Abstract
Design
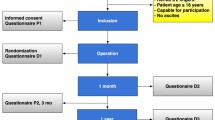
This trial is a randomized controlled, patient-blinded, multicentre, superiority trial.
Methods
All patients ≥18 years with a single, symptomatic and primary umbilical or epigastric hernia (<2 fingers) qualified for participation in the study. Flat polypropylene mesh repair was compared to patch repair (PROCEED® Ventral Patch) (PVP). The objective of this trial was to identify a superior method for umbilical and epigastric hernia repair in terms of complication rates.
Results
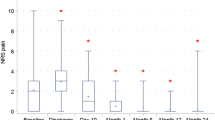
A total of 352 patients were randomized in this trial; 348 patients received the intervention (n = 177 PVP vs. n = 171 mesh). No peri-operative complications occurred. PVP placement was significantly faster compared to mesh placement (30 min, SD 11 vs. 35 min, SD 11) and was scored as an easier procedure. At 1-month follow-up, 76 patients suffered any kind of complication. There was no significant difference in the proportion of complications (24.9% for PVP and 18.7% for mesh, p = 0.195). A significant difference was seen in re-operation rate within 1 month, significantly less early re-operations in the mesh group (0.0 vs. 2.8%, p = 0.027). After 1-year follow-up, no significant differences are seen in recurrence rates (n = 13, 7.8% PVP vs. n = 5, 3.3% mesh, p = 0.08).
Conclusions
Both mesh and PVP had a comparable amount of reported complications. There was a significantly higher incidence of early re-operations due to early complications in the PVP group. No differences were seen in infection rates and the need for antibiotic treatment. No significant difference was seen in the recurrence rates.
Registration
This trial was registered in the Dutch Trail Registry (NTR) NTR2514NL33995.060.10. [12].

Similar content being viewed by others
References
Christoffersen MM (2015) Clinical outcomes after elective repair for small umbilical and epigastric hernias. Dan Med J 62(11):B5161
Christoffersen MW et al (2013) Lower reoperation rate for recurrence after mesh versus sutured elective repair in small umbilical and epigastric hernias. A nationwide register study. World J Surg 37(11):2548–2552. doi:10.1007/s00268-013-2160-0
Christoffersen MW et al (2015) Long-term recurrence and chronic pain after repair for small umbilical or epigastric hernias: a regional cohort study. Am J Surg 209(4):725–732
Westen M et al (2014) Chronic complaints after simple sutured repair for umbilical or epigastric hernias may be related to recurrence. Langenbecks Arch Surg 399(1):65–69
Ponten JE et al (2015) A consecutive series of 235 epigastric hernias. Hernia 19(5):821–825
Arroyo A et al (2001) Randomized clinical trial comparing suture and mesh repair of umbilical hernia in adults. Br J Surg 88(10):1321–1323
NZa. Open data van de Nederlandse Zorgautoriteit. http://www.opendisdata.nl/msz/zorgproduct/110401049
Nienhuijs S et al (2007) Chronic pain after mesh repair of inguinal hernia: a systematic review. Am J Surg 194(3):394–400
Colavita PD et al (2014) Umbilical hernia repair with mesh: identifying effectors of ideal outcomes. Am J Surg 208(3):342–349
Cox TC et al (2016) The cost of preventable comorbidities on wound complications in open ventral hernia repair. J Surg Res 206(1):214–222
Ponten JE et al (2014) Mesh Or Patch for Hernia on Epigastric and Umbilical Sites (MORPHEUS trial): study protocol for a multi-centre patient blinded randomized controlled trial. BMC Surg 14:33
NTR. Nederlands Trial Register. 2010. http://www.trialregister.nl/trialreg/admin/rctview.asp?TC=2514
Moher D et al (2010) CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 340:c869
Schulz KF et al (2011) CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. Int J Surg 9(8):672–677
Simons MP et al (2009) European Hernia Society guidelines on the treatment of inguinal hernia in adult patients. Hernia 13(4):343–403
Abdel-Baki NA, Bessa SS, Abdel-Razek AH (2007) Comparison of prosthetic mesh repair and tissue repair in the emergency management of incarcerated para-umbilical hernia: a prospective randomized study. Hernia 11(2):163–167
Helgstrand F et al (2013) Outcomes after emergency versus elective ventral hernia repair: a prospective nationwide study. World J Surg 37(10):2273–2279. doi:10.1007/s00268-013-2123-5
Chow S, Shao J, Wang H (2008) Sample size calculations in clinical research, 2nd edn. CRC Press, Boca Raton
Clavien PA et al (2009) The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250(2):187–196
Dindo D, Demartines N, Clavien PA (2004) Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240(2):205–213
Horan TC, Andrus M, Dudeck MA (2008) CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am J Infect Control 36(5):309–332
Nguyen MT et al (2014) Comparison of outcomes of synthetic mesh vs suture repair of elective primary ventral herniorrhaphy: a systematic review and meta-analysis. JAMA Surg 149(5):415–421
Winsnes A et al (2016) Surgical outcome of mesh and suture repair in primary umbilical hernia: postoperative complications and recurrence. Hernia 20(4):509–516
Berger RL et al (2013) Development and validation of a risk-stratification score for surgical site occurrence and surgical site infection after open ventral hernia repair. J Am Coll Surg 217(6):974–982
Abramov D et al (1996) Antibiotic prophylaxis in umbilical and incisional hernia repair: a prospective randomised study. Eur J Surg 162(12):945–948 discussion 949
Aslani N, Brown CJ (2010) Does mesh offer an advantage over tissue in the open repair of umbilical hernias? A systematic review and meta-analysis. Hernia 14(5):455–462
Muysoms FE et al (2016) A prospective, multicenter, observational study on quality of life after laparoscopic inguinal hernia repair with ProGrip laparoscopic, self-fixating mesh according to the European Registry for Abdominal Wall Hernias Quality of Life Instrument. Surgery 160(5):1344–1357
Acknowledgements
The authors would like to thank T.A. Mulders, MD and Ph.D., for his help and statistical knowledge, but above all, his friendship. Special thanks to Jax Café, New York City, USA, where the first lines of this manuscript were written, and Hotel Armentarola, Alto Adige, Italy, where this manuscript was finished. Both places are ideal writing environments.
Funding
The MORPHEUS trial started as a non-funded trial, because of slow inclusion rate in participating centres due to high device costs; an investigator initiated grant proposal was deposited. An Investigator Sponsored-Studies (ISS) proposal, which is investigator, initiated applied for and granted by the Johnson & Johnson Company. This funding was used to create levelling in costs for participating hospitals between the two mesh devices used. The Johnson & Johnson Company was not involved in the analysis of the data and the drafting of the manuscript.
Protocol
The protocol was approved and published in an open access, peer reviewed journal [11].
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ponten, J.E.H., Leenders, B.J.M., Leclercq, W.K.G. et al. Mesh Versus Patch Repair for Epigastric and Umbilical Hernia (MORPHEUS Trial); One-Year Results of a Randomized Controlled Trial. World J Surg 42, 1312–1320 (2018). https://doi.org/10.1007/s00268-017-4297-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-017-4297-8