Abstract
Objectives
Renal blood flow (RBF) has been shown to predict disease progression in autosomal dominant polycystic kidney disease (ADPKD). We investigated the feasibility and accuracy of phase-contrast RBF by MRI (RBFMRI) in ADPKD patients with a wide range of estimated glomerular filtration rate (eGFR) values.
Methods
First, we validated RBFMRI measurement using phantoms simulating renal artery hemodynamics. Thereafter, we investigated in a test-set of 21 patients intra- and inter-observer coefficient of variation of RBFMRI. After validation, we measured RBFMRI in a cohort of 91 patients and compared the variability explained by characteristics indicative for disease severity for RBFMRI and RBF measured by continuous hippuran infusion.
Results
The correlation in flow measurement using phantoms by phase-contrast MRI was high and fluid collection was high (CCC=0.969). Technical problems that precluded RBFMRI measurement occurred predominantly in patients with a lower eGFR (34% vs. 16%). In subjects with higher eGFRs, variability in RBF explained by disease characteristics was similar for RBFMRI compared to RBFHip, whereas in subjects with lower eGFRs, this was significantly less for RBFMRI.
Conclusions
Our study shows that RBF can be measured accurately in ADPKD patients by phase-contrast, but this technique may be less feasible in subjects with a lower eGFR.
Key points
• Renal blood flow (RBF) can be accurately measured by phase-contrast MRI in ADPKD patients.
• RBF measured by phase-contrast is associated with ADPKD disease severity.
• RBF measurement by phase-contrast MRI may be less feasible in patients with an impaired eGFR.
Similar content being viewed by others
Explore related subjects
Find the latest articles, discoveries, and news in related topics.Avoid common mistakes on your manuscript.
Introduction
Most autosomal dominant polycystic kidney disease (ADPKD) subjects show progressive kidney function decline and develop end-stage renal disease (ESRD) between their 4th to 7th decade of life [1]. With renoprotective treatments emerging, such as vasopressin V2-receptor antagonists and somatostatin analogues [2, 3], it will be important to assess disease severity. Especially those patients with a high likelihood of disease progression towards ESRD will need treatment. Assessing disease severity and prognosis in ADPKD is not without difficulty. Glomerular filtration rate (GFR) may not be the best measure in this respect, because GFR remains within normal limits for an extended period of time [4]. Therefore, total kidney volume (TKV) assessed by MRI has been investigated as an alternative [5]. TKV measurement is, however, laborious and the association between TKV and disease severity is limited at an individual patient level. Renal blood flow (RBF) has been proposed as a third marker to assess disease severity [6]. A decrease in RBF flow paralleled TKV growth in the observational CRISP cohort study [6] and is thought to precede decline in GFR [7].
However, the value of RBF has been investigated in a single study, using gadolinium-contrast to obtain a reference image of the renal artery to position the phase-contrast perpendicular to the vessel contour and included only ADPKD subjects with a creatinine clearance > 70 ml/min [6, 7]. Confirmation, especially in subjects with lower kidney function, is therefore needed. Moreover, a method without gadolinium administration would be preferable to avoid the risks of developing gadolinium-associated contrast-induced nephropathy [8] and nephrogenic systemic fibrosis [9, 10]. Besides MRI, RBF can also be measured by infusion of para-aminohippuric acid (RBFHip) [11, 12].
Given these considerations, the aim of the present study was to investigate whether measurement of RBF by MRI without using gadolinium contrast (RBFMRI) could be performed accurately and precisely in a group of ADPKD patients with a wide range in kidney function. First, we validated RBFMRI measurement using phantoms simulating renal artery hemodynamics. Thereafter, we investigated in a test-set of patients the intra- and inter-observer variability. After validation, we compared RBFMRI with RBFHip, and investigated associations of RBFMRI and RBFHip with characteristics indicative for disease severity in ADPKD subgroups.
Methods and Materials
Patient population
For this study, all consecutive patients with ADPKD visiting our out-patient clinic from January 2007 until September 2012 were asked to participate. The diagnosis of APDKD was made based upon the revised Ravine criteria [13]. Subjects were considered ineligible if they received renal replacement therapy, had undergone renal surgery, were unable to undergo MRI, and had other systemic diseases or treatments potentially affecting kidney function. One hundred forty six subjects met these criteria, and were invited for an extensive, one-day screening. This screening included, among others, an MRI and assessment of effective renal plasma flow (ERPF), which were used to measure RBFMRI and RBFHip. Thirteen patients refused to participate and in thirteen patients, RBFMRI was not measured due to logistical reasons, leaving 120 patients for the present analyses. This study was conducted in accordance with the guidelines of the Helsinki Declaration. All participants gave written informed consent.
Measurement and calculations
All participants collected at home a 24-hour urine sample the day preceding the screening at our facility, in which albuminuria was measured. During the visit, blood pressure was measured (Dinamap) and fasting blood samples were drawn for determination of osmolality, copeptin and creatinine. GFR was estimated using the CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) equation [14, 15]. MRI and measurement of renal hemodynamic parameters were performed per protocol on the same day.
Renal blood flow measured by MRI
Imaging was performed on a 1.5-Tesla Magnetom Avento (Siemens, Erlangen, Germany). Two coils were placed on the anterior and posterior abdominal walls directly over the kidneys. Two fast, respiratory-triggered, coronal and transversal T2-weighted image sets were acquired to determine the kidney volumes [16]. To localize the renal arteries, coronal and transversal data sets were scanned during the breath hold using a T2-weighted TrueFISP sequence. RBF was assessed with phase-contrast MRI (PC-MRI), using a two-dimensional fast gradient echo phase-contrast sequence with retrospective cardiac gating (30 cardiac phases) and flow compensation [17]. Acquisition parameters TR/TE/FA were 43.6 ms/4 ms/30 °. A single slice was positioned perpendicular to the renal artery. Slice thickness was 5 mm, FOV was 32 cm, and the number of phase-encoding steps was 260-384, depending on patient size. Acquisition time was 150-160 seconds. The encoding velocity (VENC) was 100 cm/s and flow acquisition was obtained in the slice direction. Supplementary Figure 1 shows a representative sample of data recorded during the cardiac cycle and shows a realistic renal arterial waveform. RBFMRI measurement lasted between 150 and 160 seconds. Flow analysis was performed using ARGUS flow software (Siemens, Erlangen, Germany) for phase-contrast imaging. Images were magnified to 5.0 and renal artery contours were drawn manually on anatomical images to define the region of interest for the corresponding phase-contrast images at all phases of the cardiac cycle. The drawing of these contours required 5-10 minutes per kidney. Flow rates of all renal arteries (left, right and any additional arteries) were added to calculate total RBFMRI. Flow measurements were judged technically unsatisfactory when they met one or more of the following criteria: indistinct vessel contour (blurred as a result of motion), artefacts in the vessel area, ghosting (movement artefacts), or non-physiologic, heterogeneous measurement or weak signal [18]. TKV was measured on T2-weighted coronal images using Analyze Direct 8.0 (Analyze Direct, Inc., Overland Park, KS, USA) and divided by height. Intra- and inter-observer coefficients of variation for TKV measurement were 1.8 and 2.3 %, respectively.
Validation of flow measured by MRI in a phantom
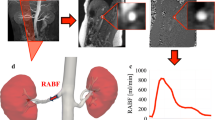
Two flexible silicon phantoms, each representing the aorta and two renal arteries perpendicular to the aorta (Elastrat, Geneva, Switzerland) (Fig. 1) were used to assess accuracy of the PC-MRI measurements. The four renal arteries in these two phantoms had diameters of 5, 6, 7 and 8 mm, representing the normal range of renal artery diameters. The phantoms were suspended in a container filled with water. An MRI-compatible pump (Cardioflow 1000MR, Shelly Medical Imaging Technologies, London, Canada) was positioned proximally; one renal artery was connected to a container, the other renal artery and distal aorta were connected to a reservoir (Fig. 1). A mix of 40-% glycerol and 60-% purified water was used as flow medium to mimic blood viscosity (~3.5 mPa/s) [19]. A synchronized trigger pulse signal was generated by the MRI to simulate electrocardiographic (ECG) gating at a heart rate of 60 beats per minute. The flow in the phantom was determined simultaneously by PC-MRI according to the protocol described above and by fluid collection (volume pumped through the renal artery was collected in a container). These flow collections were considered the gold-standard measure to evaluate the accuracy of the MR-calculated flow. Importantly, the system was positioned in the horizontal plane and the container was placed floating in the reservoir to prevent any steady flow bias due to the principle of communicating vessels (Fig. 1). During our phantom experiment, the pump was set at different flow rates (800, 1000, 1200 and 1400 ml/min) and acted as “cardiac output”. In this phantom, “cardiac output” is divided over two renal arteries and the aorta. Thus, the blood flow rates in the phantom renal arteries were dependent on the pump rates (“cardiac output”) and the diameters of the phantom renal arteries and aorta. Flow was measured in one renal artery at a time, but several times to assess reproducibility.
Variability of RBFMRI measurement
RBFMRI measurements were performed by two research physicians (ES and EM) whom were trained to perform RBFMRI measurements. During their training period, they measured 60 RBFMRI values under supervision of an experienced MRI technician (PK). Subsequently, they measured RBFMRI on 2 occasions, blinded to previous results, in 21 patients (i.e., 42 kidneys) to determine intra- and inter-observer variability.
RBF measured by 131I-hippuran
Measurement of renal hemodynamic parameters was performed using a constant infusion method with 131I-hippuran to measure ERPF [11, 20]. During these assessments, patients were not water-loaded, but allowed to drink ad libitum. At 08.00 am, a priming solution of 0.04 mL/kg body weight was administered, followed by a constant infusion of the tracer at a rate depending on eGFR for 5.5 hours. After a stabilisation period of 1.5 hours, two 2-hour clearance periods followed, in which ERPF was measured as (I*V)/P, where I*V represents the infusion of the tracer and P represents the tracer concentration in plasma. Intra-subject day-to-day variability using this method is 5 % [11]. RBFHip was calculated using the formula ERPF/(1-hematocrit).
Statistical analysis
Lin’s concordance correlation coefficient (CCC) and Bland-Altman analyses were used to assess agreement between the gold standard fluid collection and the flow measured by MRI in the phantom. Reproducibility was assessed by CCC, Bland-Altman analyses and intra- and inter-observer coefficient of variation (CV).
Subsequently, RBFMRI and RBFHip were measured in an ADPKD patient cohort of which baseline characteristics were calculated for the overall population and for participants with an eGFR >70 and ≤70 ml/min*1.73m2, separately. Normality was assessed by a Q-Q-plot. Normal distributed variables were expressed as the mean ± standard deviation (SD), whereas non-normal distributed variables were expressed as the median (interquartile range). Difference in baseline characteristics between two eGFR subgroups was tested using a Chi-square test for categorical data, and a Student’s t-test when normally distributed or a Mann-Whitney test when not normally distributed.
Disease characteristics were correlated to RBFHip and to RBFMRI in the overall cohort and in subcohorts stratified for eGFR >70 and ≤70 ml/min*1.73m2. To investigate the best fit for RBFHip and RBFMRI, we performed a stepwise backwards regression analysis. Clinically important disease characteristics were entered and deleted stepwise from the model in order of weakest association until a model with the best fit (i.e., adjusted R2) was obtained. Model discrimination was determined by bootstrapping (2000 times) to calculate the difference in adjusted R2 of two multivariate linear regression models [21].
All analyses were performed with SPSS version 20.0 (SPSS Inc., Chicago, IL, USA). A two-sided p-value less than 0.05 was considered to indicate statistical significance.
Results
Validation of flow measured by MRI
Figure 2 shows a scatter-plot comparing pulsatile flow measured by fluid collection and flow measured by PC-MRI, combined for all flow rates and vessel diameters. The 5-mm diameter renal artery images were judged technically unsatisfactory, because of artefacts in the vessel area and measurement heterogeneity. An excellent agreement between flow measured by the gold standard of fluid collection and by PC-MRI was observed. The CCC for 6 to 8 mm was 0.969 (p < 0.001), indicating high agreement. Bland-Altman analysis shows that flow measured by MRI on average underestimated flow measured by fluid collection by 2.9 % (Fig. 2). A significant correlation was noted between the mean (flow measured by fluid collection and by MRI) and the absolute difference between these two measures (r = -0.47, p = 0.004).
Results of the phantom experiment. The upper panel shows a scatter plot with flow assessed by fluid collection vs. measured by MRI. The dotted line shows the line of identity. The lower panel shows a corresponding Bland-Altman plot. The CCC was 0.977, 0.989 and 0.933 for the 6, 7 and 8 mm phantoms, respectively (p < 0.001 for all)
Reproducibility of RBFMRI measurement in a test-set of ADPKD patients
Figure 3 shows the agreement between blood flow measured by observers ES and EM in 21 ADPKD patients. The average intra-observer CV was 2.3 % (ES 2.0 % and EM 2.6 %). The estimated reliability, i.e., intra-class correlation coefficient, for observers ES and EM were 0.997 and 0.995, respectively (both p < 0.001). The inter-observer CV was 3.5 % with a reliability coefficient of 0.991 (p < 0.001). In patients with an eGFR >70 ml/min*1.73m2, the intra- and inter-observer CVs were 1.5 and 3.5 %, respectively, whereas in patients with an eGFR ≤70 ml/min*1.73m2, these values were 3.2 and 3.5 %, respectively.
Intra-observer and inter-observer agreement of renal blood flow (RBF) measurement (ml/min). A The intra-class correlation coefficient for observer A was 0.997 and 0.995 for observer B (p < 0.001 for both; n = 21 ADPKD patients); the intra-observer coefficient of variation was 2.0 % and 2.6 %, respectively. B The inter-observer coefficient of variation was 3.5 % with a reliability coefficient of 0.991 (p < 0.001)
RBFMRI measurement in an ADPKD patient cohort
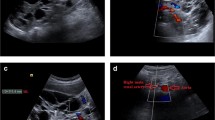
Out of the 120 patients, 29 (24 %) had RBF measurements that were judged technically unsatisfactory, and these patients were excluded for further analysis, leaving 91 ADPKD patients for analyses (in patients with an eGFR ≤70 and >70 ml/min*1.73m2, this held for 18 (34 %) and 11 (16 %) patients, respectively). The excluded patients had a significantly lower eGFR and ERPF compared to the patients that could be studied [eGFR 79.2 vs. 58.1 ml/min*1.73m2 (p = 0.001)], and ERPF 263.5 vs. 201.7 ml/min*1.73m2 (p = 0.004). No significant difference in TKV was observed between the groups (969 vs. 1526 mL/m, p = 0.28). The included patients were relatively young with a mean age of 39.8 ± 11.4 years and their eGFR ranged from 15 to 136 ml/min*1.73m2. Nine patients (9.9 %) had multiple renal arteries (one bilateral, seven unilateral and one unilateral with two additional renal arteries). RBFMRI ranged from 244 to 1547 ml/min*1.73m2. Right artery RBFMRI was significantly higher than left artery RBFMRI (456 vs. 358 ml/min*1.73m2, p < 0.001). The smallest renal artery diameter was 3.9 and the largest was 11.4 mm. The additional renal arteries had smaller diameters, with a mean of 6 mm compared to 7 mm of ‘’single” renal arteries. As expected, RBF was significantly lower in patients with lower eGFR whereas age, TKV and use of antihypertensives were significantly higher in this subgroup (Table 1). Figure 4 shows examples of PC-MRI flows that were judged technically satisfactory and unsatisfactory.
Phase-contrast magnetic resonance angiogram without gadolinium of the renal arteries in ADPKD. a The phase-contrast angiogram was performed perpendicular to the renal artery on the coronal image b Cross-sectional image through the renal artery that was judged technically satisfactory and c unsatisfactory to measure renal blood flow, because of indistinct vessel jcontour (blurred as a result of motion)
Associations of RBFMRI and RBFHip with disease characteristics.
RBFMRI correlated significantly with RBFHip (r = 0.81, p < 0.001), although RBFMRI was higher than RBFHip over the entire range of RBFMRI. In univariate regression analyses, both RBFMRI as well as RBFHip were positively correlated with eGFR and urinary sodium excretion, and negatively with age and TKV (Table 2). A stepwise backward multiple regression model showed that RBFHip and RBFMRI were significantly and especially associated with eGFR (both <0.001). In the whole group, the variability explained by the model for RBFMRI was significantly lower than for RBFHip (adjusted R2 = 0.61 and 0.77, respectively; p < 0.001). In patients with an eGFR >70 ml/min*1.73m2, the adjusted R2 was not significantly different for RBFMRI than RBFHip (0.36 and 0.51, respectively; p = 0.19), whereas in patients with an eGFR ≤70 ml/min*1.73m2, disease characteristics explained a smaller fraction of variability in RBFMRI compared to RBFHip (0.17 and 0.72, respectively; p < 0.001; Table 3).
In addition, we performed a sensitivity analysis in which we excluded patients with additional renal arteries in order to investigate whether multiple renal arteries caused methodological problems. Multiple renal arteries were found only in patients with eGFR >70 ml/min*1.73m2. We found similar variability in measurement of RBFMRI and MRIHip as in the whole group (adjusted R2 = 0.63 and 0.77, respectively), and the variability explained by the model increased slightly for RBFMRI (adjusted R2 = 0.42) and remained similar for RBFHip (adjusted R2 = 0.53).
Discussion
In this study, we investigated whether measurement of RBF by MRI without using gadolinium contrast (RBFMRI) could be performed accurately and precisely. First, we validated RBFMRI measurement using phantoms simulating renal artery hemodynamics. Thereafter, we investigated in a test-set of patients the reproducibility of RBFMRI. After validation, we measured RBFMRI in a cohort of ADPKD patients and compared RBFMRI and RBF measured by hippuran infusion with characteristics indicative for disease severity.
In our phantom experiments, mimicking the clinical renal hemodynamic profile, RBFMRI showed perfect agreement to the gold-standard for flow measurement, i.e., fluid collection. We observed no differences in accuracy in RBFMRI between the “renal arteries” varying in diameter or between flow velocities, although it should be noted that measurement of RBFMRI was not feasible in the “artery” with the smallest diameter (5 mm). Our findings are in line with the values obtained by Dambreville et al [19], who showed that steady and pulsatile flow can be measured reliably in polyvinyl alcohol tubes with different diameters. These authors also experienced difficulties in measuring MRI flow in tubes with small diameters. In our test set of 21 patients, reproducibility was high and comparable with values obtained in the literature with contrast-measured RBF [6, 19]. These data indicate that RBFMRI can be measured reliably in vitro and in vivo. In patients without any additional renal arteries, we found similar variability in measurement of RBFMRI and MRIHip compared to all patients. These data indicate that the presence of additional renal arteries also does not influence the reliability of RBF measurement.
We used MRI to measure RBFMRI in a cohort of ADPKD patients. Our technical success rate was 76 %, which is comparable with previous literature on this method [18]. We compared RBFMRI with RBFHip in these patients. It should be noted that RBFHip is another indirect measurement of RBF. RBFHip was lower compared to RBFMRI in patients with relatively preserved as well as impaired kidney function. It is known that RBFHip underestimates RBF, because the extraction of hippuran by tubular cells is not 100 % and dependent on kidney function [12]. In two previous studies, it was shown that RBFMRI correlated well with RBF determined by clearance of para-amino-hippurate (r = 0.94 and r = 0.93, respectively) [22, 23]. These studies used healthy subjects. In the present study we applied hippuran instead of para-amino-hippurate [12]. We showed that RBFMRI correlated well with RBFHip (r = 0.90) and that RBFMRI was associated with anatomical, hemodynamic and laboratory parameters indicating disease severity. This corroborates the findings by the CRISP consortium that found the same associations and additionally described that RBFMRI predicted kidney function decline [6]. Measurement of RBF can, therefore, be important to assess disease severity, to predict prognosis and potentially also act as a surrogate endpoint to assess the efficacy of renoprotective interventions early in the disease. RBF could also be used in combination with GFR to assess the filtration fraction [24].
Although on average we showed that RBF could be measured reliably by MRI without using contrast, there are a number of issues rendering such measurement less feasible in ADPKD patients with impaired kidney function. First, RBFMRI measurement in the smallest phantom artery (5 mm), which is a surrogate for more severe ADPKD, was not possible. Second, in the phantom experiment, we found a significant correlation between mean flow and difference in flow measured by MRI and fluid collection, with a larger difference in the lower flow range. This indicates that systematic bias increases at the lower flow range. Third, variability was higher in patients with a low eGFR than in patients with a high eGFR. Fourth, RBFMRI measurement in patients with lower eGFRs resulted less often in interpretable images. MRI images were judged technically unsatisfactory in 34 % of patients with an eGFR ≤70 vs. in 16 % of patients with an eGFR >70 ml/min*1.73m2. Fifth, in subjects with a lower eGFR, only a small fraction of variability in RBFMRI was explained by disease characteristics. We speculate that due to the lower RBF in smaller renal arteries the phase-contrast measurement may give a (too) weak signal, causing the lower success rate. Two methods could be used to overcome this problem. The spatial resolution could be increased or the VENC could be adjusted. However, for this study, we used the protocol of the CRISP consortium [6, 7, 19] with pre-determined variables (FOV 32 cm, slide thickness 5 mm, VENC of 100cm/s) as to maintain the protocol and to compare results without bias. In addition, renal arteries with larger diameters allow better phase-contrast positioning and easier measurement of the flow. Notwithstanding these observations, we think that this does not necessarily disqualify the use of RBFMRI measurement for ADPKD staging and risk classification. The clinical need for risk classification in this patient group is especially present early in the disease, when GFR remains within the normal limits for an extended period of time [1]. At a later stage of the disease, when kidney function starts to decline, eGFR in association with age can be used as a parameter to assess disease severity and prognosis. It should be stated that in patients with an eGFR >70 ml/min*1.73 m2, gadolinium-enhanced techniques can also be used to measure RBF, because in this patient subgroup there is a low risk of toxicity. Lastly, there are other unenhanced techniques to measure flow, such as arterial spin labelling [25], but this technique is especially useful to measure regional variations in renal perfusion, and investigating this technique was beyond the scope of the present study. Of note, a pilot study showed no difference for RBF values between arterial spin labelling and RBFMRI measurement [26].
Limitations of our study are, first, that we studied renal hemodynamical parameters in a cross-sectional setting. Therefore, we were not able to investigate associations with disease progression. Second, this study assessed reliability and accuracy of RBFMRI using a phantom simulating the clinical situation. However, it lacks a gold standard method for RBF measurement in vivo. Such a method would necessitate a flow probe directly introduced in the renal arteries, which, because of its invasive character, is not feasible. We, therefore, assessed reliability of RBFMRI in an indirect way, by studying the degree of variability of RBFMRI that was explained by accepted characteristics of ADPKD severity, and by comparing this with the degree of variability of RBFHip explained by the same disease characteristics. Strengths of our study were that we measured RBFMRI and ERPF by gold-standard techniques on the same day. These measurements were performed in a relatively large, well-phenotyped cohort of ADPKD patients with relatively preserved as well as impaired kidney function. Furthermore, our method avoids use of gadolinium, which is important because of the potential side effects of this contrast agent in patients with kidney dysfunction [27].
In conclusion, our study shows that in ADPKD patients, RBF can be measured by MRI accurately and reliably without using gadolinium contrast, but that assessment of RBF by PC-MRI may be less feasible in patients with impaired kidney function.
References
Grantham JJ (2008) Clinical practice. autosomal dominant polycystic kidney disease. N Engl J Med 359:1477–85
Torres VE, Chapman AB, Devuyst O et al (2012) Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367:2407–18
Caroli A, Perico N, Perna A et al (2013) Effect of long-acting somatostatin analogue on kidney and cyst growth in autosomal dominant polycystic kidney disease (ALADIN): A randomised, placebo-controlled, multicentre trial. Lancet 382:1485–95
Grantham JJ, Mulamalla S, Swenson-Fields KI (2011) Why kidneys fail in autosomal dominant polycystic kidney disease. Nat Rev Nephrol 7:556–66
Grantham JJ, Torres VE, Chapman AB et al (2006) Volume progression in polycystic kidney disease. N Engl J Med 354:2122–30
King BF, Torres VE, Brummer ME et al (2003) Magnetic resonance measurements of renal blood flow as a marker of disease severity in autosomal-dominant polycystic kidney disease. Kidney Int 64:2214–21
Torres VE, King BF, Chapman AB et al (2007) Magnetic resonance measurements of renal blood flow and disease progression in autosomal dominant polycystic kidney disease. Clin J Am Soc Nephrol 2:112–20
Ergun I, Keven K, Uruc I et al (2006) The safety of gadolinium in patients with stage 3 and 4 renal failure. Nephrol Dial Transplant 21:697–700
Bernstein EJ, Schmidt-Lauber C, Kay J (2012) Nephrogenic systemic fibrosis: A systemic fibrosing disease resulting from gadolinium exposure. Best Pract Res Clin Rheumatol 26:489–503
Penfield JG, Reilly RF Jr (2007) What nephrologists need to know about gadolinium. Nat Clin Pract Nephrol 3:654–68
Donker AJ, van der Hem GK, Sluiter WJ, Beekhuis H (1977) A radioisotope method for simultaneous determination of the glomerular filtration rate and the effective renal plasma flow. Neth J Med 20:97–103
Battilana C, Zhang HP, Olshen RA, Wexler L, Myers BD (1991) PAH extraction and estimation of plasma flow in diseased human kidneys. Am J Physiol 261:F726–33
Pei Y, Obaji J, Dupuis A et al (2009) Unified criteria for ultrasonographic diagnosis of ADPKD. J Am Soc Nephrol 20:205–12
Levey AS, Stevens LA, Schmid CH et al (2009) A new equation to estimate glomerular filtration rate. Ann Intern Med 150:604–12
Levey AS, Greene T, Kusek J, Beck GJ A simplified equation to predict glomerular filtration rate for serum creatinine [abstract]. 2000:11:115A.
Bae KT, Commean PK, Lee J (2000) Volumetric measurement of renal cysts and parenchyma using MRI: Phantoms and patients with polycystic kidney disease. J Comput Assist Tomogr 24:614–9
de Haan MW, Kouwenhoven M, Kessels AG, van Engelshoven JM (2000) Renal artery blood flow: Quantification with breath-hold or respiratory triggered phase-contrast MR imaging. Eur Radiol 10:1133–7
Bax L, Bakker CJ, Klein WM, Blanken N, Beutler JJ, Mali WP (2005) Renal blood flow measurements with use of phase-contrast magnetic resonance imaging: Normal values and reproducibility. J Vasc Interv Radiol 16:807–14
Dambreville S, Chapman AB, Torres VE et al (2010) Renal arterial blood flow measurement by breath-held MRI: Accuracy in phantom scans and reproducibility in healthy subjects. Magn Reson Med 63:940–50
Apperloo AJ, de Zeeuw D, Donker AJ, de Jong PE (1996) Precision of glomerular filtration rate determinations for long-term slope calculations is improved by simultaneous infusion of 125I-iothalamate and 131I-hippuran. J Am Soc Nephrol 7:567–72
Cameron AC, Windmeijer FAG (1996) R-squared measures for count data regression models with applications to health-care utilization. J Bus Econ Stat 14:209–20
Sommer G, Corrigan G, Fredrickson J et al (1998) Renal blood flow: Measurement in vivo with rapid spiral MR imaging. Radiology 208:729–34
Wolf RL, King BF, Torres VE, Wilson DM, Ehman RL (1993) Measurement of normal renal artery blood flow: Cine phase-contrast MR imaging vs clearance of p-aminohippurate. AJR Am J Roentgenol 161:995–1002
Cutajar M, Hilton R, Olsburgh J et al (2015) Renal blood flow using arterial spin labelling MRI and calculated filtration fraction in healthy adult kidney donors pre-nephrectomy and post-nephrectomy. Eur Radiol. doi:10.1007/s00330-015-3594-6
Ferre JC, Bannier E, Raoult H, Mineur G, Carsin-Nicol B, Gauvrit JY (2013) Arterial spin labeling (ASL) perfusion: Techniques and clinical use. Diagn Interv Imaging 94:1211–23
Cutajar M, Thomas DL, Hales PW, Banks T, Clark CA, Gordon I (2014) Comparison of ASL and DCE MRI for the non-invasive measurement of renal blood flow: Quantification and reproducibility. Eur Radiol 24:1300–8
Anonymous (12/23/2010) FDA drug safety communication: New warnings for using gadolinium-based contrast agents in patients with kidney dysfunction. :http://www.fda.gov/Drugs/DrugSafety/ucm223966.htm
Acknowledgements
The scientific guarantor of this publication is Ron T. Gansevoort. The authors of this manuscript declare relationships with the following companies: Otzuka. The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article. The authors state that this work has not received any funding. One of the authors has significant statistical expertise. Institutional review board approval was obtained. Written informed consent was obtained from all subjects (patients) in this study.
Some study subjects or cohorts have been previously reported in
• Clin J Am Soc Nephrol. 2010 Jun;5(6):1091-8. doi: 10.2215/CJN.00360110
• Am J Kidney Dis. 2013 Sep;62(3):531-40. doi: 10.1053/j.ajkd.2013.03.030.
Methodology: retrospective, cross sectional study, performed at one institution.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(DOCX 59 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Spithoven, E.M., Meijer, E., Borns, C. et al. Feasibility of measuring renal blood flow by phase-contrast magnetic resonance imaging in patients with autosomal dominant polycystic kidney disease. Eur Radiol 26, 683–692 (2016). https://doi.org/10.1007/s00330-015-3877-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-015-3877-y