Abstract
Effective combination antiretroviral therapy (cART) has lead to a significant reduction in the prevalence and incidence of central nervous system (CNS) HIV-associated brain disease, particularly CNS opportunistic infections and HIV encephalitis. Despite this, cognitive deficits in people living with HIV, also known as HIV-associated neurocognitive disorders (HAND) have become more prevalent in recent years. The pathogenesis of HAND is likely to be multifactorial, however recent evidence suggests that brain microglial activation is the most likely pathogenic mechanism. Recent developments in positron emission tomography (PET) brain neuroimaging using novel brain radioligands targeting a variety of physiological changes in the brains of HIV-positive individuals have improved our understanding of the mechanisms associated with the development of HAND. This review will highlight recent PET brain neuroimaging studies in the cART era, focusing on physiological and neurochemical changes associated with HAND in people living with HIV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The introduction of effective combination antiretroviral (cART) therapy has led to dramatic reductions in the incidence and prevalence of HIV-associated central nervous system (CNS) opportunistic infections and HIV encephalopathy [1, 2]. Despite the significant impact of cART on HIV-associated CNS disease, clinically significant cognitive deficits affecting people living with HIV have become increasingly apparent in recent years [3]. Prevalence rates of HIV-associated neurocognitive disorders, also known as HAND of between 15 to 50% have been observed across clinical and geographical settings, with effectively treated HIV-positive individuals having a higher prevalence of cognitive impairment compared to the general population [4–9]. HAND diagnosis is based on neuropsychometric testing and includes asymptomatic neurocognitive impairment (ANI), mild neurocognitive impairment (MCI), and the most severe form, HIV-associated dementia (HAD) [10]. In countries where cART is widely available, HIV-associated dementia has dramatically declined, however milder forms of cognitive impairment persist despite the availability of potent cART. HAND in people living with HIV has several possible pathogenic mechanisms including poor drug concentration of antiretrovirals in CNS, legacy effect of CNS damage sustained during early stages of HIV infection of the brain, antiretroviral neurotoxicity, persistent brain immune activation, and comorbidities such as cerebrovascular disease, syphilis, and hepatitis C co-infection [11–18].
Neuroimaging techniques that evaluate brain structure, metabolism, neurochemistry, and brain function have been used widely in clinical settings to aid in the diagnosis, and description of neurological disorders associated with HIV. Recent technical advances in these techniques offer new perspectives for studying the neuropathogenesis of HIV-associated brain disease in the era of cART. Positron emission tomography (PET) is now well established for clinical use in neurodegenerative disorders, especially in the diagnosis of dementia. The development of PET brain radiotracers that allow imaging of neuroinflammation, functional activity, neurotransmitter systems, and amyloid plaque deposition might have the potential to advance our knowledge of the pathophysiology of HAND. This review will highlight PET radiotracers used to study HIV-associated brain disease, focusing on PET neuroimaging studies that have contributed to our understanding of HAND in the era of combination antiretroviral therapy (Table 1).
PET-FDG brain imaging in HAND
Fluorodeoxyglucose [18F]-FDG PET brain imaging has been widely used in clinical and research settings for the evaluation of several neurological diseases, including HIV-associated brain diseases [30]. [18F]-FDG PET allows the in vivo quantification of cerebral glucose metabolism, which reflects neuronal and synaptic activity. [18F]-FDG PET has the ability to quantify regional differences in cerebral glucose metabolism, which can provide information about the distribution and activation of brain patterns. [18F]-FDG PET has proven to be useful as a diagnostic tool in the clinical evaluation of CNS lesions in patients with HIV, particularly in those with CNS opportunistic infections where [18F]-FDG PET can be used to distinguish between CNS malignancy and infection [31, 32]. [18F]-FDG PET has also been used to explore regional cerebral glucose metabolism patterns in HIV-positive patients with and without cognitive impairment. Early pre-cART studies using [18F]-FDG PET have shown consistently the presence of subcortical hypermetabolism in the basal ganglia, striatum, and thalamus of HIV-positive patients with early stages of HIV-associated dementia, as well as asymptomatic HIV-positive individuals, suggesting that increased glucose metabolism of subcortical structures, like the basal ganglia, are characteristic of HAND in patients without cART [33, 34]. A limited number of studies have investigated the impact of cART on cerebral glucose metabolism using [18F]-FDG PET. They have demonstrated changes in [18F]-FDG PET uptake despite effective virological suppression with cART. Changes include reductions in [18F]-FDG PET uptake in frontal regions in HIV-positive patients without cognitive impairment [19], and subtle basal ganglia hypermetabolism despite virological control with cART [20]. In summary, [18F]-FDG PET is helpful in the clinical evaluation of HIV positive patients with space-occupying lesions demonstrated on MRI or CT. However, the role of [18F]-FDG PET in the clinical assessment of HAND is less clear, as [18F]-FDG PET brain uptake correlates poorly with clinical and cognitive parameters in patients with different forms of HAND. Further research is needed to determine the role of [18F]-FDG PET in this context.
PET imaging of neurotransmitters in HAND
The presence of acute-onset parkinsonism in HIV-positive patients with HIV-associated dementia suggested a vulnerability of the dopaminergic system to the effects of HIV infection of the brain [35, 36]. This clinical observation was followed by reports of hypersensitivity to dopamine blockers, and the reduced concentration of CSF dopamine levels in HIV-positive patients with and without cognitive deficits [37]. Histopathological and [18F]-FDG PET imaging support the propensity of HIV-1 to affect basal ganglia structures [38, 39], which are known to have the highest density of dopaminergic terminals. Dopamine function can be assessed using PET imaging. [11C]-cocaine allows the assessment of presynaptic dopaminergic transporters (DAT) availability, while [11C]-raclopride measures D2 dopaminergic receptor availability, which mostly reflects postsynaptic sites [40]. Using these radioligands, Wang et al. found that although there were no differences in D2 receptors between HIV-positive patients and controls in any brain region, a significant decrease in DAT availability was observed in the putamen and ventral striatum of HIV-positive patients with HIV-associated dementia compared with HIV-negative controls. This small study (n = 15, HIV-positive individuals) was the first to suggest that decreased DAT might contribute towards the pathogenesis of HIV-associated dementia (Fig. 1) [21]. These findings were validated in a larger cohort of HIV-positive patients (n = 35) with cognitive impairment, but no HIV-associated dementia. In this study, compared to HIV-negative controls, HIV-positive individuals with cognitive impairment had reduced dopaminergic function (lower DAT), and this was associated with poorer cognitive performance [22]. Interestingly, in both studies, despite the significant decreases in dopaminergic function in the basal ganglia structures, there were only mild motor symptoms such as bradykinesia and rigidity, including intermittent choreiform movements in only three HIV-positive individuals with HIV-associated dementia. Further studies are needed to determine the effect of cART on DAT and D2 availability in HIV-positive individuals with and without cognitive impairment and its relationship with cognitive function.
Distribution volume ratio images of PET with 11C-cocaine (DA transporter) at the level of the basal ganglia. A significant decrease in DAT availability was observed in the putamen and ventral striatum of HIV-positive patients with HIV-associated dementia compared with HIV-negative controls. The images are scaled with respect to the maximum value obtained in the control subject and presented using the rainbow scale from [21] Wang et al. with permission
PET imaging of neuroinflammation in HAND
Chronic activation of brain microglia has been suggested to be a major contributor towards HIV-associated brain disease [41]. In HIV, activation of microglia has been associated with several factors, including persistent low-level HIV RNA replication, reduced concentration of cART in the CSF, cART neurotoxicity, co-infections (hepatitis C, syphilis) and lifestyle factors (smoking alcohol and recreational drug use). All of these factors might lead to activation of microglia despite effective control of HIV RNA with cART [42]. PET imaging allows in vivo quantification of neuroinflammation by measuring the density of the translocator protein 18 kDa (TSPO). TSPO is highly expressed in the mitochondria of microglia and astrocytes [43]. Following activation through host responses to cellular injury, microglia and astrocytes increase expression of TSPO [44]. Increased binding of a TSPO radioligand therefore provides a proxy measure of brain microglial activation that can be assessed in vivo with PET. Few studies have investigated microglial activation using TSPO PET in people living with HIV, with the majority of studies employing [11C]-PK11195, a first-generation TSPO radioligand. The findings have been contradictory with some studies demonstrating differences in [11C]-PK11195 binding between HIV-positive individuals with and without cognitive impairment [27, 45] and others showing no significant differences between groups [26]. Possible explanations for these discrepancies could be related to the difficulties in making accurate measures of brain binding with this “first-generation” ligand because of a lower proportion of the signal that arises from specifically bound [11C]-PK11195 [46].
Second-generation TSPO radioligands such as [11C]-DPA-713 and [11C]-PBR28 have higher affinity for TSPO and significantly better sensitivity compared with [11C] PK11195 [47]. One of the limitations of second-generation radioligands is that affinity for the target protein is determined by the rs6971 single-nucleotide polymorphism (SNP) in the TSPO gene [48, 49] that leads to an amino acid substitution (Ala147Thr), which is associated with in vitro affinity of TSPO in platelets [50, 51]. Three patterns of TSPO binding phenotypes have been identified in humans: high affinity binders (HABs) who are those subjects without the polymorphism (HH), low affinity binders (LABs) who are homozygotes (LL) and mixed affinity binders (MABs) who are heterozygotes (HL) expressing both low- and high-affinity binding to TSPO. HABs and LABs express a single binding site for TSPO with either high or low affinity, whereas MABs express equal amounts of HAB and LAB binding sites. A genotype analysis in plasma is available to characterize expected TSPO affinity that allows the incorporation of genotypic data enabling more accurate quantitative interpretation of TSPO PET data [48]. Another limitation relates to the TSPO itself. TSPO is regulated by a number of physiological factors including stress, steroid, and cholesterol metabolism, which can affect the analysis of TSPO imaging studies, and might contribute to poor tests reproducibility [29]. To control for some of these factors, analysis of second-generation TSPO data using a brain region that serves as a region of reference has been suggested. Brain regions of reference are selected because they are believed to be brain regions were binding activity is nonspecific in the disease process studied, however when postmortem and receptor imaging studies are performed, a significant percentage of specific binding is encountered, complicating the interpretation of the data [52]. Studies using this model of analysis have employed the gray matter because of the uniform pattern of binding displayed in healthy subjects, but not HIV-positive patients using the first-generation TSPO radioligand [11C] PK11195.
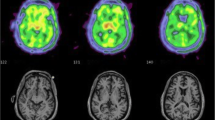
Two studies using “second-generation” TSPO radioligands investigated microglial activation in people living with HIV on cART using the gray white matter (GM) as a region of reference. Coughlin et al. used [11C]-DPA-713 to evaluate regional brain distribution of TSPO in HIV-positive patients compared with seronegative controls [29]. The study compared 23 HIV-positive individuals with and without HAND with 12 HIV-negative controls. HIV-positive individuals demonstrated significantly higher TSPO normalized volume of distribution (VTGM) in the white matter, cingulate cortex, and the supramarginal gyrus. An increase in TSPO VTGM within the frontal cortex was specifically linked to individuals with HIV-associated dementia. Using [11C]-PBR28 PET, Vera et al. found evidence of increases in brain TSPO radioligand uptake, with the greatest uptake of [11C]PBR28 in subcortical brain grey matter, particularly in the basal ganglia (globus pallidus, caudate and striatum) in a group of cognitively healthy HIV-positive individuals without HAND and effective cART [18] (Fig. 2). In this study a significant relationship between increased TSPO distribution volume ratios VTDVR, in the basal ganglia and poorer cognitive performance in tasks assessing verbal and visual memory was observed, as well as white matter microstructural abnormalities on diffusion tensor imaging MRI [18]. These findings evidence that suggest that effective cART, HIV-positive patients microglial and possibly, astroglial activation is present and that microglial activation could potentially lead to the development of HIV-associated brain disease and HAND. More research is required to determine the feasibility of using TSPO PET in the clinical assessment of patients with HAND.
Volume of distribution parametric maps (0–90 min) of [11C]PBR28 TSPO PET in a 55-year-old HIV-positive man without cognitive impairment on the left and a 53-year-old control subject on the right. Both individuals were high affinity binders. Images are transverse, coronal, and sagittal, from top to bottom
PET imaging of neuropathology in HAND
Neurodegeneration has been suggested as one the pathogenic mechanisms associated with HAND. However, until recently, it has not been possible to examine in vivo biomarkers of neurodegeneration in HIV-positive patients effectively treated with cART. The deposition of Aβ42−amyloid is considered a key marker of neuronal degeneration in Alzheimer’s disease (AD). Several reports have described a significant increase in brain Aβ42-amyloid deposition in patients with acquired immunodeficiency syndrome (AIDS) in brain pathology following post-mortem examinations [53, 54], as well as reductions in Aβ42-amyloid in CSF of treated HIV-positive patients with HAND [55–57], indicating increased deposition of Aβ42-amyloid in brain parenchyma. With the improvements in life expectancy of people living with HIV, increasing concerns have arisen as to whether HIV-positive patients are at increased risk of developing AD, and the challenges that clinicians will face differentiating between HAND and other neurodegenerative disorders in aging HIV-infected populations. The development of radiotracers such as the amyloid binding agents [11C]-labeled Pittsburgh Compound B [11C]-PIB, [18F]-flobetapir, [18F]-florbetaben, and [18F]-flutametamol offer the possibility to measure in vivo, the degree and distribution of Aβ42-amyloid deposition in the brains of HIV-positive individuals with cognitive impairment, and to distinguished between HAND and AD. In HIV-negative individuals, [11C]-PiB has demonstrated amyloid deposition in preclinical AD [58]. Two studies have used [11C]-PiB in HIV-positive individuals [23, 24] with unexpected results, as both studies were unable to demonstrate the presence of amyloid accumulation in patients with HAND. There are several possible explanations for these results. First, [11C]-PiB has significantly better affinity for fibrillar plaques rather than diffuse amyloid plaques, which have been traditionally associated with HAND. Second, there might be differences in amyloid metabolism between AD and HAND that [11C]-PiB is unable to detect. Finally, relatively younger individuals were included in these studies (the oldest was 67 years old) [59 ]. Indeed, Turner et al. reported for the first time the presence of in vivo amyloid deposition in a 71-year-old HIV-positive individual with HAND using [18F]-florbetaben, which has better affinity for diffuse amyloid plaques. [18F]-florbetaben PET CT showed abnormal scan appearances with pronounced cortical radiotracer deposition suggesting for the first time the presence of in vivo Aβ42-amyloid deposition in a person living with HIV [25]. Further research is required to determine the role of amyloid imaging in the clinical assessment of people living with HIV with cognitive impairment.
Conclusions and future directions
The introduction of cART has dramatically changed the pattern of brain disease associated with HIV infection. In settings where cART is readily available, CNS opportunistic infections and HIV-associated dementia are now rare, while patients on effective treatment are presenting with cognitive symptoms associated with HAND. This change in the natural history of HIV-associated CNS brain disease along with an aging HIV population at risk of neurodegenerative diseases generates new challenges to clinicians and researchers alike, as defining the cause of cognitive deficits in older HIV-positive patients can be difficult, due to the multifactorial nature of HAND. PET brain neuroimaging using novel radioligands might provide insight into different pathophysiological changes in the brain, which then combined with other neuroimaging techniques such as MRI could increase our knowledge of the relationship between structural, chemical, and functional changes in the brains of people living with HIV. In this respect, radioligands targeting microglial and/or astrocyte activation may be of particular importance, as neuroinflammation in HIV appears to be a major contributing factor for the development of HAND [60, 61]. PET brain tracers targeting inflammation, dopaminergic function, and possibly amyloid deposition have the potential to both serve as biomarkers supporting the diagnosis and management of HAND, and to facilitate the evaluation of therapeutic interventions. Considerably more research is needed to evaluate and establish the role of PET brain imaging techniques in the diagnosis and management of HAND in people living with HIV.
References
d’Arminio Monforte A, Cinque P, Mocroft A, Goebel FD, Antunes F, Katlama C, et al. Changing incidence of central nervous system diseases in the EuroSIDA cohort. Ann Neurol. 2004;55:320–8.
Dore GJ, Correll PK, Li Y, Kaldor JM, Cooper DA, Brew BJ, et al. Changes to AIDS dementia complex in the era of highly active antiretroviral therapy. AIDS. 1999;13:1249–53.
McArthur JC, McDermott MP, McClernon D, St Hillaire C, Conant K, Marder K, et al. Attenuated central nervous system infection in advanced HIV/AIDS with combination antiretroviral therapy. Arch Neurol. 2004;61:1687–96.
McArthur JC, Haughey N, Gartner S, Conant K, Pardo C, Nath A, et al. Human immunodeficiency virus-associated dementia: an evolving disease. J Neurovirol. 2003;9:205–21.
Sacktor N. The epidemiology of human immunodeficiency virus-associated neurological disease in the era of highly active antiretroviral therapy. J Neurovirol. 2002;8 Suppl 2:115–21.
Heaton RK, Clifford DB, Franklin DR Jr, Woods SP, Ake C, Vaida F, et al. HIV-associated neurocognitive disorders persist in the era of potent antiretroviral therapy: CHARTER Study. Neurology. 2010;75:2087–96.
Robertson KR, Nakasujja N, Wong M, Sacktor N. Pattern of neuropsychological performance among HIV-positive patients in Uganda. BMC Neurol. 2007;7:8.
Simioni S, Cavassini M, Annoni JM, Rimbault Abraham A, Bourquin I, Schiffer V, et al. Cognitive dysfunction in HIV patients despite long-standing suppression of viremia. AIDS. 2010;24:1243–50.
Garvey L, Surendrakumar V, Winston A. Low rates of neurocognitive impairment are observed in neuro-asymptomatic HIV-infected subjects on effective antiretroviral therapy. HIV Clin Trials. 2011;12:333–8.
Antinori A, Arendt G, Becker JT, Brew BJ, Byrd DA, Cherner M, et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007;69:1789–99.
Grima P, Fabbiani M, Ciccarelli N, Tana M, Farina S, Colafigli M, et al. Increased ophthalmic artery resistance index is associated with cognitive impairment in HIV-infected patients. J Infect. 2012;65(5):439–46.
Negin J, Martiniuk A, Cumming RG, Naidoo N, Phaswana-Mafuya N, Madurai L, et al. Prevalence of HIV and chronic comorbidities among older adults. AIDS. 2012;26 Suppl 1:S55–63.
Wallace MR, Heaton RK, McCutchan JA, Malone JL, Velin R, Nelson J, et al. Neurocognitive impairment in human immunodeficiency virus infection is correlated with sexually transmitted disease history. Sex Transm Dis. 1997;24:398–401.
Sun B, Abadjian L, Rempel H, Monto A, Pulliam L. Differential cognitive impairment in HCV coinfected men with controlled HIV compared to HCV monoinfection. J Acquir Immune Defic Syndr. 2013;62:190–6.
Winston A, Garvey L, Sabin CA. Superior neurocognitive function is associated with central nervous system antiretroviral drug penetration only in regimens containing more than three antiretroviral agents. AIDS. 2011;25:1014–5.
Marra CM, Zhao Y, Clifford DB, Letendre S, Evans S, Henry K, et al. Impact of combination antiretroviral therapy on cerebrospinal fluid HIV RNA and neurocognitive performance. AIDS. 2009;23:1359–66.
Robertson K, Liner J, Meeker RB. Antiretroviral neurotoxicity. J Neurovirol. 2012;18(5):388–99.
Vera JH, Guo Q, Cole JH, Boasso A, Greathead L, Kelleher P, et al. Neuroinflammation in treated HIV-positive individuals: A TSPO PET study. Neurology. 2016;86:1425–32.
Andersen AB, Law I, Krabbe KS, Bruunsgaard H, Ostrowski SR, Ullum H, et al. Cerebral FDG-PET scanning abnormalities in optimally treated HIV patients. J Neuroinflammation. 2010;7:13.
von Giesen HJ, Antke C, Hefter H, Wenserski F, Seitz RJ, Arendt G. Potential time course of human immunodeficiency virus type 1-associated minor motor deficits: electrophysiologic and positron emission tomography findings. Arch Neurol. 2000;57:1601–7.
Wang GJ, Chang L, Volkow ND, Telang F, Logan J, Ernst T, et al. Decreased brain dopaminergic transporters in HIV-associated dementia patients. Brain J Neurol. 2004;127:2452–8.
Chang L, Wang GJ, Volkow ND, Ernst T, Telang F, Logan J, et al. Decreased brain dopamine transporters are related to cognitive deficits in HIV patients with or without cocaine abuse. NeuroImage. 2008;42:869–78.
Ances BM, Christensen JJ, Teshome M, Taylor J, Xiong C, Aldea P, et al. Cognitively unimpaired HIV-positive subjects do not have increased 11C-PiB: a case–control study. Neurology. 2010;75:111–5.
Ances BM, Benzinger TL, Christensen JJ, Thomas J, Venkat R, Teshome M, et al. 11C-PiB imaging of human immunodeficiency virus-associated neurocognitive disorder. Arch Neurol. 2012;69:72–7.
Turner RS, Chadwick M, Horton WA, Simon GL, Jiang X, Esposito G. An individual with human immunodeficiency virus, dementia, and central nervous system amyloid deposition. Alzheimers Dement (Amst). 2016;4:1–5.
Wiley CA, Lopresti BJ, Becker JT, Boada F, Lopez OL, Mellors J, et al. Positron emission tomography imaging of peripheral benzodiazepine receptor binding in human immunodeficiency virus-infected subjects with and without cognitive impairment. J Neurovirol. 2006;12:262–71.
Hammoud DA, Endres CJ, Chander AR, Guilarte TR, Wong DF, Sacktor NC, et al. Imaging glial cell activation with [11C]-R-PK11195 in patients with AIDS. J Neurovirol. 2005;11:346–55.
P. N. Garvey L, Politis M, RamlackhansinghA, Taylor-Robinson S, Brooks D, and Winston A, Microglial cell activation is visualized with [11C]-PK11195 positron emission tomography in neurologi- cally asymptomatic HIV-infected subjects on effective ART. CROI 2012. Oral Abstract: 78LB.
Coughlin JM, Wang Y, Ma S, Yue C, Kim PK, Adams AV, et al. Regional brain distribution of translocator protein using [(11)C]DPA-713 PET in individuals infected with HIV. J Neurovirol. 2014;20:219–32.
Singhal T. Positron emission tomography applications in clinical neurology. Semin Neurol. 2012;32:421–31.
Davison JM, Subramaniam RM, Surasi DS, Cooley T, Mercier G, Peller PJ. FDG PET/CT in patients with HIV. AJR Am J Roentgenol. 2011;197:284–94.
Lewitschnig S, Gedela K, Toby M, Kulasegaram R, Nelson M, O’Doherty M, et al. (1)(8)F-FDG PET/CT in HIV-related central nervous system pathology. Eur J Nucl Med Mol Imaging. 2013;40:1420–7.
Pascal S, Resnick L, Barker WW, Loewenstein D, Yoshii F, Chang JY, et al. Metabolic asymmetries in asymptomatic HIV-1 seropositive subjects: relationship to disease onset and MRI findings. J Nucl Med. 1991;32:1725–9.
Hinkin CH, van Gorp WG, Mandelkern MA, Gee M, Satz P, Holston S, et al. Cerebral metabolic change in patients with AIDS: report of a six-month follow-up using positron-emission tomography. J Neuropsychiatry Clin Neurosci. 1995;7:180–7.
Hriso E, Kuhn T, Masdeu JC, Grundman M. Extrapyramidal symptoms due to dopamine-blocking agents in patients with AIDS encephalopathy. Am J Psychiatry. 1991;148:1558–61.
Factor SA, Podskalny GD, Barron KD. Persistent neuroleptic-induced rigidity and dystonia in AIDS dementia complex: a clinico-pathological case report. J Neurol Sci. 1994;127:114–20.
Berger JR, Kumar M, Kumar A, Fernandez JB, Levin B. Cerebrospinal fluid dopamine in HIV-1 infection. AIDS. 1994;8:67–71.
Kure K, Weidenheim KM, Lyman WD, Dickson DW. Morphology and distribution of HIV-1 gp41-positive microglia in subacute AIDS encephalitis. Pattern of involvement resembling a multisystem degeneration. Acta Neuropathol. 1990;80:393–400.
Rottenberg DA, Sidtis JJ, Strother SC, Schaper KA, Anderson JR, Nelson MJ, et al. Abnormal cerebral glucose metabolism in HIV-1 seropositive subjects with and without dementia. J Nucl Med. 1996;37:1133–41.
Volkow ND, Wang GJ, Fowler JS, Fischman M, Foltin R, Abumrad NN, et al. Methylphenidate and cocaine have a similar in vivo potency to block dopamine transporters in the human brain. Life Sci. 1999;65:PL7–12.
González-Scarano F, Martín-García J. The neuropathogenesis of AIDS. Nat Rev Immunol. 2005;5:69–81.
Winston A, Duncombe C, Li PC, Gill JM, Kerr SJ, Puls R, et al. Does choice of combination antiretroviral therapy (cART) alter changes in cerebral function testing after 48 weeks in treatment-naive, HIV-1-infected individuals commencing cART? A randomized, controlled study. Clin Infect Dis. 2010;50:920–9.
Papadopoulos V, Baraldi M, Guilarte TR, Knudsen TB, Lacapère JJ, Lindemann P, et al. Translocator protein (18kDa): new nomenclature for the peripheral-type benzodiazepine receptor based on its structure and molecular function. Trends Pharmacol Sci. 2006;27:402–9.
Stephenson DT, Schober DA, Smalstig EB, Mincy RE, Gehlert DR, Clemens JA. Peripheral benzodiazepine receptors are colocalized with activated microglia following transient global forebrain ischemia in the rat. J Neurosci. 1995;15:5263–74.
Garvey LJ, Pavese N, Politis M, Ramlackhansingh A, Brooks DJ, Taylor-Robinson SD, et al. Increased microglia activation in neurologically asymptomatic HIV-infected patients receiving effective ART. AIDS. 2014;28(1):67–72.
Matthews PM, Datta G. Positron-emission tomography molecular imaging of glia and myelin in drug discovery for multiple sclerosis. Expert Opin Drug Discovery. 2015;10:557–70.
Chauveau F, Boutin H, Van Camp N, Dollé F, Tavitian B. Nuclear imaging of neuroinflammation: a comprehensive review of [11C]PK11195 challengers. Eur J Nucl Med Mol Imaging. 2008;35:2304–19.
Owen DR, Yeo AJ, Gunn RN, Song K, Wadsworth G, Lewis A, et al. An 18-kDa translocator protein (TSPO) polymorphism explains differences in binding affinity of the PET radioligand PBR28. J Cereb Blood Flow Metab. 2012;32:1–5.
Kreisl WC, Jenko KJ, Hines CS, Lyoo CH, Corona W, Morse CL, et al. A genetic polymorphism for translocator protein 18 kDa affects both in vitro and in vivo radioligand binding in human brain to this putative biomarker of neuroinflammation. J Cereb Blood Flow Metab. 2013;33:53–8.
Owen DR, Gunn RN, Rabiner EA, Bennacef I, Fujita M, Kreisl WC, et al. Mixed-affinity binding in humans with 18-kDa translocator protein ligands. J Nucl Med. 2011;52:24–32.
Guo Q, Owen DR, Rabiner EA, Turkheimer FE, Gunn RN. Identifying improved TSPO PET imaging probes through biomathematics: the impact of multiple TSPO binding sites in vivo. NeuroImage. 2012;60:902–10.
Frankle WG, Cho RY, Prasad KM, Mason NS, Paris J, Himes ML, et al. In vivo measurement of GABA transmission in healthy subjects and schizophrenia patients. Am J Psychiatry. 2015;172:1148–59.
Green DA, Masliah E, Vinters HV, Beizai P, Moore DJ, Achim CL. Brain deposition of beta-amyloid is a common pathologic feature in HIV positive patients. AIDS. 2005;19:407–11.
Soontornniyomkij V, Moore DJ, Gouaux B, Soontornniyomkij B, Tatro ET, Umlauf A, et al. Cerebral beta-amyloid deposition predicts HIV-associated neurocognitive disorders in APOE epsilon4 carriers. AIDS. 2012;26:2327–35.
Clifford DB, Fagan AM, Holtzman DM, Morris JC, Teshome M, Shah AR, et al. CSF biomarkers of Alzheimer disease in HIV-associated neurologic disease. Neurology. 2009;73:1982–7.
Krut JJ, Zetterberg H, Blennow K, Cinque P, Hagberg L, Price RW, et al. Cerebrospinal fluid Alzheimer’s biomarker profiles in CNS infections. J Neurol. 2013;260:620–6.
Gisslén M, Krut J, Andreasson U, Blennow K, Cinque P, Brew BJ, et al. Amyloid and tau cerebrospinal fluid biomarkers in HIV infection. BMC Neurol. 2009;9:63.
Cohen AD, Klunk WE. Early detection of Alzheimer’s disease using PiB and FDG PET. Neurobiol Dis. 2014;72(Pt A):117–22.
Ortega M, Ances BM. Role of HIV in amyloid metabolism. J Neuroimmune Pharmacol. 2014;9:483–91.
Minagar A, Shapshak P, Fujimura R, Ownby R, Heyes M, Eisdorfer C. The role of macrophage/microglia and astrocytes in the pathogenesis of three neurologic disorders: HIV-associated dementia, Alzheimer disease, and multiple sclerosis. J Neurol Sci. 2002;202:13–23.
Anthony IC, Ramage SN, Carnie FW, Simmonds P, Bell JE. Influence of HAART on HIV-Related CNS Disease and Neuroinflammation. J Neuropathol Exp Neurol. 2005;64(6):529–36.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest and source of funding
JHV has received honoraria from Merck and Janssen Cilag, and sponsorship to attend scientific conferences from Janssen Cilag, Gilead Sciences and AbbVie and Merck. SD received honoraria from Avid Radiopharmaceuticals and research sponsorship from eLilly.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Vera, J.H., Ridha, B., Gilleece, Y. et al. PET brain imaging in HIV-associated neurocognitive disorders (HAND) in the era of combination antiretroviral therapy. Eur J Nucl Med Mol Imaging 44, 895–902 (2017). https://doi.org/10.1007/s00259-016-3602-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-016-3602-3