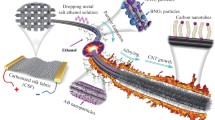
Abstract
This study presents a new controlled approach to deep perforation of millimeter-long carbon nanotube arrays (CNTAs) by fast oxidative cutting. The approach is based on decorating CNTAs with silver (Ag) nanoparticles, followed by heating Ag-decorated CNTAs with microwave radiation (2.48 GHz, 300 W). The perforation was evaluated using different techniques such as transmission electron microscopy, X-ray photoelectron spectroscopy, and Brunauer–Emmett–Teller method. The results of the oxidation of carbonaceous materials indicated that the relative amount of oxygen functional groups increased without total oxidation of carbon up to ~ 60 s. After 60 s, the amount of functional groups decreased as the total oxidation started suddenly. Afterwards, at around 120 and 420 s, the oxidation of Ag-decorated CNTAs reached the point of total perforation and total cutting, respectively. Though carbon decomposition terminated at around 420 s, the total pore volume and surface area increased continuously. This was attributed to the steady growth of Ag nanoparticles located between CNTAs.








Similar content being viewed by others
Change history
14 May 2018
This study presents a new controlled approach to deep perforation of millimeter-long carbon nanotube arrays (CNTAs) by fast oxidative cutting. The approach is based on decorating CNTAs with silver (Ag) nanoparticles, followed by heating Ag-decorated CNTAs with microwave radiation (2.48 GHz, 300 W).
References
Y.-Q. Cai, G.-B. Jiang, J.-F. Liu, Q.-X. Zhou, Anal. Chim. Acta 494, 149 (2003)
L. Jiang, L. Gao, J. Sun, J. Colloid Interface Sci. 260, 89 (2003)
L. Ting, W. Pang, X.Y. Ren RP, Han, J. Comput. Theor. Nanosci. 10(10), 2385 (2013)
S.M. Sanip, A.F. Ismail, P.S. Goh, T. Soga, M. Tanemura, H. Yasuhiko, Sep. Purif. Technol. 78, 208 (2011)
T. Chen, L. Dai, Mater. Today 16, 272 (2013)
C. Wang, M. Waje, X. Wang, J.M. Tang, R.C. Haddon, Y.S. Yan, Nano Lett. 4, 345 (2004)
S. S. Shigeki Hasegawa, Yoshihiro Shinozaki, Masahiro Imanishi, (2010)
G. Chen, S. Sakurai, M. Yumura, K. Hata, D.N. Futaba, Carbon N. Y. 107, 433 (2016)
T. Hiraoka, A. Izadi-Najafabadi, T. Yamada, D.N. Futaba, S. Yasuda, O. Tanaike, K. Hata, Compact and light supercapacitor electrodes from a surface-only solid by opened carbon nanotubes with 2 200 m2 g – 1 surface area. Adv. Func. Mater. 20(3), 422–428 (2010)
M.V. Shuba, A.G. Paddubskaya, P.P. Kuzhir, S.A. Maksimenko, V.K. Ksenevich, G. Niaura, D. Seliuta, I. Kasalynas, G. Valusis, Nanotechnology 23, 495714 (2012)
R. Liu, Dai, Hafner, Bradley, Boul, Lu, Iverson, Shelimov, Huffman, Rodriguez-Macias, Shon, Lee, Colbert, and Smalley. Science 280, 1253 (1998)
J.W. Jang, C.E. Lee, C.J. Lee, Solid State Commun. 135, 683 (2005)
D. Wu, L. Wu, W. Zhou, Y. Sun, M. Zhang, J. Polym. Sci. Part B Polym. Phys. 48, 479 (2010)
W. Bauhofer, J.Z. Kovacs, Compos. Sci. Technol. 69, 1486 (2009)
M. Zhang, M. Yudasaka, A. Koshio, C. Jabs, T. Ichihashi, S. Iijima, Appl. Phys. A 74, 7 (2002)
C. Wang, S. Guo, X. Pan, W. Chen, X. Bao, J. Mater. Chem. 18, 5782 (2008)
S.Y. Lee, D.-H. Kim, S.C. Choi, D.-J. Lee, J.Y. Choi, H.-D. Kim, Microporous Mesoporous Mater. 194, 46 (2014)
R. Pelalak, M. Baniadam, M. Maghrebi, Appl. Phys. A Mater. Sci. Process. 111, 951 (2013)
J.C. Maxwell, Oxford Clarendon Press 360 (1873)
R. Jin, Y.C. Cao, E. Hao, G.S. Métraux, G.C. Schatz, C.A. Mirkin, Nature 425, 487 (2003)
W. Chiang, B.E. Brinson, A.Y. Huang, P.A. Willis, M.J. Bronikowski, J.L. Margrave, R.E. Smalley, R.H. Hauge, J. Phys. Chem. B 105, 8297 (2001)
F. Xin, L. Li, Compos. Part A Appl. Sci. Manuf. 42, 961 (2011)
T. Susi, T. Pichler, P. Ayala, Beilstein J. Nanotechnol. 6, 177 (2015)
M. Deng, G. Zhao, Q. Xue, L. Chen, Y. Lu, Appl. Catal. B Environ. 99, 222 (2010)
J.W. Niemantsverdriet, Diffraction and Extended X-Ray Absorption Fine Structure (EXAFS) (2007)
B.J. Hinds, N. Chopra, T. Rantell, R. Andrews, V. Gavalas, L.G. Bachas, Science 303, 62 (2004)
Y. Saito, T. Yoshikawa, J. Cryst. Growth 134, 154 (1993)
Acknowledgements
The authors are grateful to Iran Nanotechnology Initiative Council for financial support.
Author information
Authors and Affiliations
Corresponding author
Appendices
Appendix 1: Detailed XPS data
Figures 9 and 10 show the deconvulation of the O1s and Ag 3d spectra, respectively. The deconvolution of the O1s peak (Fig. 9) shows the existence of some carboxylic and hydroxyl groups on Ag-CNTAs at 522.2 and 531.9 eV, respectively. The small peak at 530.6 eV can be related to physically-adsorbed oxygen atoms. Furthermore, according to Fig. 10, Ag 3d5/2 and Ag 3d3/2 were observed at 368.1 and 374 eV, respectively. No bond was observed between oxygen and Ag.
Appendix 2: Comparison of the total surface area of Ag-CNTAs after 0 and 60 s of heating
In this appendix, the variation of the surface area of Ag-CNTAs (SAg−CNTA) after 0 and 60 s of heating is studied. As shown in Fig. 8, the surface area of Ag-CNTAs declined during the first step (0–60 s) despite the lack of any decomposition. To compare these variations more accurately, the surface area of Ag-CNTAs should be mathematically modeled. Here, Ag nanoparticles are considered to be as a hemisphere located on CNTAs. Figure 11 helps to evaluate SAg−CNTA, which is formulated in Eq. (1).
where A1 and A2 represent the exposed surface area of Ag nanoparticle and CNTA, respectively. A3 refers to the surface area of sites on CNTA, which is covered by Ag nanoparticles. n is also the number of Ag nanoparticles on the CNTA’s sidewall.
In view of the constant value of A2, the observed decrease of SAg−CNTA during 0–60 s could be attributed to A1 and A3, which can be obtained as follows:
The substitution of A1 and A3 in (1) gives:
Since the Ag is not consumed during the experiment, the total volume of Ag nanoparticles is assumed to be constant between 0 and 60 s. Therefore, the volume of an individual Ag nanoparticle can be simply calculated by the following equation:
also,
\({V_{{\text{Ag}}@~t=0\,{\text{s}}}}={V_{{\text{Ag}}\,@~\,t=60\,{\text{s}}}}\) (based on the above information)
So, the total volume can be obtained by the product of the number of Ag particles and their individual volume.
where r and R are the radius of Ag nanoparticles after 0 and 60 s of heat treatment. The values of r and R were measured using TEM images as 3.5 and 6 nm, respectively.
According to Eq. (3), SAg−CNTA after 0 and 60 s can be written as:
The above equation can be simplified by re-writing Eq. (5) as:
As can be seen, the mathematical comparison of the total surface area of Ag nanoparticles at 0 and 60 s could justify the drop in the total surface area of Ag during 0–60 s as observed in Fig. 8.
Rights and permissions
About this article
Cite this article
Ojaghi, N., Mokhtarifar, M., Sabaghian, Z. et al. Rapid and controllable perforation of carbon nanotubes by microwave radiation. Appl. Phys. A 124, 367 (2018). https://doi.org/10.1007/s00339-018-1787-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00339-018-1787-y