5 mM concentrations and samples were harvested after 24 h and 48 h. Cellulase in five different concentrations (5, 25, 50, 100, and 200 µg mL−1) was used and samples were harvested after 1 day, 2 days, 3 days, 5 days and 7 days. Mannan was used at 10, 50 and 100 mg L−1 concentrations for 3 days, 7 days and 10 days. PEG, CdCl2, and mannan solutions were prepared in Milli-Q water, whereas cellulase was dissolved in citrate buffer. Glycyrrhizin content and biomass yield were determined in treated and non-treated hairy roots.
Protein Extraction for Antioxidant Activity
Total protein was extracted from 30-day-old hairy roots (one hairy root line along with three biological replicates) and control roots to analyze antioxidant enzyme activities. Extraction buffer consisted of 10 mM potassium phosphate buffer (pH 7.8) with 1% PVP (w/v), 0.5% triton X-100 (v/v), and 0.1 mM EDTA. The homogenate was centrifuged at 10,000 rpm for 30 min at 4 °C. The supernatant was collected and stored at − 80 °C for further analysis. The total protein was determined by Bradford’s (1976) method. A known concentration of bovine serum albumin (BSA, Sigma-Aldrich, USA) was used for the standard curve preparation.
Antioxidant Activity Assay
Superoxide dismutase (SOD, EC1.15.1.1) activity was determined using the protocol of Beyer and Fridovich (1987). The reaction mixture was made of 100 mM potassium phosphate buffer with pH 7.8, 10 mM l-methionine, 0.025% (v/v) Triton X-100 and 0.57 mM nitro blue tetrazolium chloride (NBT). 2 mL of reaction mixture were transferred into glass tubes. Along with 20 µL of protein, 4.4% (w/v) riboflavin was also added to the tube just before measuring the OD. After the initial OD, tubes were illumined for 7 min in light. In both cases, OD was measured at 560 nm on a Perkin Elmer, Lambda 35, UV/Vis spectrophotometer.
Ascorbate peroxidase (APX, EC1.11.1.11) activity was measured according to the Nakano and Asada (1981) protocol. For the assessment of ascorbate activity, the reaction mixture was prepared consisting of 50 mM potassium phosphate buffer, 5 mM ascorbate, 1 mM EDTA and 100 µL of sample. 1 mM hydrogen peroxide was added just before the OD was recorded at 290 nm continuously for 3 min.
Guaiacol peroxidase (GPX, EC1.11.1.7) activity was measured according to the Zheng and Van Huystee (1992) protocol. The reaction mixture was made of 10 mM sodium phosphate buffer (pH 5.8), 1% guaiacol (v/v) and 100 mM H2O2. Absorbance was recorded at 470 nm, kinetics were measured every 10 s for 1 min. Catalase (CAT, 1.11.1.6) activity was analyzed according to the Aebi (1984) protocol. For the catalase test, 50 mM sodium phosphate buffer (pH 7.0), 10 µL enzyme extract and 2 mM H2O2 were used in the reaction mixture. The activity was examined by observing the decrease in absorbance at 240 nm due to the decomposition of H2O2.
Statistical Analysis
All the experiments were performed three times. The data are represented as mean values. The error bars in the figures represent the standard error in biological replicates. To statistically analyze the significant differences between antioxidant activities and expression profiles of transcripts student’s t tests were performed. Dunnett’s multiple comparison test was performed for determining the statistical differences between control roots and hairy root lines for glycyrrhizin content. Student’s t test and the Dunnett test were performed by means of Graphpad Prism 5.03 software. Significant differences between nontreated and stress-treated hairy root samples were determined using a Duncan’s new multiple range test (DMRT) using SPSS software. Significance was defined as P < 0.05.
Results
Establishment, Proliferation and Molecular Confirmation of Hairy Roots
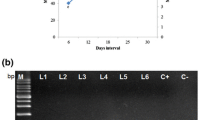
In vitro leaves were used as explants for transformation. Among three age groups of leaves used, 4-week-old leaves responded best with higher transformation efficiency (45–55%). Hairy roots were not induced from very young leaves even after 1 month of transformation. However, more than 4-week-old leaves responded but with lower transformation efficiency (< 20%). The 2-day pre-culture period was beneficial for transformation, a further increase up to 4 days did not show any significant change and explants started to necrose under prolonged preculture periods. Two to three days of co-cultivation was sufficient for the visibility of infection. Half MS medium supplemented with cefotaxime (250 mg L−1) was best suited for induction of hairy roots. Although, MS medium was also capable of hairy root induction, further growth of the roots was less compared to ½ MS medium. Hairy roots emerged after 2–3 weeks of transformation (Fig. 1a). 3-cm-long hairy roots (along with some lateral branches) were cut from the explants and subcultured in the liquid medium. The roots having healthy tips grew well. Each root emerging from a separate wounding site was treated as an independent line. Approximately one month was needed for full growth of hairy roots in MS liquid medium without growth hormones (Fig. 1b). On the basis of morphological characters, such as elongation and branching potential, some better appearing putative hairy root lines were selected. DNA was extracted from these lines to confirm the transgenic nature of roots. PCR analysis with TL region primers was conducted to assess the genetic transformation status of hairy root lines. The predicted products of 650 bp were obtained in positive lines (Fig. 1c).
Comparative Growth Kinetics and Glycyrrhizin Production in Hairy Root Lines
The growth kinetics of hairy roots were analyzed until 40 days and a continuous increase in fresh weight was recorded after every 10 days. The initial inoculum was set at 100 mg (Fig. 2a). Enhancement in fresh weight was recorded in 30-day-old hairy roots (11.5-fold in L1, 12.7-fold in L2, and 9.7-fold in line L3). Maximum enhancement of fresh weight was observed at 40 days (19.1-fold in line L1, 21.8-fold in line L2 and 19.2-fold in line L3) but hairy roots exhibited remarkable browning at this stage. Lines L1, L2 and L3 were analyzed in comparison to control roots for glycyrrhizin content through HPLC analysis. Glycyrrhizin content in control roots was 0.0897 µg mg−1 dry weight (DW). Among all the three tested lines, line L2 showed the highest glycyrrhizin content, that is, 5.2-fold (0.4698 µg mg−1 DW), L1 showed 4.8-fold (0.4347 µg mg−1 DW), and L3 showed 4.1-fold (0.3765 µg mg−1 DW) as compared to control roots (Fig. 2b).
Transcriptional Response of MVA Pathway Genes Involved in Glycyrrhizin Biosynthesis
Glycyrrhizin biosynthesis occurred through the mevalonate pathway (MVA) (Fig. 3). The transcript level of important glycyrrhizin biosynthetic pathway genes was examined through qRT-PCR. Higher expression level of HMGR (5.6-fold), FPPS (2.4-fold), SQS (1.5-fold), bAS (1.6-fold), and CYP88D6 (4.9-fold) occurred in hairy roots as compared to control roots (Fig. 4).
Effect of Abiotic Stress on Glycyrrhizin Content
For elicitation of glycyrrhizin yield through abiotic stress, PEG 6000 (drought stress) and CdCl2 (heavy metal) were used. Glycyrrhizin content in non elicited hairy roots was 0.4294 µg mg−1 DW. At 1% concentration of PEG, glycyrrhizin content was enhanced to 5.4-fold (2.3415 µg mg−1 DW), 3.8-fold (1.6367 µg mg−1 DW) and 2.1-fold (0.9228 µg mg−1 DW) after 24 h, 48 h and 72 h of treatments, respectively. Comparatively less enhancement in glycyrrhizin content, that is, 1.7-fold (0.7470 µg mg−1 DW), was observed at 5% concentration of PEG used for 24 h. At 15% concentration of PEG, a slight increase was observed in glycyrrhizin content, that is, up to 1.7-fold (0.7429 µg mg−1 DW), 1.4-fold (0.6185 µg mg−1 DW), and 1.5-fold (0.6726 µg mg−1 DW) for 24, 48 and 72 h of treatments, respectively (Fig. 5a). Hairy roots were also subjected to CdCl2 treatment, but this treatment was not appropriate for enhancing glycyrrhizin content (Fig. 5b).
Effect of Biotic Stress on Glycyrrhizin Content
In the presence of 100 µg mL−1 cellulase, glycyrrhizin yield was enhanced to 1.6-fold (0.1096 µg mg−1), 2.5-fold (0.1665 µg mg−1), and 1.5-fold (0.1011 µg mg−1), whereas at 200 µg mL−1 cellulase concentration, production of glycyrrhizin was up to 2.5-fold (0.1703 µg mg−1), 4.7-fold (0.3165 µg mg−1), and 8.6-fold (0.5738 µg mg−1) after 3 days, 5 days and 7 days of treatments, respectively (Fig. 5c). Cellulase proved to be an effective elicitor for enhancing glycyrrhizin content.
Hairy roots were also subjected to mannan treatment. Mannan at 50 and 100 mg L−1 concentrations enhanced the yield of glycyrrhizin up to 2.0-fold (0.8771 µg mg−1 DW) and 3.7-fold (1.6207 µg mg−1 DW), respectively, after 3 days of treatment. An increase of 4.9-fold (1.9587 µg mg−1 DW), 6.1-fold (2.4131 µg mg−1 DW), and 5.9-fold (2.3425 µg mg−1 DW) was recorded at 10, 50 and 100 mg L−1 doses of mannan, respectively, after 7 days of treatment. Whereas a 2.0-fold (0.8727 µg mg−1 DW) and 1.6-fold (0.6753 µg mg−1 DW) increase occurred after 10 days of stress at 50 and 100 mg L−1, respectively (Fig. 5d). The highest production of glycyrrhizin, that is, 7.8-fold (3.3089 µg mg−1 DW), was recorded at 10 mg L−1 concentration of mannan after 10 days of stress.
Effect of Biotic and Abiotic Stress on Biomass Accumulation
In controlled shake-flask cultures, the biomass of G. glabra hairy roots displayed a stable and linear growth tendency during the biomass-growth period. Drought and heavy metal stress were negatively distressing the biomass. However, cellulase and mannan increased the biomass, but significant differences were not obtained in comparison to non elicited cultures.
Effect of Elicitation on Antioxidant Enzyme Activity
Antioxidant activity assays were performed in control roots and hairy roots. In hairy roots, APX 1.5-fold, GPX 1.3-fold, CAT 2.1-fold and SOD 2.6-fold was found elevated in comparison to control roots (Fig. 6). SOD is the main antioxidant enzyme and the highest enhancement was reported in its activity when compared to other enzyme activities. Therefore, SOD activity was also measured in elicitor treated hairy roots. PEG at 1% concentration, enhanced 2.6-fold SOD activity in hairy roots after 24 h of treatment (Fig. 7a). Enhancement in SOD activity, that is, 5.2-fold was also obtained at 200 µg mL−1 cellulase concentration after 7 days post stress (Fig. 7b). Mannan at 10 mg L−1 concentration increased SOD activity to 1.6-fold after 10 days of treatment (Fig. 7c).
Discussion
In the present study, a comparative analysis between control roots and hairy roots was performed. Glycyrrhizin yield was estimated through HPLC and expression of genes involved in glycyrrhizin biosynthesis was observed via qRT-PCR. A five-fold enhancement has been observed in glycyrrhizin content in hairy roots in comparison to control roots. Expressions of HMGR, SQS, FPPS, bAS and CYP88D6 genes were higher in hairy roots. This high expression of important genes of the glycyrrhizin biosynthetic pathway was supported by a high yield of glycyrrhizin in hairy roots. Production of secondary metabolites and expression of their corresponding biosynthetic genes have a strong correlation (Matsuda et al. 2010). The expression of rol genes (rolA, rolB, and rolC) is responsible for enhanced levels of secondary metabolites in hairy roots (Shkryl et al. 2008). The rol genes enhanced the production of phytoalexins by activating the expression of defense-related genes. The mode of activation of these genes was slightly different from the regular calcium-dependent NADPH oxidase pathway and other plant defense-related hormonal pathways (Bulgakov 2008).
High expression of HMGR, FPPS, and SQS leads to enhancement of triterpenoid accumulation (Kim et al. 2010; Munoz-Bertomeu et al. 2007; Seo et al. 2005; Singh et al. 2016). SQS and bAS, design the backbone of triterpenes by production and modification of different intermediates of the pathway (Hayashi et al. 1999, 2001). Biosynthesis of glycyrrhizin begins from initial cyclization of 2,3-oxidosqualene through bAS (Tamura et al. 2017). CYP88D6 converted beta-amyrin to 11-oxo-b-amyrin through two oxidation steps. 11-oxo-b-amyrin is an expected intermediate between beta-amyrin and glycyrrhizin (Seki et al. 2008, 2011). HMGR is the rate limiting enzyme of the MVA pathway and CYP88D6 is the last step enzyme of glycyrrhizin biosynthesis (Nieto et al. 2009; Seki et al. 2008).
Plant species responded differently towards different stresses and there is a need to optimize stress conditions in terms of concentration and duration of stress treatments (Krasensky and Jonak 2012). Plant adaptation towards environmental conditions is a multifaceted phenomenon. Many protein phosphatases, kinases and various signaling molecules, participate in the whole process. The responses of plant or plants cells are an overall result of all activities (Hirayama and Shinozaki 2010).
We have observed that PEG at 1% concentration has a pronounced effect on glycyrrhizin yield in hairy roots, however, in another study severe drought conditions were required to enhance the glycyrrhizin production in stolons of G. glabra (Nasrollahi et al. 2014). Enhancement in triterpenoid yield was also reported after drought stress (Selmar and Kleinwächter 2013). Drought started the formation of reactive oxidation species such as hydrogen peroxide (H2O2), hydroxyl radical (HO−), superoxide (O2−−) and singlet oxygen (1O2) to cause a disturbance in normal cell homeostasis (Ozkur et al. 2009). To overcome these situations, plants depend on enzymatic and non-enzymatic antioxidant systems. Triterpenoids are associated with antioxidant activities (Okubo and Yoshiki 2000). Glycyrrhizin (triterpenoid) acting as a ROS depreciator, plays a crucial role in contesting the oxidative stress thus minimizes the negative effect of drought stress (Kim and Lee 2008). Isopentenyl pyrophosphate, a precursor of terpene biosynthesis, was enhanced under drought stress (Turtola et al. 2003). Glycyrrhizin production was also enhanced through methyl jasmonate treatment in G. inflata (Wongwicha et al. 2011). MeJa and SA enhanced the yield of glycyrrhizin by 3.8 and 4.1-fold, respectively, in the roots of G. glabra (Shabani et al. 2009).
In the present work, cellulase (cellulolytic enzyme) and mannan were used as biotic elicitors that proved to be efficient inducers of enhanced glycyrrhizin content. In previous reports, mannan enhanced pseudohypericin production up to 2.8-fold and hypericin production up to 1.7-fold in seedlings of Hypericum adenotrichum (Yamaner et al. 2013). Cellulase was also effective in enhancing the yield of capsidiol in suspension cultures of Capsicum annuum (Ma 2008). The exact mechanism of action of elicitation by cellulase and mannan is still not clearly known, but it may be presumed that they activate the genes of secondary metabolite biosynthetic pathways. The possible mode of action of the cellulase mechanism is that it either depolarizes the plant cell or activates the endogenous ion channels. Probably, due to the pore forming ability of cellulase, the ions are filtered with ease by the membrane avoiding any need for channel activation and receptor binding (Klusener and Weiler 1999).
The rol genes are responsible for characteristic features of hairy roots (Georgiev et al. 2010). Infection of A. rhizogenes activates the plant defense system through enhanced production of secondary metabolites and PR protein synthesis (Bulgakov et al. 2013). Integration of the rolB gene activates the NADPH oxidase system and initiates the formation of ROS. The enzymatic and non-enzymatic antioxidant systems of cells were activated to protect the cells from the detrimental effects of ROS activities (Shkryl et al. 2010). SOD acts like a first line of defense, changing O2− into H2O2, after which H2O2 immediately converts into O2 and H2O by other antioxidant systems (Chen et al. 2017b). In the present work, SOD activity was also estimated in elicited and non elicited roots.
Drought stress is associated with oxidative stress, which results in enhanced accumulation of ROS in chloroplasts, peroxisomes, and mitochondria. A correlation exists between the efficiency of plants to overcome the oxidative stress and stimulation of SOD activity which is substrate dependent as well. The enhancement in ROS levels promoted genes encoding SOD and on the other hand, SOD acts like a scavenger in degrading ROS levels (Abedi and Pakniyat 2010). The enhancement in SOD activity has been reported under drought stress in many other plant species such as sunflower (Gunes et al. 2008), wheat (Bakalova et al. 2004) and G. uralensis (Pan et al. 2006).
This study addresses the enhancement of glycyrrhizin yield by elicitation in hairy roots of G. glabra. Elicitation strategies ought to be helpful in large-scale production of glycyrrhizin from hairy root cultures of G. glabra in a bioreactor system. This could be empirically utilized in the commercialized mass production of glycyrrhizin. Concentration and duration of elicitation are plant and elicitor specific, therefore, optimized conditions could be helpful in future molecular studies regarding pathway analysis of glycyrrhizin biosynthesis. Moreover, glycyrrhizin is a valuable pharmaceutical compound and due to its root centric nature, extraction of this compound severely destroys the whole plant thereby endangering its existence and affecting biodiversity. By applying the above-mentioned approaches, high concentrations of glycyrrhizin could be obtained without loss of biodiversity and in a short time span.
References
Abedi T, Pakniyat H (2010) Antioxidant enzyme changes in response to drought stress in ten cultivars of oilseed rape (Brassica napus L.). Czech J Genet Plant Breed 46:27–34
Aebi H (1984) Catalase in vitro. Methods Enzymol 105:121–126
Agostini E, Talano MA, Gonzalez PS, Oller AL, Medina MI (2013) Application of hairy roots for phytoremediation: what makes them an interesting tool for this purpose? Appl Microbiol Biotechnol 97:1017–1030
Alam P, Foudah AI, Zaatout HH, Kamal YT, Abdel-Kader MS (2017) Quantification of glycyrrhizin biomarker in Glycyrrhiza glabra rhizome and baby herbal formulations by validated RP-HPTLC methods. Afr J Tradit Complement Altern Med 14(2):198–205
Baíza AM, Quiroz-Moreno A, Ruíz JA, Loyola-Vargas VM (1999) Genetic stability of hairy root cultures of Datura stramonium. Plant Cell Tissue Organ Cult 59:9–17
Bakalova S, Nikolova A, Nedeva D (2004) Isoenzyme profiles of peroxidase, catalase and superoxide dismutase as affected by dehydration stress and ABA during germination of wheat seeds. J Plant Physiol 30:64–77
Beyer WF Jr, Fridovich I (1987) Assaying for superoxide dismutase activity: some large consequences of minor changes in conditions. Anal Biochem 161:559–566
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Bulgakov VP (2008) Functions of rol genes in plant secondary metabolism. Biotechnol Adv 26:318–324
Bulgakov VP, Shkryl YN, Veremeichik GN, Gorpenchenko TY, Vereshchagina YV (2013) Recent advances in the understanding of Agrobacterium rhizogenes-derived genes and their effects on stress resistance and plant metabolism. Adv Biochem Eng Biotechnol 134:1–22
Chandra S, Chakraborty N, Chakraborty A, Rai R, Bera B, Acharya K (2014) Abiotic elicitor-mediated improvement of innate immunity in Camellia sinensis. J Plant Growth Regul 33:849–859
Chen YE, Su YQ, Zhang CM, Ma J, Mao HT, Yang ZH, Yuan M, Zhang ZW, Yuan S, Zhang HY (2017a) Comparison of photosynthetic characteristics and antioxidant systems in different wheat strains. J Plant Growth Regul 37:347–359
Chen Z, Yang B, Hao Z, Zhu J, Zhang Y, Xu T (2017b) Exogenous hydrogen sulfide ameliorates seed germination and seedling growth of Cauliflower under Lead stress and its antioxidant role. J Plant Growth Regul 37:5–15
Gantait A, Pandit S, Nema NK, Mukjerjee PK (2010) Quantification of glycyrrhizin in Glycyrrhiza glabra extract by validated HPTLC densitometry. J AOAC Int 93:492–495
Georgiev VG, Weber J, Kneschke EM, Denev PN, Bley T, Pavlov AI (2010) Antioxidant activity and phenolic content of betalain extracts from intact plants and hairy root cultures of the red beetroot Beta vulgaris cv. Detroit dark red. Plant Foods Hum Nutr 65:105–111
Gunes A, Pilbeam DJ, Inal A, Coban S (2008) Influence of silicon on sunflower cultivars under drought stress, I: growth, antioxidant mechanisms, and lipid peroxidation. Commun Soil Sci Plant Anal 39:1885–1903
Hatami M, Ghorbanpour M (2014) Defense enzymes activity and biochemical variations of Pelargonium zonale in response to nanosilver particles and dark storage. Turk J Biol 38:130–139
Hayashi H, Hirota A, Hiraoka N, Ikeshiro Y (1999) Molecular cloning and characterization of two cDNAs for Glycyrrhiza glabra squalene synthase. Biol Pharm Bull 22:947–950
Hayashi H, Huang P, Kirakosyan A, Inoue K, Hiraoka N, Ikeshiro Y, Kushiro T, Shibuya M, Ebizuka Y (2001) Cloning and characterization of a cDNA encoding beta-amyrin synthase involved in glycyrrhizin and soyasaponin biosyntheses in licorice. Biol Pharm Bull 24:912–916
Hirayama T, Shinozaki K (2010) Research on plant abiotic stress responses in the post-genome era: past, present and future. Plant J 61:1041–1052
https://www.transparencymarketresearch.com/licorice-extracts-market.html
Hussain MS, Fareed S, Ansari S, Rahman MA, Ahmad IZ, Saeed M (2012) Current approaches toward production of secondary plant metabolites. J Pharm Bioallied Sci 4:10–20
Ito M, Nakashima H, Baba M, Pauwels R, De Clercq E, Shigeta S, Yamamoto N (1987) Inhibitory effect of glycyrrhizin on the in vitro infectivity and cytopathic activity of the human immunodeficiency virus [HIV (HTLV-III/LAV)]. Antivir Res 7:127–137
Ito M, Sato A, Hirabayashi K, Tanabe F, Shigeta S, Baba M, De Clercq E, Nakashima H, Yamamoto N (1988) Mechanism of inhibitory effect of glycyrrhizin on replication of human immuno deficiency virus (HIV). Antivir Res 10:289–298
Kim YJ, Lee CS (2008) Glycyrrhizin attenuates MPTP neurotoxicity in Mouse and MPP-induced cell death in PC12 Cells. Korean J Physiol Pharmacol 12:65–71
Kim OT, Kim SH, Ohyama K, Muranaka T, Choi YE, Lee HY, Kim MY, Hwang B (2010) Upregulation of phytosterol and triterpene biosynthesis in Centella asiatica hairy roots overexpressed ginseng farnesyl diphosphate synthase. Plant Cell Rep 29:403–411
Klusener B, Weiler EW (1999) Pore-forming properties of elicitors of plant defense reactions and cellulolytic enzymes. FEBS Lett 459:263–266
Kovalenko P, Antonjuk V, Maliuta S (2004) Secondary metabolites synthesis in transformed cells of Glycyrrhiza glabra L. and Potentilla alba L. as producents of radioprotective compounds. Ukr Bioorg Acta 1:13–22
Krasensky J, Jonak C (2012) Drought, salt, and temperature stress-induced metabolic rearrangements and regulatory networks. J Exp Bot 63:1593–1608
Lu HY, Liu JM, Zhang HC, Yin T, Gao SL (2008) Ri-mediated transformation of Glycyrrhiza uralensis with a squalene synthase gene (GuSQS1) for production of glycyrrhizin. Plant Mol Biol Rep 26:1–11
Ma CJ (2008) Cellulase elicitor induced accumulation of capsidiol in Capsicum annuum L. suspension cultures. Biotechnol Lett 30:961–965
Matsuda F, Hirai MY, Sasaki E, Akiyama K, Yonekura-Sakakibara K, Provart NJ, Sakurai T, Shimada Y, Saito K (2010) AtMetExpress development: a phytochemical atlas of Arabidopsis development. Plant Physiol 152:566–578
Mehrotra S, Kukreja AK, Khanuja SPS, Mishra BN (2008) Genetic transformation studies and scale up of hairy root culture of Glycyrrhiza glabra in bioreactor. Electron J Biotechnol 11:69–75
Moharrami F, Hosseini B, Sharafi A, Farjaminezhad M (2017) Enhanced production of hyoscyamine and scopolamine from genetically transformed root culture of Hyoscyamus reticulatus L. elicited by iron oxide nanoparticles. In Vitro Cell Dev Biol Plant 53:104–111
Munoz-Bertomeu J, Sales E, Ros R, Arrillaga I, Segura J (2007) Up-regulation of an N-terminal truncated 3-hydroxy-3-methylglutaryl CoA reductase enhances production of essential oils and sterols in transgenic Lavandula latifolia. Plant Biotechnol J 5:746–758
Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with Tobacco tissue cultures. Physiol Plant 15:473–497
Nakano Y, Asada K (1981) Hydrogen peroxide is scavenged by ascorbate-specific peroxidase in spinach chloroplasts. Plant Cell Physiol 22:867–880
Nasrollahi V, Mirzaie-asl A, Piri K, Nazeri S, Mehrabi R (2014) The effect of drought stress on the expression of key genes involved in the biosynthesis of triterpenoid saponins in liquorice (Glycyrrhiza glabra). Phytochemistry 103:32–37
Nieto B, Fores O, Arro M, Ferrer A (2009) Arabidopsis 3-hydroxy-3-methylglutaryl-CoA reductase is regulated at the post-translational level in response to alterations of the sphingolipid and the sterol biosynthetic pathways. Phytochemistry 70:53–59
Okubo K, Yoshiki Y (2000) The role of triterpenoid on reactive oxygen scavenging system: approach from the new chemiluminescence system (XYZ system). Biofactors 13:219–223
Ozkur O, Ozdemir F, Bor M, Turkan I (2009) Physiochemical and antioxidant responses of the perennial xerophyte Capparis ovata Desf. to drought. Environ Exp Bot 66:487–492
Pan Y, Wu LJ, Yu ZL (2006) Effect of salt and drought stress on antioxidant enzymes activities and SOD isoenzymes of liquorice (Glycyrrhiza uralensis Fisch). Plant Growth Regul 49:157–165
Satdive RK, Fulzele DP, Eapen S (2007) Enhanced production of azadirachtin by hairy root cultures of Azadirachta indica A. Juss by elicitation and media optimization. J Biotechnol 128:281–289
Sawai S, Saito K (2011) Triterpenoid biosynthesis and engineering in plants. Front Plant Sci 2:1–8
Seki H, Ohyama K, Sawai S, Mizutani M, Ohnishi T, Sudo H, Akashi T, Aoki T, Saito K, Muranaka T (2008) Licorice beta-amyrin 11-oxidase, a cytochrome P450 with a key role in the biosynthesis of the triterpene sweetener glycyrrhizin. Proc Natl Acad Sci USA 105:14204–14209
Seki H, Sawai S, Ohyama K, Mizutani M, Ohnishi T, Sudo H, Fukushima EO, Akashi T, Aoki T, Saito K, Muranaka T (2011) Triterpene functional genomics in licorice for identification of CYP72A154 involved in the biosynthesis of glycyrrhizin. Plant Cell 23:4112–4123
Selmar D, Kleinwächter M (2013) Influencing the product quality by deliberately applying drought stress during the cultivation of medicinal plants. Ind Crops Prod 42:558–566
Seo JW, Jeong JH, Shin CG, Lo SC, Han SS, Yu KW, Harada E, Han JY, Choi YE (2005) Overexpression of squalene synthase in Eleutherococcus senticosus increases phytosterol and triterpene accumulation. Phytochemistry 66:869–877
Shabani L, Ehsanpour A, Asghari G, Emami J (2009) Glycyrrhizin production by in vitro cultured Glycyrrhiza glabra elicited by methyl jasmonate and salicylic acid. Russ J Plant Physiol 56:621–626
Sharma S, Thokchom R (2014) A review on endangered medicinal plants of India and their conservation. J Crop Weed 10(2):205–218
Shirazi Z, Piri K, Asl AM, Hasanloo T (2012) Glycyrrhizin and isoliquiritigenin production by hairy root culture of Glycyrrhiza glabra. J Med Plant Res 6:4640–4646
Shkryl YN, Veremeichik GN, Bulgakov VP, Tchernoded GK, Mischenko NP, Fedoreyev SA, Zhuravlev YN (2008) Individual and combined effects of the rolA, B, and C genes on anthraquinone production in Rubia cordifolia transformed calli. Biotechnol Bioeng 100:118–125
Shkryl YN, Veremeichik GN, Bulgakov VP, Gorpenchenko TY, Aminin DL, Zhuravlev YN (2010) Decreased ROS level and activation of antioxidant gene expression in Agrobacterium rhizogenes pRiA4-transformed calli of Rubia cordifolia. Planta 232:1023–1032
Singh G, Tiwari M, Singh SP, Singh S, Trivedi PK, Misra P (2016) Silencing of sterol glycosyltransferases modulates the withanolide biosynthesis and leads to compromised basal immunity of Withania somnifera. Sci Rep 6:25562
Srivastava M, Purshottam DK, Srivastava AK, Misra P (2013) In vitro conservation of Glycyrrhiza glabra by slow growth culture. Int J Biotechnol Res 3:49–58
Srivastava M, Sharma S, Misra P (2016) Elicitation based enhancement of secondary metabolites in Rauwolfia serpentina and Solanum khasianum hairy root cultures. Pharmacogn Mag 12(46):315–320
Tamura K, Seki H, Suzuki H, Kojoma M, Saito K, Muranaka T (2017) CYP716A179 functions as a triterpene C-28 oxidase in tissue-cultured stolons of Glycyrrhiza uralensis. Plant Cell Rep 36:437–445
Tenea GN, Calin A, Gavrila L, Cucu N (2008) Manipulation of root biomass and biosynthetic potential of Glycyrrhiza glabra L. plants by Agrobacterium rhizogenes mediated transformation. Roum Biotechnol Lett 13:3922–3932
Theboral J, Sivanandhan G, Subramanyam K, Arun M, Selvaraj N, Manickavasagam M, Ganapathi A (2014) Enhanced production of isoflavones by elicitation in hairy root cultures of Soybean. Plant Cell Tissues Organ 117:477–481
Thwe A, Arasu M, Li X, Park CH, Kim SJ, Al-Dhabi NA, Park SU (2016) Effect of different Agrobacterium rhizogenes strains on hairy root induction and phenylpropanoid biosynthesis in Tartary Buckwheat (Fagopyrum tataricum Gaertn). Front Microbiol 7:1–10
Tohma HS, Gulçin I (2010) Antioxidant and radical scavenging activity of aerial parts and roots of Turkish liquorice (Glycyrrhiza glabra L.). Int J Food Prop 13:657–671
Torkamani M, Jafari M, Abbaspour N, Heidary R, Safaie N (2014) Enhanced production of valerenic acid in hairy root culture of Valeriana officinalis by elicitation. Open Life Sci 9:853–863
Turtola S, Manninen AM, Rikala R, Kainulainen P (2003) Drought stress alters the concentration of wood terpenoids in Scots pine and Norway spruce seedlings. J Chem Ecol 29:1981–1995
Wongwicha W, Tanaka H, Shoyama Y, Putalun W (2011) Methyl jasmonate elicitation enhances glycyrrhizin production in Glycyrrhiza inflata hairy roots cultures. Z Naturforsch C 66:423–428
Yamaner Ö, Erdağ B, Gökbulut C (2013) Stimulation of the production of hypericins in in vitro seedlings of Hypericum adenotrichum by some biotic elicitors. Turk J Bot 37:153–159
Zhao J, Davis LC, Verpoorte R (2005) Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv 23:283–333
Zheng X, Van Huystee R (1992) Peroxidase-regulated elongation of segments from peanut hypocotyls. Plant Sci 81:47–56
Acknowledgements
The authors are thankful to the Director, CSIR-National Botanical Research Institute, Lucknow, for providing the facilities. The authors are also thankful to Indian Institute of Integrated Medicine, Jammu, for providing elite material of G. glabra (rhizome). MS and GS are thankful to CSIR, New Delhi, for providing Senior Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Srivastava, M., Singh, G., Sharma, S. et al. Elicitation Enhanced the Yield of Glycyrrhizin and Antioxidant Activities in Hairy Root Cultures of Glycyrrhiza glabra L.. J Plant Growth Regul 38, 373–384 (2019). https://doi.org/10.1007/s00344-018-9847-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00344-018-9847-2