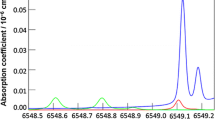
Abstract
In atmospheric and environmental sciences, optical spectrometers are used for the measurements of greenhouse gas mole fractions and the isotopic composition of water vapor or greenhouse gases. The large sample cell volumes (tens of milliliters to several liters) in commercially available spectrometers constrain the usefulness of such instruments for applications that are limited in sample size and/or need to track fast variations in the sample stream. In an effort to make spectrometers more suitable for sample-limited applications, we developed a low-volume analyzer capable of measuring mole fractions of methane and carbon monoxide based on a commercial cavity ring-down spectrometer. The instrument has a small sample cell (9.6 ml) and can selectively be operated at a sample cell pressure of 140, 45, or 20 Torr (effective internal volume of 1.8, 0.57, and 0.25 ml). We present the new sample cell design and the flow path configuration, which are optimized for small sample sizes. To quantify the spectrometer’s usefulness for sample-limited applications, we determine the renewal rate of sample molecules within the low-volume spectrometer. Furthermore, we show that the performance of the low-volume spectrometer matches the performance of the standard commercial analyzers by investigating linearity, precision, and instrumental drift.









Similar content being viewed by others
Notes
In the following, we will use the unit cubic centimeters (cc) for volumes, standard cubic centimeters (scc) for effective volumes (pressure-dependent volumes; see Sect. 3.3), and standard cubic centimeters per minute (sccm) for volumetric flow rates.
Invar is an alloy (64 % iron and 36 % nickel) with a very low coefficient of thermal expansion.
It is noteworthy that not only the volume of different components, but also their internal shape determines the renewal rate of the instrument. The volume in a straight piece of tubing, for instance, is swept more efficiently than a spherical volume of the same size. The filters are a good example of poorly swept components due to their large internal diameter (compared to the tubing).
CH4 (CO) raw data are reported ca. every 4.1 s (8.3 s); the software of the CRDS automatically averages over 4.1 s (8.3 s) of ring-down time data.
For the sake of clarity, we mention only t 90 whenever the other two parameters t 99 and ν 50 support the same result. Table 2 gives an overview of all parameters.
References
M.W. Sigrist, Air Monitoring by Spectroscopic Techniques (Wiley, New York, 1994)
P. Bousquet, A. Gaudry, P. Ciais, V. Kazan, P. Monfray, P.G. Simmonds, S.G. Jennings, T.C. O’Connor, Phys. Chem. Earth 21, 5–6 (1996)
F. D’Amato, P. Mazzinghi, F. Castagnoli, Appl. Phys. B 75, 2–3 (2002)
D. Romanini, M. Chenevier, S. Kassi, M. Schmidt, C. Valant, M. Ramonet, J. Lopez, H.-J. Jost, Appl. Phys. B 83, 4 (2006)
J. Winderlich, H. Chen, C. Gerbig, T. Seifert, O. Kolle, J. V. Lavric, C. Kaiser, A. Hoefer, M. Heimann, Atmos. Meas. Tech. 3, 1113–1128 (2010)
Earth Networks—Greenhouse Gas Network, http://www.earthnetworks.com/OurNetworks/GreenhouseGasNetwork.aspx (last visited: November 2012)
L. Haszpra, Z. Barcza, P.S. Bakwin, B.W. Berger, K.J. Davis, T. Weidinger, J. Geophys. Res. 106, D3 (2001)
A.N. Parsons, J.E. Barrett, D.H. Wall, R.A. Virginia, Ecosystems 7, 3 (2004)
X.A. Padin, M. Vázquez-Rodríguez, A.F. Rios, F.F. Pérez, J. Mar. Syst. 66, 1–4 (2007)
C. Wille, L. Kutzbach, T. Sachs, D. Wagner, E. Pfeiffer, Glob. Change Biol. 14, 6 (2008)
C.R. Webster, A.J. Heymsfield, Science 302, 5651 (2003)
E.R.T. Kerstel, R.Q. Iannone, M. Chenevier, S. Kassi, H.-J. Jost, D. Romanini, Appl. Phys. B 85, 2–3 (2006)
G. Friedrichs, J. Bock, F. Temps, P. Fietzek, A. Körtzinger, D. W. R. Wallace, Limnol. Oceanogr. Methods 8, 539–551 (2010)
F. Keppler, S. Laukenmann, J. Rinne, H. Heuwinkel, M. Greule, M. Whiticar, J. Lelieveld, Environ. Sci. Technol. 44, 13 (2010)
V. Gkinis, T.J. Popp, S.J. Johnsen, T. Blunier, Atmos. Meas. Tech. 4, 2531–2542 (2011)
N.C. Munksgaard, C.M. Wurster, A. Bass, M.I. Bird, Hydrol. Process. 26, 23 (2012)
S.B. Joye, A. Boetius, B.N. Orcutt, J.P. Montoya, H.N. Schulz, M.J. Erickson, S.K. Lugo, Chem. Geol. 205, 3–4 (2004)
T. Güllük, H. Wagner, F. Slemr, Rev. Sci. Instrum. 68, 230 (1997)
C. Stowasser, C. Buizert, V. Gkinis, J. Chappellaz, S. Schüpbach, M. Bigler, X. Faïn, P. Sperlich, M. Baumgartner, A. Schilt, T. Blunier, Atmos. Meas. Tech. 5, 999–1013 (2012)
R.H. Rhodes, X. Faïn, C. Stowasser, T. Blunier, J. Chappellaz, J.R. McConnell, D. Romanini, L.E. Mitchell, E.J. Brook, Earth Planet Sci. Lett. 368, 9–19 (2013)
A.E. Andrews, J.D. Kofler, M.E. Trudeau, J.C. Williams, D.H. Neff, K.A. Masarie, D.Y. Chao, D.R. Kitzis, P.C. Novelli, C.L. Zhao, E.J. Dlugokencky, P.M. Lang, M.J. Crotwell, M.L. Fischer, M.J. Parker, J.T. Lee, D.D. Baumann, A.R. Desai, C.O. Stanier, S.F.J. de Wekker, D.E. Wolfe, J.W. Munger, P.P. Tans, Atmos. Meas. Tech. Discuss. 6, 1461–1553 (2013)
E.R. Crosson, Appl. Phys. B 92, 3 (2008)
X.F. Wen, X.H. Lee, X.M. Sun, J.L. Wang, Y.K. Tang, S.G. Li, G.R. Yu, J. Atmos. Ocean Tech. 29, 2 (2012)
G. Berden, R. Peeters, G. Meijer, Int. Rev. Phys. Chem. 19, 4 (2000)
M. Mazurenka, A.J. Orr-Ewing, R. Peverall, G.A.D. Ritchie, Annu. Rep. Prog. Chem. Sect. C Phys. Chem. 101, 100–142 (2005)
L.S. Rothman, I.E. Gordon, A. Barbe, D.C. Benner, P.F. Bernath, M. Birk, V. Boudon, L.R. Brown, A. Campargue, J.-P. Champion, K. Chance, L.H. Coudert, V. Dana, V.M. Devi, S. Fally, J.-M. Flaud, R.R. Gamache, A. Goldman, D. Jacquemart, I. Kleiner, N. Lacome, W. Lafferty, J.-Y. Mandin, S.T. Massie, S.N. Mikhailenko, C.E. Miller, N. Moazzen-Ahmadi, O.V. Naumenko, A.V. Nikitin, J. Orphal, V.I. Perevalov, A. Perrin, A. Predoi-Cross, C.P. Rinsland, M. Rotger, M. Simeckova, M.A.H. Smith, K. Sung, S.A. Tashkun, J. Tennyson, R.A. Toth, A.C. Vandaele, J. Vander Auwera, J. Quant. Spectrosc. Ra. 110, 533–572 (2009)
H. Chen, A. Karion, C.W. Rella, J. Winderlich, C. Gerbig, A. Filges, T. Newberger, C. Sweeney, P.P. Tans, Atmos. Meas. Tech. 6, 1031–1040 (2013)
W.H. Press, B.P. Flannery, S.A. Teukolsky, W.T. Vetterling, Numerical Recipes: The Art of Scientific Computing (Cambridge University Press, New York, 1986)
A.E. Siegman, Lasers (University Science Books, Mill Valley, 1986)
H. Huang, K.K. Lehmann, Opt. Express 15, 8745–8759 (2007)
D. Allan, Proc. IEEE 54, 221–230 (1966)
P. Werle, Appl. Phys. B 10, 251–253 (2011)
V. Gkinis, T.J. Popp, T. Blunier, M. Bigler, S. Schüpbach, E. Kettner, S.J. Johnsen, Isot. Environ. Health Sci. 46, 4 (2010)
P. Barriga, C. Zhao, D.G. Blair, Gen. Relativ. Gravit. 37, 1609–1619 (2005)
M. Krystek, M. Anton, Meas. Sci. Technol. 18, 3438–3442 (2007)
Acknowledgments
We like to express our gratitude to the many helping hands at Picarro especially in the clean room and in integration. Many thanks to Christian Ladeby for his excellent work in machining the cavity body. Finally, we thank Vasileios Gkinis and David Balslev-Clausen for fruitful discussions.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Resonant mode frequency calculation
In order for the two modes to build up in the cavity, their resonant frequencies must be degenerate. Using the standard notation, the resonant frequency of a mode with mode numbers n, m, q is given by:
where FSR = c/(2 n 0 L) is the free spectral range of the cavity, n 0 is the index of refraction of the gas inside the cavity and g = 1 − L/R. The curious factor of 1–(−1)m accounts for the lack of symmetry for odd-numbered horizontal transverse modes after one round trip through the three-mirror resonator [34]. The fundamental cavity mode (n = m = 0 and q = q 0) resonates at
where q 0 is given by the operating wavelength at the molecular transition of interest. A high-order mode is said to be degenerate with the fundamental mode if the difference in frequency between the two modes is within the linewidth (full-width at half-maximum) of the laser, LW. It follows that
Using the expressions for the frequencies \(\nu_{n,m,q}\) and \(\nu_{{0,0,q_{0} }}\), the equation can be rewritten as
or
Since \(q - q_{0}\) has to be an integer, the calculation of the number of degenerate modes is simplified by looking at the fractional part (〈〉) only:
Appendix 2: Linearity
Figures 10a and 11a show the calibration curve of the low-volume CRDS for CH4 and CO, respectively. Calibration curves are measured for all operating pressures (140, 45, and 20 Torr). In each calibration curve, the true mole fractions of the two gas mixtures CA08274 and CA08292 (Table 3, hereafter referred to as low and high gas mixtures) are plotted against the mole fractions as reported by the low-volume CRDS (black markers in Figs. 10a and 11a). Each gas mixture is injected into the low-volume CRDS for ca. 1 h at a flow rate of 20 sccm (140 Torr), 10 sccm (45 Torr), and 2 sccm (20 Torr), respectively. Every data point in the calibration curve represents the mean value of these measurements series. Mean values and measurement precision (1σ) of all measurement series are listed in Table 4.
a Low-volume CRDS calibration curves for measurements of CH4 mole fractions based on the high and low gas standards at the three available operating pressures (black markers) and the corresponding linear fits (black lines). The fit parameters a and b as well as their uncertainties are listed in the lower right corner. Measurements of the “check” are shown as red crosses. b Close-ups of the calibration curves confirm linearity, since fit and check overlap within their uncertainties. Residuals R are listed for each operating pressure
a Low-volume CRDS calibration curves for measurements of CO mole fractions based on the high and low gas standards at the three available operating pressures (black markers) and the corresponding linear fits (black lines). The fit parameters a and b as well as their uncertainties are listed in the lower right corner. Measurements of the “check” are shown as red crosses. b Close-ups of the calibration curves confirm linearity, since fit and check overlap within their uncertainties. Residuals R are listed for each operating pressure
A linear fit is applied to the measurements of the low and high gas mixtures (black lines) using a weighted total least-squares algorithm where every data point has individual uncertainties in both coordinates [35]. Here, the individual uncertainties are the uncertainty of the gas mixture (abscissa, Table 3) and the standard deviation of the measurement series (ordinate, Table 4). The algorithm yields the fit parameters (a and b) as well as their uncertainties (1σ, listed at the bottom right in Fig. 10a and 11a). The uncertainty of the linear fit is too small to display in Figs. 10a and 11a, but is shown in Figs. 10b and 11b (cyan).
To confirm linearity, the third gas mixture CA08280 (hereafter referred to as “check”) is measured. The true mole fraction of the check is plotted against the mole fraction as measured by the low-volume CRDS (red crosses). Figures 10b (CH4) and 11b (CO) show close-ups of the calibration curve around the check for an operating pressure of 140 (top panel), 45 (middle panel), and 20 Torr (bottom panel), respectively. The uncertainty on the absolute mole fraction of the gas mixture is shown as a red error bar (abscissa, Table 3); the measurement precision is too small to be indicated with an error bar (ordinate, Table 4). We can conclude that linearity of CH4 and CO mole fraction measurements is confirmed for all operating pressures, since the check and the linear fit overlap within their uncertainties.
Both calibration curves are pressure-dependent, as seen by the varying slope (a) and offset (b) fit parameters. For CH4, the pressure dependence is small and can be explained by the fact that we adjusted the fitting algorithm for the three operating pressures, but used the standard (140 Torr) internal instrument calibration. By adjusting the instrument calibration as well, the differences between the three calibration curves for CH4 can be minimized.
The pressure dependence of the CO calibration curves is more pronounced. We found that a single set of model functions obtained at 140 Torr was sufficient to capture the effect of CO2 and H2O on the quantification of the CO absorption line (see Sects. 2.1 and 2.2). Thus, we did not adjust the CO fitting algorithm for the three operating pressures. Using one fitting algorithm for different operating pressures results in the strong pressure dependence of the CO calibration curves, which reflects the effect of pressure broadening. For CO at 20 Torr, 1 ppm of CO in air will produce an absorption feature with a peak height of 0.79 ppb/cm of absorption loss. At 45 Torr, this peak height is about 1.41 ppb/cm, which is greater by a factor of 1.78 than the 20 Torr value, with a pressure increase of 2.25 times. At 140 Torr (which is 7 times 20 Torr), the peak height is just 2.34 ppb/cm for the same 1 ppm concentration, for a factor of 2.96.
Rights and permissions
About this article
Cite this article
Stowasser, C., Farinas, A.D., Ware, J. et al. A low-volume cavity ring-down spectrometer for sample-limited applications. Appl. Phys. B 116, 255–270 (2014). https://doi.org/10.1007/s00340-013-5528-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00340-013-5528-9