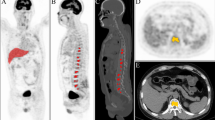
Abstract
Purpose
Fluorodeoxyglucose-positron emission tomography/computed tomography (FDG-PET/CT) is included in the International Myeloma Working Group (IMWG) imaging guidelines for the work-up at diagnosis and the follow-up of multiple myeloma (MM) notably because it is a reliable tool as a predictor of prognosis. Nevertheless, none of the published studies focusing on the prognostic value of PET-derived features at baseline consider tumor heterogeneity, which could be of high importance in MM. The aim of this study was to evaluate the prognostic value of baseline PET-derived features in transplant-eligible newly diagnosed (TEND) MM patients enrolled in two prospective independent European randomized phase III trials using an innovative statistical random survival forest (RSF) approach.
Methods
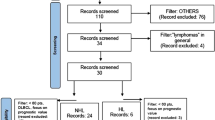
Imaging ancillary studies of IFM/DFCI2009 and EMN02/HO95 trials formed part of the present analysis (IMAJEM and EMN02/HO95, respectively). Among all patients initially enrolled in these studies, those with a positive baseline FDG-PET/CT imaging and focal bone lesions (FLs) and/or extramedullary disease (EMD) were included in the present analysis. A total of 17 image features (visual and quantitative, reflecting whole imaging characteristics) and 5 clinical/histopathological parameters were collected. The statistical analysis was conducted using two RSF approaches (train/validation + test and additional nested cross-validation) to predict progression-free survival (PFS).
Results
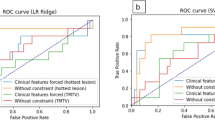
One hundred thirty-nine patients were considered for this study. The final model based on the first RSF (train/validation + test) approach selected 3 features (treatment arm, hemoglobin, and SUVmaxBone Marrow (BM)) among the 22 involved initially, and two risk groups of patients (good and poor prognosis) could be defined with a mean hazard ratio of 4.3 ± 1.5 and a mean log-rank p value of 0.01 ± 0.01. The additional RSF (nested cross-validation) analysis highlighted the robustness of the proposed model across different splits of the dataset. Indeed, the first features selected using the train/validation + test approach remained the first ones over the folds with the nested approach.
Conclusion
We proposed a new prognosis model for TEND MM patients at diagnosis based on two RSF approaches.
Trial registration
IMAJEM: NCT01309334 and EMN02/HO95: NCT01134484





Similar content being viewed by others
Notes
The scalar output of an RSF, known as “ensemble mortality,” provides an estimator of “the number of deaths expected under the null hypothesis of similar survival behavior” [13]. In this paper, the RSF mortality does not refer to deaths but to progression events.
References
Cavo M, Terpos E, Nanni C, Moreau P, Lentzsch S, Zweegman S, et al. Role of 18F-FDG positron emission tomography/computed tomography in the diagnosis and management of multiple myeloma and other plasma cell dyscrasias: a consensus statement by the International Myeloma Working Group. Lancet Oncol. 2017;18:e206–17.
Moreau P, Attal M, Caillot D, Macro M, Karlin L, Garderet L, et al. Prospective evaluation of magnetic resonance imaging and [(18)F]fluorodeoxyglucose positron emission tomography-computed tomography at diagnosis and before maintenance therapy in symptomatic patients with multiple myeloma included in the IFM/DFCI 2009 trial: results of the IMAJEM Study. J Clin Oncol. 2017;35:2911–8.
Bartel TB, Haessler J, Brown TL, Shaughnessy JD Jr, van Rhee F, Anaissie E, et al. F18-fluorodeoxyglucose positron emission tomography in the context of other imaging techniques and prognostic factors in multiple myeloma. Blood. 2009;114:2068–76.
Zamagni E, Patriarca F, Nanni C, Zannetti B, Englaro E, Pezzi A, et al. Prognostic relevance of 18-F FDG PET-CT in newly diagnosed multiple myeloma patients treated with up-front autologous transplantation. Blood. 2011;118:5989–95.
Usmani SZ, Mitchell A, Waheed S, Crowley J, Hoering A, Petty N, et al. Prognostic implications of serial 18-fluoro-deoxyglucose emission tomography in multiple myeloma treated with total therapy 3. Blood. 2013;121:1819–23.
McDonald JE, Kessler MM, Gardner MW, Buros AF, Ntambi JA, Waheed S, et al. Assessment of total lesion glycolysis by (18) F FDG PET/CT significantly improves prognostic value of GEP and ISS in myeloma. Clin Cancer Res. 2017;23:1981–7.
Fonti R, Larobina M, Del Vecchio S, De Luca S, Fabbricini R, Catalano L, et al. Metabolic tumor volume assessed by 18F-FDG PET/CT for the prediction of outcome in patients with multiple myeloma. J Nucl Med. 2012;53:1829–35.
Terao T, Machida Y, Tsushima T, Miura D, Narita K, Kitadate A, et al. Pre-treatment metabolic tumour volume and total lesion glycolysis are superior to conventional positron-emission tomography/computed tomography variables for outcome prediction in patients with newly diagnosed multiple myeloma in clinical practice. Br J Haematol. 2020. https://doi.org/10.1111/bjh.16633
Fonti R, Pellegrino S, Catalano L, Pane F, Del Vecchio S, Pace L. Visual and volumetric parameters by 18F-FDG-PET/CT: a head to head comparison for the prediction of outcome in patients with multiple myeloma. Ann Hematol. 2020;99:127–35.
Rasche L, Chavan SS, Stephens OW, Patel PH, Tytarenko R, Ashby C, et al. Spatial genomic heterogeneity in multiple myeloma revealed by multi-region sequencing. Nat Commun. 2017;8:268.
Yip SSF, Aerts HJWL. Applications and limitations of radiomics. Phys Med Biol. 2016;61:R150–66.
Hatt M, Tixier F, Pierce L, Kinahan PE, Cheze Le Rest C, Visvikis D. Characterization of PET/CT images using texture analysis: the past, the present…any future? Eur J Nucl Med Mol Imaging. 2017;44:151–65.
Ishwaran H, Kogalur U, Blackstone E, Lauer M. Random survival forest. Ann Appl Stat. 2008;2:841–60.
Morvan L, Carlier T, Jamet B, Bailly C, Bodet-Milin C, Moreau P, et al. Leveraging RSF and PET images for prognosis of multiple myeloma at diagnosis. Int J Comput Assist Radiol Surg. 2020;15:129–39.
Schaefer A, Vermandel M, Baillet C, Dewalle-Vignion AS, Modzelewski R, Vera P, et al. Impact of consensus contours from multiple PET segmentation methods on the accuracy of functional volume delineation. Eur J Nucl Med Mol Imaging. 2016;43:911–24.
Bailly C, Bodet-Milin C, Couespel S, Necib H, Kraeber-Bodéré F, Ansquer C, et al. Revisiting the robustness of PET-based textural features in the context of multi-centric trials. PLoS ONE. 2016;11:e0159984.
Desseroit MC, Tixier F, Weber WA, Siegel BA, Cheze Le Rest C, Visvikis D, et al. Reliability of PET/CT shape and heterogeneity features in functional and morphologic components of non–small cell lung cancer tumors: a repeatability analysis in a prospective multicenter cohort. J Nucl Med. 2016;58:406–11.
Van Velden FHP, Kramer GM, Frings V, Nissen IA, Mulder ER, de Langen AJ, et al. Repeatability of radiomic features in non-small-cell lung cancer [18F] FDG-PET/CT studies: impact of reconstruction and delineation. Mol Imaging Biol. 2016;18:788–95.
Zwanenburg A, Vallières M, Abdalah MA, Aerts HJWL, Andrearczyk V, Apte A, et al. The image biomarker standardization initiative: standardized quantitative radiomics for high-throughput image-based phenotyping. Radiology. 2020;295:328–38.
Van Griethuysen JJM, Fedorov A, Parmar C, Hosny A, Aucoin N, Narayan V, et al. Computational radiomics system to decode the radiographic phenotype. Cancer Res. 2017;77:e104–e7.
Hao H, Zhou Z, Li S, Maquilan G, Folkert MR, Iyengar P, et al. Shell feature: a new radiomics descriptor for predicting distant failure after radiotherapy in non-small cell lung cancer and cervix cancer. Phys Med Biol. 2018;63:095007.
Lucia F, Visvikis D, Vallières M, Desseroit MC, Miranda O, Robin P, et al. External validation of a combined PET and MRI radiomics model for prediction of recurrence in cervical cancer patients treated with chemoradiotherapy. Eur J Nucl Med Mol Imaging. 2019;46:864–77.
Stein CK, Qu P, Epstein J, Buros A, Rosenthal A, Crowley J, et al. Removing batch effects from purified plasma cell gene expression microarrays with modified ComBat. BMC Bioinformatics. 2015;16:63.
Orlhac F, Soussan M, Maisonobe JA, Garcia CA, Vanderlinden B, Buvat I. Tumor texture analysis in 18F-FDG PET: Relationships between texture parameters, histogram indices, standardized uptake values, metabolic volumes, and total lesion glycolysis. J Nucl Med. 2014;55:414–22.
Mukaka MM. Statistics corner: a guide to appropriate use of correlation coefficient in medical research. Malawi Med. 2012;24:69–71.
Dietrich S, Floegel A, Troll M, Kühn T, Rathmann W, Peters A, et al. Random survival forest in practice: a method for modelling complex metabolomics data in time to event analysis. Int J Epidemiol. 2016;45:1406–20.
Wang H, Gang L. A selective review on random survival forests for high dimensional data. Quant Biosci. 2017;36:85–96.
Lambin P, Leijenaar RTH, Deist TM, Peerlings J, de Jong EEC, van Timmeren J, et al. Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol. 2017;14:749–62.
Attal M, Lauwers-Cances V, Hulin C, Leleu X, Caillot D, Escoffre M, et al. Lenalidomide, bortezomib, and dexamethasone with transplantation for myeloma. N Engl J Med. 2017;376:1311–20.
Cavo M, Gay F, Beksac M, Pantani L, Petrucci MT, Dimopoulos MA, et al. Autologous haematopoietic stem-cell transplantation versus bortezomib-melphalan-prednisone, with or without bortezomib-lenalidomide-dexamethasone consolidation therapy, and lenalidomide maintenance for newly diagnosed multiple myeloma (EMN02/HO95): a multicentre, randomised, open-label, phase 3 study. Lancet Haematol. 2020;7:e456–68.
Aljama MA, Sidiqi MH, Buadi FK, Lacy MQ, Gertz MA, Dispenzieri A, et al. Utility and prognostic value of 18F-FDG positron emission tomography-computed tomography scans in patients with newly diagnosed multiple myeloma. Am J Hematol. 2018;93:1518–23.
Da-ano R, Masson I, Lucia F, Doré M, Robin P, Alfieri J, et al. Performance comparison of modified ComBat for harmonization of radiomic features for multicenter studies. Sci Rep. 2020;10:10248.
Da-ano R, Visvikis D, Hatt M. Harmonization strategies for multicenter radiomics investigations. Phys Med Biol. 2020. https://doi.org/10.1088/1361-6560/aba798.
Zhou Y, McArdle JJ. Rationale and applications of survival tree and survival ensemble methods. Psychometrika. 2015;80:811–33.
Gregorutti B, Michel B, Saint-Pierre P. Correlation and variable importance in random forests. Stat Comput. 2017;3:659–78.
Moreau P, Zweegman S, Perrot A, Hulin C, Caillot D, Facon T, et al. Evaluation of the prognostic value of positron emission tomography-computed tomography (PET-CT) at diagnosis and follow-up in transplant-eligible newly diagnosed multiple myeloma (TE NDMM) patients treated in the phase 3 CASSIOPEIA Study: results of the CASSIOPET Companion Study. Oral communication. ASH annual congress 2019.
Moreau P, Attal M, Hulin C, Arnulf B, Belhadj K, Benboubker L, et al. Bortezomib, thalidomide, and dexamethasone with or without daratumumab before and after autologous stem-cell transplantation for newly diagnosed multiple myeloma (CASSIOPEIA): a randomised, open-label, phase 3 study. Lancet. 2019;394:29–38.
Funding
This work has been supported in part by grants from the French National Agency for Research called “Investissements d’Avenir” IRON Labex no. ANR-11-LABX-0018-01, INCa-DGOS-Inserm_12558 (SIRIC ILIAD), and the European Regional Development Fund, the Pays de la Loire region on the Connect Talent scheme MILCOM, Nantes Métropole (Convention 2017-10470).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Advanced Image Analyses (Radiomics and Artificial Intelligence)
Electronic supplementary material
ESM 1
(PDF 1951 kb)
Rights and permissions
About this article
Cite this article
Jamet, B., Morvan, L., Nanni, C. et al. Random survival forest to predict transplant-eligible newly diagnosed multiple myeloma outcome including FDG-PET radiomics: a combined analysis of two independent prospective European trials. Eur J Nucl Med Mol Imaging 48, 1005–1015 (2021). https://doi.org/10.1007/s00259-020-05049-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-05049-6