Abstract
We have assessed the effect of copepod chemical cues on the diel feeding rhythms of heterotrophic and mixotrophic marine protists. All phagotrophic protists studied exhibited relatively high diurnal feeding rates. The magnitude of the diel feeding rhythm, expressed as the quotient of day and night ingestion rates, was inversely related to the time that phagotrophic protists were maintained in the laboratory in an environment without predators. In the case of the recently isolated ciliate Strombidium arenicola, the rhythm was lost after a few months. When challenged with chemical alarm signals (copepodamides) from the copepod Calanus finmarchicus at realistic concentrations (0.6–6 pM), S. arenicola partially re-established diurnal feeding. Conversely, the amplitude of the diel feeding rhythm for the ciliate Mesodinium rubrum was not affected by copepodamides, although the 24-h integrated food intake increased by approximately 23%. For the dinoflagellates Gyrodinium dominans and Karlodinium armiger, copepodamides significantly reduced the amplitude of their diel feeding rhythms; significant positive effects on total daily ingestion were only observed in G. dominans. Finally, the dinoflagellate Oxyrrhis marina, isolated >20 years ago, showed inconsistent responses to copepodamides, except for an average 6% increase in its total ingestion over 24 h. Our results demonstrate that the predation risk by copepods affects the diel feeding rhythm of marine protists and suggests a species-specific response to predation threats.




Similar content being viewed by others
References
Calbet A, Saiz E (2005) The ciliate-copepod link in marine ecosystems. Aquat Microb Ecol 38:157–167. https://doi.org/10.3354/ame038157
Gifford DJ (1991) The protozoan-metazoan trophic link in pelagic ecosystems. J Protozool 38:81–86. https://doi.org/10.1111/j.1550-7408.1991.tb04806.x
Arias A, Saiz E, Calbet A (2017) Diel feeding rhythms in marine microzooplankton: effects of prey concentration, prey condition, and grazer nutritional history. Mar Biol 164:205. https://doi.org/10.1007/s00227-017-3233-7
Jakobsen HH, Strom SL (2004) Circadian cycles in growth and feeding rates of heterotrophic protist plankton. Limnol Oceanogr 49:1915–1922. https://doi.org/10.4319/lo.2004.49.6.1915
Ng WHA, Liu H (2015) Diel variation of the cellular carbon to nitrogen ratio of Chlorella autotrophica (Chlorophyta) growing in phosphorus- and nitrogen-limited continuous cultures. J Phycol 51:82–92. https://doi.org/10.1111/jpy.12254
Ng WHA, Liu H, Zhang S (2017) Diel variation of grazing of the dinoflagellate Lepidodinium sp. and ciliate Euplotes sp. on algal prey: the effect of prey cell properties. J Plankton Res 39:450–462. https://doi.org/10.1093/plankt/fbx020
Strom SL (2001) Light-aided digestion, grazing and growth in herbivorous protists. Aquat Microb Ecol 23:253–261. https://doi.org/10.3354/ame023253
Arias A, Saiz E, Calbet A (2019) Towards an understanding of diel feeding rhythms in marine protists: consequences of light manipulation. Microb Ecol 79:64–72. https://doi.org/10.1007/s00248-019-01390-y
Broglio E, Johansson M, Jonsson PR (2001) Trophic interaction between copepods and ciliates: effects of prey swimming behavior on predation risk. Mar Ecol Prog Ser 220:179–186. https://doi.org/10.3354/meps220179
Lima SL, Dill LM (1990) Behavioral decisions made under the risk of predation: a review and prospectus. Can J Zool 68:619–640. https://doi.org/10.1139/z90-092
Tiselius P, Jonsson P, Verity P (1993) A model evaluation of the impact of food patchiness on foraging strategy and predation risk in zooplankton. Bull Mar Sci 53:247–264
Titelman J (2001) Swimming and escape behavior of copepod nauplii: implications for predator-prey interactions among copepods. Mar Ecol Prog Ser 213:203–213. https://doi.org/10.3354/meps213203
Bollens S, Frost B (1989) Predator-induced diet vertical migration in a planktonic copepod. J Plankton Res 11:1047–1065. https://doi.org/10.1093/plankt/11.5.1047
Bollens SM, Frost BW (1991) Diel vertical migration in zooplankton: rapid individual response to predators. J Plankton Res 13:1359–1365. https://doi.org/10.1093/plankt/13.6.1359
Selander E, Heuschele J, Nylund GM, Pohnert G, Pavia H, Bjærke O, Pender-Healy LA, Tiselius P, Kiørboe T (2016) Solid phase extraction and metabolic profiling of exudates from living copepods. PeerJ 4:e1529. https://doi.org/10.7717/peerj.1529
Selander E, Kubanek J, Hamberg M, Andersson MX, Cervin G, Pavia H (2015) Predator lipids induce paralytic shellfish toxins in bloom-forming algae. Proc Natl Acad Sci U S A 112:6395–6400. https://doi.org/10.1073/pnas.1420154112
Selander E, Berglund E, Engström P, Berggren F, Eklund J, Harðardóttir S, Lundholm N, Grebner W, Andersson M (2019) Copepods drive large-scale trait-mediated effects in marine plankton. Sci Adv 5:eaat5096. https://doi.org/10.1126/sciadv.aat5096
Lindström J, Grebner W, Rigby K, Selander E (2017) Effects of predator lipids on dinoflagellate defence mechanisms-increased bioluminescence capacity. Sci Rep 7:1–9. https://doi.org/10.1038/s41598-017-13293-4
Prevett A, Lindström J, Xu J, Karlson B, Selander E (2019) Grazer-induced bioluminescence gives dinoflagellates a competitive edge. Curr Biol 29:R564–R565. https://doi.org/10.1016/j.cub.2019.05.019
Guillard (1975) Culture of phytoplankton for feeding marine invertebrates. In: Smith WLCMH (ed) Culture of marine invertebrate animals. Springer, Boston, pp 29–60
Calbet A, Isari S, Martínez RA, Saiz E, Garrido S, Peters J, Borrat RM, Alcaraz M (2013) Adaptations to feast and famine in different strains of the marine heterotrophic dinoflagellates Gyrodinium dominans and Oxyrrhis marina. Mar Ecol Prog Ser 483:67–84. https://doi.org/10.3354/meps10291
Anderson SR, Menden-Deuer S (2017) Growth, grazing, and starvation survival in three heterotrophic dinoflagellate species. J Eukaryot Microbiol 64(2):213–225
Senft-Batoh CD, Dam HG, Shumway SE, Wikfors GH (2015) A multi-phylum study of grazer-induced paralytic shellfish toxin production in the dinoflagellate Alexandrium fundyense: a new perspective on control of algal toxicity. Harmful Algae 44:20–31
Tsuda A, Furuya K, Nemoto T (1989) Feeding of micro- and macrozooplankton at the subsurface chlorophyll maximum in the subtropical North Pacific. J Exp Mar Biol Ecol 132:41–52
Grebner W, Berglund EC, Berggren F, Eklund J, Harðadóttir S, Andersson MX, Selander E (2018) Induction of defensive traits in marine plankton—new copepodamide structures. Limnol Oceanogr 64:820–831. https://doi.org/10.1002/lno.11077
Calbet A, Saiz E, Irigoien X, Alcaraz M, Trepat I (1999) Food availability and diel feeding rhythms in the marine copepods Acartia grani and Centropages typicus. J Plankton Res 21:1009–1015. https://doi.org/10.1093/plankt/21.5.1009
Bollens SM, Stearns DE (1992) Predator-induced changes in the diel feeding cycle of a planktonic copepod. J Exp Mar Biol Ecol 156:179–186. https://doi.org/10.1016/0022-0981(92)90244-5
Kiørboe T, Saiz E, Tiselius P, Andersen KH (2018) Adaptive feeding behavior and functional responses in zooplankton. Limnol Oceanogr 63:308–321. https://doi.org/10.1002/lno.10632
Olivares, M., Calbet, A., & Saiz, E. (2020). Effects of multigenerational rearing, ontogeny and predation threat on copepod feeding rhythms. Aquatic Ecology, 1-13.
Hairston NG, Olds EJ (1987) Population differences in the timing of diapause: a test of hypotheses. Oecologia 71:339–344. https://doi.org/10.1007/BF00378705
Jersabek CD, Luger MS, Schabetsberger R, Grill S, Strickler JR (2007) Hang on or run? Copepod mating versus predation risk in contrasting environments. Oecologia 153:761–773. https://doi.org/10.1007/s00442-007-0768-1
Svensson J-E (1997) Fish predation on Eudiaptomus gracilis in relation to clutch size, body size, and sex: a field experiment. Hydrobiologia 344:155–161. https://doi.org/10.1023/A:1002966614054
Hansson L-A (2004) Plasticity in pigmentation induced by conflicting threats from predation and UV radiation. Ecology 85:1005–1016. https://doi.org/10.1890/02-0525
Ringelberg J (1991) Enhancement of the phototactic reaction in Daphnia hyalina by a chemical mediated by juvenile perch (Perca fluviatilis). J Plankton Res 13:17–25. https://doi.org/10.1093/plankt/13.1.17
Tollrian R (1995) Predator-induced morphological defenses: costs, life history shifts, and maternal effects in Daphnia pulex. Ecology 76:1691–1705. https://doi.org/10.2307/1940703
Weider LJ, Pijanowska J (1993) Plasticity of Daphnia life histories in response to chemical cues from predators. Oikos 67:385–392. https://doi.org/10.2307/3545351
Gilbert JJ (1999) Kairomone-induced morphological defenses in rotifers. In: Tollrian R, Harvell CD (eds) The Ecology and Evolution of Inducible Defenses. Princeton University Press, Princeton, New Jersey, pp 127–141
Selander E, Thor P, Toth G, Pavia H (2006) Copepods induce paralytic shellfish toxin production in marine dinoflagellates. Proc R Soc B 273:1673–1680. https://doi.org/10.1098/rspb.2006.3502
Jakobsen HH (2001) Escape response of planktonic protists to fluid mechanical signals. Mar Ecol Prog Ser 214:67–78. https://doi.org/10.3354/meps214067
Jonsson PR, Tiselius P (1990) Feeding behaviour, prey detection and capture efficiency of the copepod Acartia tonsa feeding on planktonic ciliates. Mar Ecol Prog Ser 60:35–44. https://doi.org/10.3354/meps060035
Lindholm T (1985) Mesodinium rubrum—a unique photosynthetic ciliate. Adv Aquat Microbiol 3:1–48
Fenchel T, Hansen PJ (2006) Motile behaviour of the bloom-forming ciliate Mesodinium rubrum. Mar Biol Res 2:33–40. https://doi.org/10.1080/17451000600571044
Urban MC (2007) The growth–predation risk trade-off under a growing gape-limited predation threat. Ecology 88:2587–2597. https://doi.org/10.1890/06-1946.1
Pauwels K, Stoks R, De Meester L (2010) Enhanced anti-predator defence in the presence of food stress in the water flea Daphnia magna. Funct Ecol 24:322–329. https://doi.org/10.1111/j.1365-2435.2009.01641.x
Rose R, Warne MSJ, Lim R (2001) Factors associated with fish modify life history traits of the cladoceran Ceriodaphnia cf. dubia. J Plankton Res 23:11–17. https://doi.org/10.1093/plankt/23.1.11
Watts PC, Martin LE, Kimmance SA, Montagnes DJ, Lowe CD (2011) The distribution of Oxyrrhis marina: a global disperser or poorly characterized endemic? J Plankton Res 33:579–589. https://doi.org/10.1093/plankt/fbq148
Begun AA, Orlova TY, Selina MS (2004) A “bloom” in the water of Amursky Bay (Sea of Japan) caused by the dinoflagellate Oxyrrhis marina Dujardin, 1841. Russ J Mar Biol 30(1):51–55
Johnson, M. P. (2000). Physical control of plankton population abundance and dynamics in intertidal rock pools. In Island, Ocean and Deep-Sea Biology (pp. 145-152). Springer, Dordrecht.
Berge T, Poulsen LK, Moldrup M, Daugbjerg N, Hansen PJ (2012) Marine microalgae attack and feed on metazoans. ISME J 6:1926–1936. https://doi.org/10.1038/ismej.2012.29
Rasmussen SA, Binzer SB, Hoeck C, Meier S, De Medeiros LS, Andersen NG, Place A, Nielsen KF, Hansen PJ, Larsen TO (2017) Karmitoxin: an amine-containing polyhydroxy-polyene toxin from the marine dinoflagellate Karlodinium armiger. J Nat Prod 80:1287–1293. https://doi.org/10.1021/acs.jnatprod.6b00860
Bergkvist J, Selander E, Pavia H (2008) Induction of toxin production in dinoflagellates: the grazer makes a difference. Oecologia 156:147–154. https://doi.org/10.1007/s00442-008-0981-6
Wohlrab S, Iversen MH, John U (2010) A molecular and co-evolutionary context for grazer induced toxin production in Alexandrium tamarense. PLoS One 5(11):e15039. https://doi.org/10.1371/journal.pone.0015039
Xu J, Kiørboe T (2018) Toxic dinoflagellates produce true grazer deterrents. Ecology 99(10):2240–2249. https://doi.org/10.1002/ecy.2479
Armengol L, Calbet A, Franchy G, Rodríguez-Santos A, Hernández-León S (2019) Planktonic food web structure and trophic transfer efficiency along a productivity gradient in the tropical and subtropical Atlantic Ocean. Scientific Reports 9(1):2044
Arias A, Saiz E, Tiselius P, Calbet A (2020) Trophic interactions and diel feeding rhythms of microzooplankton in a productive Swedish Fjord. ICES J. Mar. Sci.
Acknowledgements
We are grateful to all members of the research group Signals in the Sea for collaborating from the University of Gothenburg, with special thanks to Andrew Prevett for technical assistance and valuable help during laboratory work. We also want to thank Jan for the illustrations.
Graphics Program
All Figures have been elaborated using KaleidaGraph, except for Figure S2 which was elaborated with MATLAB.
Funding
This work was supported by the FERMI project (CGL2014-59227-R; MINECO/AEI/FEDER, UE) and is a contribution of the Marine Zooplankton Ecology Group (2017 SGR 87). AA was funded with an FPI fellowship (BES-2015-074092) from the MICINN of Spain.
Author information
Authors and Affiliations
Contributions
AA and AC conceived and designed the experiments. AA performed the experiments. AA, AC, ESL, and ESZ participated in the data analysis. ESL contributed reagents and materials. AA, AC, and ESZ contributed to the statistical analyses. AA, AC, ESL, and ESZ wrote and gave final approval for the publication.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with animals performed by any of the authors.
Supplementary Information
ESM 1
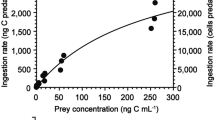
Supplementary information is available online at Microbial Ecology and includes (a) the functional response of the ciliate Strombidium arenicola, (b) a detailed explanation of the methodological process conducted to determine copepodamide effective concentrations and the resultant plot of copepodamide effective concentration throughout the incubation, and (3) a table containing day-time and night-time ingestion rates per grazer species studied in each experiment. Fig. S1 Ingestion rate of the ciliate Strombidium arenicola (μm3 grazer-1 h-1) as a function of prey concentration (μm3 mL-1). Error bars show standard error. Fig. S2 Effective concentration (nM) of copepodamides during 10h incubation. Closed circles represent the average data from the sampling time points and shaded area is the error interval (standard deviation). Table S1 Day and night ingestion rates (in terms of prey volume ingested, μm3 grazer-1 hour -1) for each of the studied grazer species as a function of copepodamides treatments (Control, 0.6 pM and 6 pM). Rates are differentiated between Experiment 1 and Experiment 2. Average ± standard error are shown. p-values from one-way ANOVA followed by a Dunnet’s test are presented to show the significance level of each copepodamides treatment with respect to the correspondent control in each phase (day and night). (DOCX 13264 kb)
Rights and permissions
About this article
Cite this article
Arias, A., Selander, E., Saiz, E. et al. Predator Chemical Cue Effects on the Diel Feeding Behaviour of Marine Protists. Microb Ecol 82, 356–364 (2021). https://doi.org/10.1007/s00248-020-01665-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-020-01665-9