Abstract
Objective
To evaluate the efficacy and safety of stent placement combined with intraluminal radiofrequency ablation (intra-RFA) and hepatic arterial infusion chemotherapy (HAIC) for patients with advanced biliary tract cancers (Ad-BTCs) and biliary obstruction (BO).
Methods
We retrospectively reviewed data for patients with Ad-BTCs and BO who underwent stent placement with or without intra-RFA and HAIC in three centres between November 2013 and November 2018. The stent patency time (SPT), overall survival (OS), and adverse events (AEs) were analysed.
Results
Of the 135 enrolled patients, 64 underwent stent placement combined with intra-RFA and HAIC, while 71 underwent only stent placement. The median SPT was significantly longer in the combination group (8.2 months, 95% confidence interval [CI]: 7.1–9.3) than in the control group (4.3 months, 95% CI: 3.6–5.0; p < 0.001). A similar result was observed for OS (combination: 13.2 months, 95% CI: 11.1–16.5; control: 8.5 months, 95% CI: 7.6–9.6; p < 0.001). The incidence of AEs related to biliary tract operation was not significantly different between the two groups (p > 0.05). The most common AE and serious AE related to HAIC were alanine aminotransferase elevation (24/64; 37.5%) and thrombocytopenia (8/64; 12.5%), respectively. All AEs were tolerable, and there was no death from AEs.
Conclusions
Stent placement combined with intra-RFA and HAIC may be a safe, potential treatment strategy for patients with Ad-BTCs and BO.
Key Points
• Advanced biliary cancers (Ad-BTCs) with biliary obstruction (BO) can rapidly result in liver failure and cachexia with an extremely poor prognosis.
• Stent placement combined with intraluminal radiofrequency ablation and hepatic arterial infusion chemotherapy may be safe and effective for patients with Ad-BTCs and BO.
• The long-term efficacy and safety of the combined treatment is promising.
Similar content being viewed by others
Introduction
Biliary tract cancers (BTCs) are classified into intrahepatic cholangiocarcinoma, perihilar cholangiocarcinoma (pCCA), distal cholangiocarcinoma, gall bladder cancer, and carcinoma of the ampulla according to the anatomical structure [1, 2]. The incidence and mortality of BTCs have progressively increased worldwide in recent decades [3, 4]. For early-stage disease, liver transplantation and radical resection seem to be potential curative options [5]. Unfortunately, approximately 60–70% of patients are diagnosed in advanced stages, and > 50% of advanced BTCs (Ad-BTCs) are complicated by biliary obstruction (BO) [6], which can rapidly result in liver failure and cachexia with an extremely poor prognosis [7]. The median survival of patients with Ad-BTCs and BO is 4.8 months, with a 5-year survival rate of 10% [8]. Metal stent placement is considered the most effective palliative treatment to improve the quality of life and survival [9]. However, 1-year recurrence rates for BO reach 68%, with the main cause for recurrence being local tumour progression [10, 11]. Therefore, tumour growth control is critical for maintaining stent patency and prolonging survival in cases of Ad-BTCs with BO.
Radiofrequency ablation (RFA) is widely used to treat solid tumours, particularly hepatocellular carcinoma [12]. Intraluminal RFA (intra-RFA), based on the same principle of energy generation, has been used to manage malignant BO (MBO) in recent years [13]. The high thermal energy generated by radiofrequency electrodes can effectively hamper intraluminal tumour growth [14]. It was reported that intra-RFA could prolong stent patency and survival in patients with BTCs and BO, although its efficacy needs improvement [15]. In a meta-analysis, the pooled weighted mean difference in stent patency was only 1.7 months [16]. Moreover, intra-RFA cannot reach residual tumours and lesions located outside the biliary tract, which may quickly progress and trigger stent re-stenosis.
Hepatic arterial infusion chemotherapy (HAIC) can effectively increase the local concentration of anti-tumour drugs and decrease the systemic distribution of agents [17]. Compared with systemic chemotherapy, HAIC can significantly reduce adverse events (AEs) and achieve more potent local anti-tumour effects [18]. Patients with advanced hepatocellular carcinoma can achieve high local tumour control rates and yield long-term benefits from HAIC [19, 20], and retrospective studies suggest that HAIC can also offer clinical benefits to patients with Ad-BTCs [21, 22]. A recent phase 2 clinical study indicated that HAIC with oxaliplatin and 5-fluorouracil for advanced pCCA can effectively control local tumour growth and provide an encouraging survival benefit [23]. Tumours that cannot be completely ablated and lesions outside the biliary tract can be simultaneously controlled by compensating HAIC. Intra-RFA combined with HAIC with oxaliplatin and 5-fluorouracil may be a promising strategy for Ad-BTCs with BO. To our knowledge, there is no report regarding this new treatment modality. Therefore, we conducted a multicentre, retrospective study to evaluate the efficacy and safety of stent placement + intra-RFA+HAIC for Ad-BTCs with BO.
Materials and methods
Study design and patients
This retrospective, multicentre cohort study was approved by the Ethics Committees of Guangdong Provincial People’s Hospital, Zhongshan Hospital of Traditional Chinese Medicine, and Guangdong Clifford Hospital, and the requirement for written informed consent was waived for all patients.
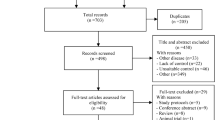
We retrospectively reviewed the medical records of consecutive patients who received interventional treatments for Ad-BTCs with BO in the three aforementioned centres between November 2013 and November 2018 (Fig. 1). According to the eighth edition of the American Joint Committee on Cancer TNM staging system, stage III and stage IV BTCs were defined as Ad-BTCs [24]. Patients who received stent placement + intra-RFA+HAIC with oxaliplatin and 5-fluorouracil were included in the combination group, while patients who underwent stent placement only were included in the control group. In the combination group, patients underwent intra-RFA and stent placement simultaneously with percutaneous transhepatic cholangiography (PTC) or after percutaneous transhepatic cholangial drainage (PTCD), followed by HAIC when Child–Pugh class A or B liver function was achieved. Patients in the control group only underwent stent placement simultaneously with PTC or after PTCD. The stent patency time (SPT) and overall survival (OS) were the primary endpoints, while treatment-related AE rates were secondary endpoints. Relevant clinical data were collected from the medical record system and follow-up records. The follow-up deadline was November 2019.
The inclusion criteria were as follows: (1) age of 18 to 80 years; (2) diagnosis of Ad-BTCs through pathology or cytology combined with MRCP, PET, or CT; (3) diagnosis of BO by serum bilirubin measurement and CT, MRCP, or PET; and (4) patient’s life expectancy > 1 month. The exclusion criteria were as follows: (1) Eastern Cooperative Oncology Group performance status > 2, (2) BO not associated with BTC, (3) other malignancies, (4) contraindications for PTC/PTCD, (5) only one HAIC cycle, and (6) missing data.
Intra-RFA and stent placement
After successful puncture of intrahepatic bile ducts using percutaneous access set (NPAS-100-RH-NT; Cook Medical) (Fig. 2c), a catheter (NHB5.0-38-40-P-NS-KMP; Cook Medical) was introduced into the biliary system using a guidewire. The site and length of the obstruction were measured according to the cholangiography findings. If the guidewire failed to pass through the obstructed segment, PTCD was performed.
Intra Intraluminal radiofrequency ablation and double stent-by-stent deployment. a, b MRCP and contrast-enhanced CT showed perihilar cholangiocarcinoma presented malignant obstructive jaundice. c–e By adding a hard-exchange guide wire and sending into the ablation catheter, radiofrequency ablation (red arrow) was performed on the left and right bile duct stenosis segment. f, g After balloon expansion, the uncovered metal stent was implanted, and angiography showed that the stent was expanded well, and the common bile duct was restored unobstructed
In the combination group, intra-RFA was performed before stent placement. In order to prevent biliary tract infections, all patients received prophylactic antibiotics the day before intra-RFA. An ablation catheter (Habib EndoHPB; EMcision Ltd.) was inserted into the targeted section under the guidewire passed through the obstructed segment. Subsequently, the ablation catheter probe was connected to a frequency generator (RITA 1500X; AngioDynamics), and ablation was performed with a power of 10 W for 120 s (Fig. 2d, e). If the stenosis length was > 2.0 cm, step-by-step intra-RFA was performed from the distal to the proximal end. For patients with multiple intrahepatic stenoses, the bile ducts simultaneously underwent ablation do not exceed 3 ducts. Subsequently, an uncovered metal stent (Wallstent; Boston Scientific) (Fig. 2f, g) with an 8 mm diameter was placed. In the control group, patients underwent stent placement using the same technique. In both groups, a multi-purpose drainage catheter was placed in the bile duct for 3–5 days after stent placement, and it was removed once stent patency was confirmed by cholangiography.
HAIC
A 5-French catheter (HNB5.0-38-80-P-NS-RH; Cook Medical) was inserted into the coeliac trunk or superior mesenteric artery. According to arteriography results, the catheter was selectively placed in the main feeding arteries of the tumours. To prevent obstruction by a blood clot, the end of the catheter was locked with a heparin lock (10 ml, 10,000 units, 1:1000 dilution). The peripheral part and vascular sheath were properly fixed on the groin, and the catheter tip position at the target location was confirmed by fluoroscopy. Subsequently, HAIC was initiated in the general ward using the following regimen: oxaliplatin, 130 mg/m2 from hour 3 on day 1; leucovorin, 400 mg/m2 from hour 2 on day 1; and fluorouracil, 400 mg/m2 bolus at hour 3 and 2400 mg/m2 over 46 h on days 1 and 2. For the alleviation of AEs, dexamethasone, stomach-protecting agents, and antiemetic drugs were administered to patients 30 min before HAIC. The same procedures were repeated for the next HAIC cycle. Each cycle was 3 weeks, and a total of six cycles were required. Delays and modifications were allowed for HAIC-related AEs. HAIC was discontinued in case of BO recurrence, unacceptable toxic effects, patient refusal to continue, or change to other treatments.
Assessments and follow-up
SPT was calculated from the date of stent placement to the date of BO recurrence confirmed by imaging examination. If patients were lost to follow-up, SPT was calculated from the date of stent placement to the date of the last follow-up and considered censored data. If there was no concrete evidence of BO recurrence during the patient’s lifespan, SPT was considered equivalent to OS and considered censored data. OS was calculated from the date of stent placement to the date of death or the date of the last follow-up. Treatment-related complications were defined as AEs within 2 weeks after intra-RFA, stent placement, or HAIC. AE grades were determined using the CTCAE5.0 criteria; grade 1–2 AEs were considered mild-to-moderate AEs while grade 3–4 AEs were considered serious AEs (SAEs).
In the first month, laboratory tests were completed within 1–2 weeks after stent placement in all patients. From the second month, laboratory tests were performed at intervals of 3 weeks to 2 months. If serum bilirubin levels were increased (> 5 mg/dL), CT or MRI was performed to confirm BO recurrence. To evaluate HAIC-related AEs, laboratory tests were performed every 1–2 weeks after HAIC. In cases of HAIC treatment withdrawal, patients were contacted every 2–3 months until they died or were lost to follow-up.
Statistical analysis
The independent-sample t test, the chi-squared test, and Fisher’s exact test were used, as appropriate, to determine differences in clinical variables, the clinical efficacy, and AE rates between the two groups. SPT and OS were calculated using the Kaplan–Meier method, and differences were identified using the log-rank test. Statistically significant factors (including clinical and laboratory parameters and tumour characteristics predicting SPT and OS) in univariate analysis were included in multivariate Cox regression analysis. A Cox proportional hazards model was used to estimate the hazard ratios. A p value of < 0.05 was considered statistically significant. All statistical analyses were performed using Statistical Package for SPSS software, version 25.0 (IBM) and STATA version 16.0 (Stata Corporation).
Results
Patient characteristics
In total, 206 consecutive patients with Ad-BTCs and BO underwent interventional treatments during the study period. After application of the inclusion and exclusion criteria, 135 (82 men [median age, 62.0 years; range, 35.0–84.0 years] and 53 women [median age, 61.0 years; range, 34–82 years]) were enrolled (Fig. 1). There were 64 and 71 patients in the combination and control groups, respectively, with no significant between-group differences in baseline characteristics (Table 1).
Intervention
All enrolled patients received stents that were placed simultaneously with PTC in 78 patients (57.8%) and after PTCD in 57 patients (42.2%). The stent length was > 6 cm and ≤ 6 cm in 48 (34.1%) and 87 (64.5%) patients, respectively. Post-stenting bilirubin levels at 1 and 2 weeks were significantly reduced in both groups, with no significant between-group difference (p > 0.05). In the combination group, all patients subsequently underwent successful intra-RFA, followed by successful insertion of the arterial catheter and administration of at least two HAIC cycles. A median number of 4.64 HAIC cycles (2–6) per patient was recorded for the overall cohort. All operations were performed under local anaesthesia.
Stent patency time and overall survival
The median follow-up duration was 10.6 (range: 7.2–14.8) months, and the longest duration was 33.4 months. Ten patients (7.4%) were alive at the last follow-up. BO recurred in 123 patients (91.1%). The median SPT was significantly longer in the combination group (8.2 months, 95% confidence interval [CI]: 7.1–9.3) than in the control group (4.3 months, 95% CI: 3.6–5.0; p < 0.001; Fig. 3a). The median OS showed a similar result (combination: 13.2 months, 95% CI: 11.1–16.5; control: 8.5 months, 95% CI: 7.6–9.6; p < 0.001; Fig. 3b). The 3-month survival rate (100.0% vs. 97.2%) was comparable between groups (p = 0.523). However, the 6-, 9-, and 12-month survival rates were significantly higher in the combination group (95.3% vs. 77.5%, 81.3% vs. 47.9%, 59.4% vs. 22.5%; p < 0.05).
Stent patency and overall survival. a Cumulative stent patency by the Kaplan–Meier analysis. Median stent patency was 7.8 months in the combination therapy group versus 4.0 months in the only stent group. b Significantly better overall survival rates were observed in the patients treated with combination therapy according to the Kaplan–Meier method (p < 0.001 for both)
Univariate and multivariate Cox regression analyses for factors relevant to SPT and OS are shown in Tables 2 and 3. The absence of distant metastasis, a shorter stricture (< 4 cm), and the level of BO in Bismuth types I–II were independent predictors of longer SPT and OS. These factors remained significant in multivariate analysis. Figure 4 presents the hazard ratios for OS (a) and SPT (b) according to the potential influencing baseline factors; a history of previous treatments was associated with shorter SPT and OS.
Adverse events
AEs related to the percutaneous biliary tract operation procedures are summarised in Table 4. The overall AE incidence was similar in the combination and control groups (mild-to-moderate AEs: 56.3% vs. 53.5%, SAEs: 10.9% vs. 7.0%; p = 0.598). The most frequent mild-to-moderate AE and SAE were abdominal pain (21.9% and 22.5% in the combination and control groups, respectively) and biliary infection (4.7% and 2.8% in the combination and control groups, respectively), respectively. The incidences and grades of abdominal pain, haemorrhage, pancreatitis, peritonitis, bile duct perforation, and biliary infection showed no significant between-group differences (p > 0.05). There were no AE-related deaths during the follow-up.
HAIC-related AEs are listed in Table 5. Arterial catheter-related AEs included catheter dislocation, catheter obstruction, arterial thrombosis, arterial haematoma, and catheter infection, observed in three (4.7%), two (3.1%), one (1.6%), five (7.8%), and one (1.6%) patient, respectively. Liver function and haematopoietic function were transiently affected by HAIC. The most common AE was alanine aminotransferase (ALT) elevation (n = 31; 48.4%). The most common mild-to-moderate AE and SAE were ALT elevation and thrombocytopenia, which occurred in 24 (37.5%) and eight (12.5%) patients, respectively.
Discussion
In the present study of patients with Ad-BTCs and BO, we found that SPT and OS were markedly longer in patients treated by stent placement + intra-RFA+HAIC than in those treated by stent placement alone. The absence of metastasis, a stricture of < 4 cm, and the level of BO in Bismuth types I–II were independent predictors of longer SPT and OS. All AEs could be tolerated or relieved after active symptomatic treatments.
In cases of BO caused by BTCs or other malignancies, relieving obstructive jaundice and maintaining stent patency are extremely pivotal to prolong survival and improve the quality of life. In our study, SPT and OS were significantly longer in the combination group than in the control group, with significantly higher survival rates at 6, 9, and 12 months in the former group. These encouraging outcomes can be attributed to the use of intra-RFA+HAIC for restricting local disease progression. The ablation electrode can generate high thermal energy, which induces coagulative necrosis of tumour cells in the bile duct cavity. Monga confirmed the disappearance of tumour blood vessels and lumen enlargement after intra-RFA [25]. Several studies have reported the clinical efficacy, safety, and survival benefit of intra-RFA for MBO [13, 15, 26]. In a prospective, randomised trial comparing intra-RFA + stent placement with stent placement alone for unresectable extrahepatic cholangiocarcinoma with BO, the combined treatment significantly improved the overall mean survival time and mean stent patency period (6.8 vs. 3.4 months; p = 0.02; 13.2 ± 0.6 vs. 8.3 ± 0.5 months; p < 0.001) [27]. However, intra-RFA reportedly exhibits several limitations; in particular, it cannot ablate lesions outside the biliary tract or destroy a tumour mass completely [27, 28]. If other treatment measures are not implemented in time, the residual tumour will grow rapidly, eventually leading to stent stenosis.
Most BTCs are hypovascular tumours and visualisation of tumour staining and tumour vascularity is difficult. Therefore, the therapeutic efficacies of transarterial chemoembolisation and transarterial radioembolisation are largely limited in patients with BTCs, particularly patients with extrahepatic cholangiocarcinoma. Continuous low-dose infusion of drugs can achieve a high local drug concentration; moreover, the toxicity of the drugs can be greatly reduced by hepatic first-pass metabolism. In addition, the synergistic effect of oxaliplatin and 5-fluorouracil is more effective for killing tumour cells. Oxaliplatin can effectively inhibit tumour cell growth by inducing ribosome biogenesis stress [29], while continuous arterial infusion of 5-fluorouracil may effectively kill tumour cells that recover from the mitotic block produced by oxaliplatin as they progress into the S phase [30]. Furthermore, hepatic arterial infusion of oxaliplatin can induce high local concentration ratios between peripheral tissues and tumours [31]. Because HAIC is not restricted to a single session, repeated treatments and multiple sessions can be performed to increase tumour destruction and prolong OS. HAIC is an effective treatment for various malignancies, including BTCs [22, 32, 33]. A phase 2 study reported that overall response and disease control rates of 67.6% and 89.2%, respectively, could be achieved with the use of HAIC with oxaliplatin and 5-fluorouracil for pCCA [23]. Therefore, intra-RFA+HAIC can induce potent local anti-tumour effects.
In our study, the median OS in the combination group was 13.2 months, which was slightly longer than that in a phase 3 clinical study (11.7 months) where patients with Ad-BTCs received systemic chemotherapy with cisplatin and gemcitabine [34]. The median OS (13.4 months) in a recent randomised clinical trial of gemcitabine plus S-1 therapy for Ad-BTCs was similar to that in our study [35]. Notably, the proportion of patients with BO was 44.7% and 41% in the phase 3 study and randomised trial, respectively, while it was 100% in the present study. Therefore, the survival benefit of intra-RFA + HAIC may be noteworthy. The results of HAIC therapy alone or combined systemic therapies have been reported in a few studies till date, although the outcomes are inconsistent. The median survival time reported here is similar to that reported by Kasai et al (median 14.6 months) [21] but lower than that reported by Cercek et al (median 25.0 months) [22] and Konstantinidis et al (median 30.8 months) [36]. In this study, the patients did not receive systemic chemotherapy and exhibited concurrent BO, which may have resulted in the different prognosis. In addition, BO was caused by various types of tumours; therefore, an accurate comparison of effects between different studies is difficult. We also compared the results for our combination therapy with those for intra-RFA + stent placement in previous studies by Yang et al [27] and Inoue et al [14] and found that SPT and OS in our combination group were similar to those in the former study, while the median SPT was similar to that in the latter study (7.6 months). However, SPT in our combination group was obviously longer than that in a meta-analysis of intra-RFA + stent placement, where the pooled weighted mean difference in stent patency was only 1.7 months [16]. Therefore, our future study will focus on comparing the efficacy of stent placement + intra-RFA+HAIC with that of stent placement + intra-RFA for the treatment of Ad-BTCs with BO.
The absence of metastatic diseases, a stricture of < 4 cm, and the level of BO in Bismuth types I–II were independent predictors of longer SPT and OS, probably because the ablation extent and heat penetration depth are limited in patients with larger tumour burdens and complicated anatomies. For example, in pCCA cases, intra-RFA may not achieve complete coagulation necrosis in intraluminal masses in the hilar region [37]. This limitation can be overcome by step-by-step ablation in cases with stenoses longer than 2 cm; however, complete ablation remains impossible. The higher the residual tumour burden after intra-RFA, the harder it is to control by compensating HAIC. From the perspective of biological tumour behaviour, the absence of distant metastasis may imply relatively slow growth of tumour cells and relatively less invasion [38]. In summary, these could be the reasons why the three factors were independent predictors of longer SPT and OS in this study. We also found that a history of previous treatments was associated with shorter SPT and OS. There are several reasons for this. First, the biliary structures were reconstructed by surgery, leading to a more complex anatomy and limiting the effects of intra-RFA [39]. Furthermore, chemoradiotherapy can change the biological tumour behaviour, leading to reduced sensitivity to the drugs used in HAIC. Because the therapeutic effect of intra-RFA+HAIC was restricted, SPT and OS were shorter in patients with a history of previous treatments than in those with no previous history of surgery, radiotherapy, or chemotherapy.
The common AEs caused by biliary tract operation procedures were similar to previously reported AEs, including pain, biliary infection, haemorrhage, and pancreatitis. The incidences and grades of AEs showed no significant differences between the two groups. Patients with serious biliary infection were cured with injections of potent antibiotics based on the findings of blood bacterial cultures and drug sensitivity tests. HAIC-related AEs can be divided into two categories, those caused by the arterial catheter and those caused by the toxicity of oxaliplatin and fluorouracil. The incidence of AEs in the former category was extremely low, and timely diagnosis and effective treatment can avoid their aggravation. AEs in the second category were managed by treatment interruption, dose modification, or symptomatic treatments.
To the best of our knowledge, this study is the first to report the efficacy and safety of stent placement + intra-RFA+HAIC and the associated prognostic factors in patients with Ad-BTCs and BO. However, the study has several limitations. First, it was a retrospective study, which may have resulted in unexpected deviation during data extraction and analysis. In particular, treatment heterogeneity occurred because of disease variation, patient preferences, and economic situations. Second, it is difficult to distinguish between intra-RFA and HAIC in terms of their contribution to the longer OS and SPT. Third, because the metal stent interfered with radiological response evaluation, the local tumour control rate for intra-RFA and HAIC was not explored. Finally, SPT was not accurately evaluated for patients who were lost to follow-up or did not exhibit BO recurrence during their lifespan. In the future, well-controlled, prospective studies with larger samples should investigate the effect of these two novel therapeutic options on survival benefit and stent patency.
In conclusion, our findings suggest that stent placement combined with intra-RFA and HAIC is a feasible and safe option for the palliative treatment of Ad-BTCs with BO. Further prospective, multicentre, randomised controlled studies with more samples are necessary to further validate these findings.
Abbreviations
- Ad-BTCs:
-
Advanced biliary tract cancers
- AE:
-
Adverse event
- ALT:
-
Alanine aminotransferase
- BO:
-
Biliary obstruction
- BTCs:
-
Biliary tract cancers
- CI:
-
Confidence interval
- HAIC:
-
Hepatic arterial infusion chemotherapy
- intra-RFA:
-
Intraluminal RFA
- MBO:
-
Malignant biliary obstruction
- OS:
-
Overall survival
- pCCA:
-
Perihilar cholangiocarcinoma
- PTC:
-
Percutaneous transhepatic cholangiography
- PTCD:
-
Percutaneous transhepatic cholangial drainage
- RFA:
-
Radiofrequency ablation
- SAEs:
-
Serious adverse events
- SPT:
-
Stent patency time
References
Razumilava N, Gores GJ (2014) Cholangiocarcinoma. Lancet 383:2168–2179
Banales JM, Cardinale V, Carpino G et al (2016) Expert consensus document: cholangiocarcinoma: current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat Rev Gastroenterol Hepatol 13:261–280
Khan SA, Taylor-Robinson SD, Toledano MB, Beck A, Elliott P, Thomas HC (2002) Changing international trends in mortality rates for liver, biliary and pancreatic tumours. J Hepatol 37:806–813
Taylor-Robinson SD, Toledano MB, Arora S et al (2001) Increase in mortality rates from intrahepatic cholangiocarcinoma in England and Wales 1968–1998. Gut 48:816
F R, Settmacher U (2015) Liver resection for intrahepatic cholangiocarcinoma. Der Chirurg Zeitschrift Für Alle Gebiete Der Operativen Medizen 86:487–492
Valle JW (2010) Advances in the treatment of metastatic or unresectable biliary tract cancer. Ann Oncol 21(Suppl 7):vii345–vii348
Berrad S, Oualla K, Nouikh L et al (2019) Palliative management of malignant biliary obstruction in patients with advanced biliary tract cancers. Ann Oncol 30(Suppl 4):iv103
Pinter M, Hucke F, Zielonke N et al (2014) Incidence and mortality trends for biliary tract cancers in Austria. Liver Int 34:1102–1108
Almadi MA, Barkun A, Martel M (2017) Plastic vs. self-expandable metal stents for palliation in malignant biliary obstruction: a series of meta-analyses. Am J Gastroenterol 112:260–273
Almadi MA, Barkun A, Martel M (2016) Self-expandable metal stents versus plastic stents for malignant biliary obstruction. Gastrointest Endosc 83:852–853
Conio M, Mangiavillano B, Caruso A et al (2018) Covered versus uncovered self-expandable metal stent for palliation of primary malignant extrahepatic biliary strictures: a randomized multicenter study. Gastrointest Endosc 88:283–291.e283
Doyle A, Gorgen A, Muaddi H et al (2019) Outcomes of radiofrequency ablation as first-line therapy for hepatocellular carcinoma less than 3 cm in potentially transplantable patients. J Hepatol 70:866–873
Dutta AK, Basavaraju U, Sales L, Leeds JS (2017) Radiofrequency ablation for management of malignant biliary obstruction: a single-center experience and review of the literature. Expert Rev Gastroenterol Hepatol 11:779–784
Inoue T, Ibusuki M, Kitano R et al (2020) Endobiliary radiofrequency ablation combined with bilateral metal stent placement for malignant hilar biliary obstruction. Endoscopy. https://doi.org/10.1055/a-1133-4448
Cui W, Wang Y, Fan W et al (2017) Comparison of intraluminal radiofrequency ablation and stents vs. stents alone in the management of malignant biliary obstruction. Int J Hyperthermia 33:853–861
Sofi AA, Khan MA, Das A et al (2018) Radiofrequency ablation combined with biliary stent placement versus stent placement alone for malignant biliary strictures: a systematic review and meta-analysis. Gastrointest Endosc 87:944–951 e941
Ganeshan A, Upponi S, Hon LQ, Warakaulle D, Uberoi R (2008) Hepatic arterial infusion of chemotherapy: the role of diagnostic and interventional radiology. Ann Oncol 19:847–851
Mocellin S, Pasquali S, Nitti D (2009) Fluoropyrimidine-HAI (hepatic arterial infusion) versus systemic chemotherapy (SCT) for unresectable liver metastases from colorectal cancer. Cochrane Database Syst Rev. https://doi.org/10.1002/14651858.CD007823.pub2:Cd007823
He M, Li Q, Zou R et al (2019) Sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin vs sorafenib alone for hepatocellular carcinoma with portal vein invasion: a randomized clinical trial. JAMA Oncol 5:953–960
Lyu N, Kong Y, Mu L et al (2018) Hepatic arterial infusion of oxaliplatin plus fluorouracil/leucovorin vs. sorafenib for advanced hepatocellular carcinoma. J Hepatol 69:60–69
Kasai K, Kooka Y, Suzuki Y et al (2014) Efficacy of hepatic arterial infusion chemotherapy using 5-fluorouracil and systemic pegylated interferon α-2b for advanced intrahepatic cholangiocarcinoma. Ann Surg Oncol 21:3638–3645
Cercek A, Boerner T, Tan BR et al (2019) Assessment of hepatic arterial infusion of floxuridine in combination with systemic gemcitabine and oxaliplatin in patients with unresectable intrahepatic cholangiocarcinoma: a phase 2 clinical trial. JAMA Oncol 6:60–67
Wang X, Hu J, Cao G et al (2017) Phase II study of hepatic arterial infusion chemotherapy with oxaliplatin and 5-fluorouracil for advanced perihilar cholangiocarcinoma. Radiology 283:580–589
Liao X, Zhang D (2020) The 8th Edition American Joint Committee on Cancer Staging for Hepato-pancreato-biliary cancer: a review and update. Arch Pathol Lab Med. https://doi.org/10.5858/arpa.2020-0032-RA
Monga A, Gupta R, Ramchandani M, Rao GV, Santosh D, Reddy DN (2011) Endoscopic radiofrequency ablation of cholangiocarcinoma: new palliative treatment modality (with videos). Gastrointest Endosc 74:935–937
Mizandari M, Kumar J, Pai M et al (2018) Interventional radiofrequency ablation: a promising therapeutic modality in the management of malignant biliary and pancreatic duct obstruction. J Cancer 9:629–637
Yang J, Wang J, Zhou H et al (2018) Efficacy and safety of endoscopic radiofrequency ablation for unresectable extrahepatic cholangiocarcinoma: a randomized trial. Endoscopy 50:751–760
Alis H, Sengoz C, Gonenc M, Kalayci MU, Kocatas A (2013) Endobiliary radiofrequency ablation for malignant biliary obstruction. Hepatobiliary Pancreat Dis Int 12:423–427
Bruno PM, Liu Y, Park GY et al (2017) A subset of platinum-containing chemotherapeutic agents kills cells by inducing ribosome biogenesis stress. Nat Med 23:461–471
Qin B, Tanaka R, Shibata Y et al (2006) In-vitro schedule-dependent interaction between oxaliplatin and 5-fluorouracil in human gastric cancer cell lines. Anticancer Drugs 17:445–453
Dzodic R, Gomez-Abuin G, Rougier P et al (2004) Pharmacokinetic advantage of intra-arterial hepatic oxaliplatin administration: comparative results with cisplatin using a rabbit VX2 tumor model. Anticancer Drugs 15:647–650
Furuta M, Watanabe J, Aramaki T (2018) 318PClinical utility of hepatic arterial infusion chemotherapy for heavily pretreated metastatic breast cancer patients: a review of a single institution. Ann Oncol 29
Palmieri LJ, Dermine S, Coriat R (2019) Potential areas of interest in a trial of sorafenib plus hepatic arterial infusion of oxaliplatin, fluorouracil, and leucovorin for hepatocellular carcinoma. JAMA Oncol. https://doi.org/10.1001/jamaoncol.2019.4052
Hall FM (2010) Cisplatin plus gemcitabine for biliary tract cancer. N Engl J Med 363:192 author reply 192-193
Morizane C, Okusaka T, Mizusawa J et al (2019) Combination gemcitabine plus S-1 versus gemcitabine plus cisplatin for advanced/recurrent biliary tract cancer: the FUGA-BT (JCOG1113) randomized phase III clinical trial. Ann Oncol 30:1950–1958
Konstantinidis IT, Groot Koerkamp B, Do RK et al (2016) Unresectable intrahepatic cholangiocarcinoma: Systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer 122:758–765
Cui W, Fan W, Lu M et al (2017) The safety and efficacy of percutaneous intraductal radiofrequency ablation in unresectable malignant biliary obstruction: a single-institution experience. BMC Cancer 17:288
Thews O, Riemann A (2019) Tumor pH and metastasis: a malignant process beyond hypoxia. Cancer Metastasis Rev 38:113–129
Zhou W, Qian L, Rong Y et al (2020) Prognostic factors and patterns of recurrence after curative resection for patients with distal cholangiocarcinoma. Radiother Oncol 147:111–117
Funding
None.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Zejian Zhou.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all patients.
Ethical approval
The study was approved by the Institutional Review Board of Provincial People’s Hospital/ Guangdong Academy of Medical Sciences, China.
Methodology
• Retrospective
• Diagnostic or prognostic study
• Multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gou, Q., Wu, L., Cui, W. et al. Stent placement combined with intraluminal radiofrequency ablation and hepatic arterial infusion chemotherapy for advanced biliary tract cancers with biliary obstruction: a multicentre, retrospective, controlled study. Eur Radiol 31, 5851–5862 (2021). https://doi.org/10.1007/s00330-021-07716-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-07716-0