Abstract
Understanding drivers behind patterns of functionally important groups of fishes is crucial for successful management and conservation of tropical seascapes. Herbivorous fishes are the most prominent consumers of marine primary production which can have profound effects on reef resilience. We explored environmental variables affecting distribution and foraging patterns of herbivorous and detritivorous fish assemblages (siganids, acanthurids and parrotfish) across distinct shallow-water habitats (coral reefs, macroalgae beds and seagrass meadows) during September–November 2016 at Mafia Island, Tanzania (8°00′S, 39°41′E). We performed underwater visual census to quantify fish assemblages, measured habitat features, deployed macroalgal assays and conducted inventories of grazing scars. Multi-dimensional scaling and mixed-effects linear models were used to evaluate differences in fish assemblages and environmental variables influencing abundance and foraging patterns of fishes. Fish communities of focal functional groups differed among habitats. Abundance of herbivores and detritivores as well as relative browsing and scraping was highest on coral reefs compared to macroalgae and seagrass meadows. Adult fish were more abundant on coral reefs while juveniles were abundant in macroalgal beds. Coral cover and crustose coralline algal cover had a positive effect on the abundance of fish in coral reef areas, while macroalgal cover had a negative effect. Contrastingly, in macroalgae habitats, macroalgal cover had a positive effect on the abundance of parrotfish. These results highlight the importance of considering connectivity between macroalgal beds and coral reefs through ontogenetic shifts in habitat use by primarily microphagous parrotfish and of incorporating a range of habitats within coastal management plans.
Similar content being viewed by others
Introduction
Disentangling which habitat characteristics are critical in shaping abundance, distribution, and ecological processes within the available range of coastal habitats will improve our understanding of marine community patterns and connectivity in shallow coastal seascapes. Benthic habitats provide marine organisms with food and shelter from abiotic or biotic stressors, or a combination of these (Friedlander and Parrish 1998), which can further influence competition and predation risk (Almany 2004).
In tropical reef environments, spatial heterogeneity is a strong driver of distribution and diversity patterns of reef fish (Chabanet et al. 1997; Sabater and Tofaeono 2007; Messmer et al. 2011; Samoilys et al. 2018), where characteristics such as structural complexity and rugosity are important predictors of fish assemblage structure and abundance (Bell and Galzin 1984; Gratwicke and Speight 2005; Graham and Nash 2013). In submerged macrophyte habitats, canopy height and cover have been shown to structure fish assemblages in both tropical seagrass meadows (Gullström et al. 2008; Alonso Aller et al. 2014) and macroalgal beds (Wenger et al. 2018; van Lier et al. 2018). However, environmental factors as such may impact coral fish communities on multiple spatial and temporal scales as some fish species might have shifting habitat preferences during different ontogenetic stages and hence utilize alternative habitats like seagrass meadows, mangroves and/or macroalgal beds during their juvenile life stage (Nagelkerken et al. 2000; Wilson et al. 2010; Berkström et al. 2012). For example, trade-offs between foraging success and predation risk depending on body size have been shown to explain habitat choice of juvenile groupers (Dahlgren and Eggleston 2000). Microhabitat preferences might therefore not be consistent across habitats, and environmental predictors might change depending on habitat identity.
Certain functional groups of reef fish have been identified as having a major role in maintaining reef resilience and reef health such as nominally herbivorous and detritivorous fishes (Bellwood et al. 2004; Mumby 2006; Hughes et al. 2007; Goatley and Bellwood 2010). The former group can be further classified into three groups: algivores (i.e., fish that target eukaryotic macroalgae and turfing algae), seagrass-feeders, and microphages (fish feeding on microscopic phototrophs that can be both epilithic and endolithic) (Choat et al. 2002; Clements et al. 2017; Clements and Choat 2018). Drivers predicting and influencing abundance, distribution and feeding patterns of these groups are considered imperative within the management of tropical shallow seascapes. Through feeding activities, these groups are acknowledged to be important and influential forces in shaping and structuring ecosystems through top–down control, especially by balancing algal and coral communities in favour of corals.
Although distribution patterns of herbivorous fish and herbivory have been studied extensively in coral reef habitats (see, e.g., Hay 1981; Bellwood et al. 2014; Adam et al. 2015; Russ et al. 2015; Steneck et al. 2017), fish herbivory can also have consequences in other habitats. In seagrass systems, removal of epiphytes through grazing is hypothesized to enhance seagrass production by decreasing competition for light (van Montfrans et al. 1984), and seagrass browsing species have a major role in trophodynamics as they incorporate carbon and nutrients in the food chain (Unsworth et al. 2007). In temperate macroalgal habitats, spatial patterns of algal distribution have been shown to be structured by top–down control induced by fish (Vergés et al. 2009; Taylor and Schiel 2010). However, foraging patterns of nominally herbivorous fish in naturally occurring tropical macroalgal beds have been largely understudied (but see Lim et al. 2016). Canopy-forming tropical macroalgal beds can harbour large numbers of herbivores and have only recently been acknowledged as important nursery grounds for coral reef fishes, including juvenile acanthurids, parrotfish and rabbitfish (Evans et al. 2014; Tano et al. 2017; Eggertsen et al. 2017; Fulton et al. 2019), highlighting the importance of investigating habitat-specific ecological functions and linkages to other habitats.
A prevailing perspective is that nominally herbivorous fishes are driving benthic structure and habitat differentiation through strong top–down control (Mumby 2006; Jackson et al. 2014; Bonaldo et al. 2014; Adam et al. 2015). As a large part of the studies that propose top–down control on the benthos by herbivorous and detritivorous fishes are performed in the Caribbean, similar mechanisms might or might not be applicable to the same extent in other geographical locations (Roff and Mumby 2012). Instead, bottom-up effects such as benthic characteristics might be more important in other places, considering the strong effects benthos can have on reef fish communities as a whole (Friedlander and Parrish 1998; Friedlander et al. 2003; Messmer et al. 2011). Long-term studies from the Philippines have shown strong bottom–up effects on distribution and abundances of detritivorous fish (Russ et al. 2018) and parrotfish assemblages (Russ et al. 2015), and similar patterns have been observed in the Pacific (Tootell and Steele 2016) and the Indian Ocean (Samoilys et al. 2018). Due to the inconsistencies of responses observed, which might be system-specific, further studies are needed.
In the Western Indian Ocean (WIO) region, fishing has been identified as the strongest factor influencing coral reef fish abundance, although benthic variables also had some effects (McClanahan and Arthur 2001), indicating a mix of top–down and bottom–up factors. The majority of herbivore-macroalgae-coral studies have been performed in Kenya (see, e.g., McClanahan et al. 1999, 2001; McClanahan and Arthur 2001), where top–down control in the form of herbivory has been identified as an important predictor for macroalgae abundance on coral reefs (McClanahan et al. 2001; Mörk et al. 2009). Likewise, a study in seagrass meadows from Zanzibar has shown that both top–down (fishing) and bottom–up (seagrass density) control explained fish assemblage structure (Alonso Aller et al. 2014). However, patterns as such might vary across the WIO region, in different habitat types and with different fishing pressure.
The present study therefore sets out to explore distribution and foraging patterns of herbivorous and detritivorous fishes across multiple habitats within a shallow tropical seascape in a marine protected area (MPA) in Tanzania. Additionally, the extent these patterns are driven by benthic characteristics (bottom–up controlled) is studied. In doing so, we also identify habitat variables which might be important in structuring juvenile communities in nursery/recruitment habitats. This is done by (1) quantifying detritivorous and functional groups of herbivorous fish assemblages in distinct and common shallow-water habitats (coral reefs, macroalgal beds and seagrass meadows), (2) identifying environmental variables structuring these fish assemblages and foraging patterns within the seascape and (3) quantifying browsing and scraping of algae in these habitats. We hypothesized that the observed fish assemblages (abundances and species composition) and foraging patterns would differ across habitats on a broad spatial scale and will be related to differences in habitat characteristics, resource availability, and predatory assemblages at fine spatial scales (1–25 m).
Methods
Description of study area
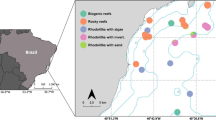
The present study was conducted within the boundaries of the Mafia Island Marine Park (MIMP), in Mafia Island, Tanzania (8°00′S, 39°41′E) (Fig. 1). The Mafia Island area is composed of a small archipelago located 20 km from the Tanzanian mainland and 120 km south of Unguja Island (Zanzibar) and has a high degree of marine biodiversity (Horrill et al. 1996; McClanahan et al. 2008). The MIMP was established in 1995 and is located on the southeastern part of the main island, covering a total area of 822 km2, of which 75% is below the high water mark (Gaspare et al. 2015). The protected area is a multi-use park, divided into zones subjected to different degrees of protection, from allowance of subsistence/artisanal fishing in some areas to complete closure in other areas. However, all destructive fishing methods and coral mining practices are forbidden (Berkström et al. 2013; Gaspare et al. 2015).
Small squares indicate where Mafia Island is located on the East African coast and large image the Southeastern side of Mafia Island where the study was conducted. Study sites are indicated with black symbols, and the Mafia Island Marine Park (MIMP) border with a black line. Tc, Thalassodendron ciliatum
The archipelagic environment is influenced by the East African Coastal Current (EACC), flowing northwards along the East African coast, and the northeast and southeast monsoon winds with one drier and sunnier period (October–March) and one rainier and more cloudy period (March–October) (Berkström et al. 2013). Tides are semi-diurnal with a mean amplitude of 3.3 m and causing strong and complex currents with velocities reaching up to 6 knots (Horrill et al. 1996). The eastern coastline of the Mafia Island is exposed to the open Indian Ocean, but protected by fringing reefs towards the southern part of the archipelago (Garpe and Öhman 2003; Gaspare et al. 2015). A large and relatively shallow bay (< 15 m deep), fringed by mangrove forests is located on the eastern side of the main island (Chole Bay), and shallow areas are also located around and towards the islands of Jibondo, Juani and Chole (Berkström et al. 2013). Shallow areas are characterized by a mosaic of macroalgae-dominated areas, sand flats, seagrass meadows and coral patch reefs (Berkström et al. 2013).
Field study
The field survey was conducted during September–November, 2016. The study coincided with the cooler season in coastal Tanzania, with mean sea surface temperatures of ~ 26 °C (McClanahan et al. 2007).
A total of 3 different habitats types were surveyed at 9 sites scattered throughout the shallow seascape (< 4 m of depth) (Fig. 1). Seagrass meadows were monospecific and consisted mainly of Thalassodendron ciliatum and macroalgal areas were dominated by the canopy-forming brown algae Sargassum aquifolium and Turbinaria conoides. While seagrass densities and cover can fluctuate with season (Robbins and Bell 2000), they generally do not undergo such dramatic changes as some Sargassum spp. (Fulton et al. 2014). Many Sargassum species from the southern Western Indian Ocean have slower growth rates during this time of the year, with cover and biomass in algal patches becoming less dense (Gillespie and Critchley 1999a). There is an increase in biomass and cover during the warmer season (December–March) (Gillespie and Critchley 1999b). Coral reef sites were comprised of a heterogenous mosaic of scleractinian corals. Habitat types were chosen on the premises where they were commonly occurring, located at approximately the same depths (1–4 m) and present in large and consistent patches (> 100 m2).
Fish and habitat surveys
Fish assemblages were surveyed by performing underwater visual census (UVCs), (n ≈ 5 habitat and site −1, ntotal = 48; ncoral = 15, nmacroalgae = 20, nseagrass = 13) along 25 × 2 m belt transects by snorkeling. All fishes were identified to lowest taxonomical level and total length (TL) was noted to closest cm, according to the method described in Tano et al. (2017). All surveys were performed between 09:30 and 16:00 and between the low and high tide peak. A snorkeler swam along the transect line ~ 0.1 m s−1, documenting all mobile fish species and then returning along the line examining substrate and plant/algae cover more thoroughly for small, cryptic species. Each snorkeler was equipped with a camera (Canon Powershot G7x Mark II and Canon WP-DC54 underwater housing), for later identification, if necessary. To avoid potential bias of length estimations, trial estimations were performed prior to the study until snorkelers were calibrated with each other and any possible bias consistent. All UVCs were performed by the same observers (M. Eggertsen and D.H. Chacin).
Every 5th meter of the transect, a 0.5 × 0.5 m square was placed and photographed from above, where benthic composition (percent cover of calcareous rubble, soft substrate, live coral cover, macroalgal cover, seagrass cover, number of macrophyte species, epilithic algal matrix (EAM) cover and crustose coralline algal (CCA) cover and rugosity) was estimated and the height of the 3 tallest macrophytes was measured to the closest cm using a ruler (n = 6 transect−1). Rugosity was visually estimated according to a 1–5 grade scale, where 1 denoted no rugosity (flat) and 5 the highest, a method suggested by Gratwicke and Speight (2005).
Depth was measured for each transect with a dive computer (Suunto Vyper Novo) and each transect was georeferenced by marking start and end point with a GPS kept at the surface and placed inside a waterproof bag. For all variables measured, a mean value per transect was calculated.
Browser assays
For estimating relative browsing pressure in different habitats, standardized browsing assays were used. Even though browsing assays only provide a relative estimation of the surrounding grazing pressure, it has been widely used within ecological studies for quantifying grazing pressure across areas and for identifying important browsing species (Fox and Bellwood 2008a, b; Ganesan et al. 2006). However, results have to be interpreted with care, as there is a profound risk that opportunistic fish might feed on assays out of curiosity, an effect which will be impossible to disentangle from data on biomass loss only (Wulff 2017). To circumvent this issue to some degree, 6 of the bioassays were documented by remote underwater video cameras (RUVs) (GoPro Hero Session) for ~ 1 h during each experimental setup (ntotal = 54), to identify which species of herbivores targeted the tethered algae. Assays were constructed by tethering thalli of Eucheuma denticulatum and Sargassum aquifolium to PVC pipes and placing them in three different habitat types (Thalassodendron ciliatum-dominated seagrass meadows, macroalgal beds, and coral reefs) during a full tidal cycle (~ 24 h). The macroalgal species were chosen because they are a common feature of the macroalgal assemblages in coastal Tanzania (Tano et al. 2016, 2017), and have been found in relatively large quantities in stomachs of main herbivorous fishes such as Siganus sutor (Eggertsen et al. unpublished), a common browser in Tanzanian coastal waters (Lugendo et al. 2007; Kimirei et al. 2011).
Fronds of E. denticulatum and S. aquifolium were collected from the field and kept in tanks with aerated seawater over night. Suitable sizes of thalli were chosen (< 5 g), dead/necrotic tissue and epiphytes were removed, and algae were spun in a salad spinner for 10 s to remove excess water. Thalli were then weighed and put into marked zip lock bags for transportation to the site. Two thalli of each species were tethered to a PVC pipe. Every browsing assay constituted a total of 4 pieces of thallus (nbrowsing assays = 18 habitat−1, nthallus = 72 habitat−1, ntotal = 648) (Fig. 2). Care was taken to place the browsing assays in continuous habitats and at a distance away from habitat edges.
Fishes removing biomass from the browsing assays were identified from the videos and herbivorous fishes within the video frame that were not feeding were also noted. RUVs have in some cases been shown to reveal more information than UVCs regarding observations of herbivorous species (Fox and Bellwood 2008a) whereas in some cases results have been equal (Longo and Floeter 2012). Both methods were included in the current study as RUVs have not been used within the study area before for monitoring herbivores. Browsing assays were retrieved after ~ 24 h, demounted from the PVC pipes and put in marked zip lock bags for transportation to land. Prior to weighing, algae samples were spun in a salad spinner for 10 s to remove excess water. To control for handling and daily growth rates of tethered macroalgae, a caged control (nthallus = 4) was placed in the experimental area at each sampling occasion.
Grazing scar inventories
To quantify grazing activity in different habitats by excavating and scraping parrotfish, an inventory of feeding scar densities was conducted. As feeding by excavating and scraping parrotfish resulted in conspicuous grazing scars in the substratum it is possible to visually quantify these (Bonaldo and Bellwood 2008, 2009; Bonaldo et al. 2011). Quadrats of 0.15 × 0.15 m were randomly placed on hard, calcareous (dead) surfaces, where all clean grazing scars were counted and measured to the nearest centimeter (n ~ 20 habitat type−1) (Fig. 3). Due to lack of suitable substrate for scrapers to feed on in seagrass habitats, this study was only performed in hard substrate habitats, i.e., coral and macroalgal habitats.
Statistical analyses
Fishes were classified into the following functional groups; seagrass and algivores (species feeding on seagrass, eukaryotic macroalgae, and turfing algae), detritivores, microphages (species feeding on endo- and epilithic microscopic phototrophs) and territorial grazers according to literature and FishBase (Montgomery et al.1989; Choat et al. 2002; Clements et al. 2017; Clements and Choat 2018; Tebbett et al. 2017; Froese and Pauly 2017; Russ et al. 2018).
Distribution of focal functional groups of fish in different habitats (coral, macroalgae and seagrass) was explored with non-metrical multidimensional scaling (nMDS) with Bray–Curtis dissimilarity index from the “vegan” package (Oksanen et al. 2017). Main and interactive effects of habitat and sites for species composition of focal fish communities were tested using PERMANOVA, Bray–Curtis dissimilarities (999 permutations) using “adonis” function from the “vegan” package (Oksanen et al. 2017). Species which occurred ≤ 3 in the UVCs were removed from the nMDS analysis. A similarity of percentage (SIMPER) analysis (Clarke 1993) from the “vegan” package was performed to identify which fish species contributed the most to dissimilarities among the three habitat types. Abundance of functional groups (herbivores, detritivores, and microphages) was analyzed with mixed linear models, “lme4” package, with “site” as random factor and habitat as a fixed factor (Bates et al. 2015). Functional group “territorial grazers” was removed from the analyses as roving herbivorous and detritivorous fishes were the main focus of the present study.
Habitat variables affecting total abundances of all focal functional groups were analyzed separately for each habitat using mixed linear models (multiple regressions) with “site” as a random factor and additional predictor variables as fixed factors (percent cover of calcareous rubble, soft substrate, live coral cover, macroalgal cover, seagrass cover, number of macrophyte species, EAM cover, CCA cover, rugosity and predatory fish abundance). Macroalgal habitats were analyzed with multiple linear regression models, as there was no difference on site level. Model selection was performed by stepwise removal of predictor variables, starting with a model containing all predictor variables allowed. Non-significant variables were then removed one by one and using function ‘step’ from “lme4” package, based on Aikaike information criterion (AIC) values. If ΔAIC ≥ 2, the model with the lowest AIC value was considered as the most parsimonious one.
Browsing assays (biomass loss for the 2 species of macroalgae) were analyzed with ANOVA, and mixed linear models (total biomass loss in different habitats) with “site” as a random factor and “habitat” as a fixed factor. Mean values of biomass loss for each macroalgal species were calculated per bioassay and the two species of algae were analyzed separately. To identify which habitats differed from each other, Tukey’s contrast test was used from package ‘multcomp’ (Hothorn et al. 2008). Total biomass loss in relation to environmental variables (macroalgal cover, macrophyte cover, rugosity, seagrass and algivorous fish abundances, EAM cover, live coral cover) was tested using mixed linear regression models with “site” and “tethered species ID” as random factors and additional predictor variables as “fixed”. Where there was no variance added due to “site” or “tethered species ID”, linear regression models were used.
All fish of the focal functional groups < 5 cm were classified as juveniles and larger individuals as adults. Size classes of pooled functional groups of fishes in different habitat types (coral, macroalgae and seagrass) were investigated using mixed linear models, and ANOVA models were performed with abundant juveniles/adults of microphagous fishes as “site” did not add any variation.
Predictor variables were checked for multicollinearity by pairwise comparison using the Spearman rank test and by evaluating variation inflation factor (VIF) values (Zuur et al. 2010). Predictor variables with VIF-values ≥ 2 were removed from the same model. Prior to model fitting, normal distributions of predictor variables were visually examined by basic diagnostic plots and if needed, transformation log(x + 1) and rescaling to size range was performed. All statistical analyses were performed in R version 3.3.1. (R Core Team 2017).
Results
Fish assemblages of focal functional groups
A total of 3,672 fishes from 34 families, 97 genera and 165 species were recorded in the UVCs during the study, of which 1141 were identified as seagrass and algivores, detritivores or microphages. Of these, 645 individuals were identified to species level (27 species), the majority of fish identified only to family level being juvenile labrid scarids. The three habitat types held distinct herbivorous fish assemblages and there was also a significant difference depending on site (PERMANOVA, Fig. 4, Table 1). The algivorous acanthurid Naso brevirostris was one of the most influential species in dissimilarities among habitats as it was abundant in coral reef sites but absent from macroalgal and seagrass habitats (SIMPER, Table 2).
NMDS ordination displaying assemblages of seagrass and algivores, detritivores and microphages in each habitat type, stress 0.19. Each symbol corresponds to a sample (1 UVC, 50 m2), and the more similar a UVC is regarding species composition, the closer they are positioned together. Fish species which are influential in clustering of habitats are displayed in the graph and named as following: AcanLeu, Acanthurus leucosternon; AcanNig, A. nigrofuscus; AcanTri, A. triostegus; AcanXan, A. xanthopterus; CentMul, Centropyge multispinis; ChaeKle, Chaetodon kleinii; ChloSor, Chlorurus sordidus; ChryBio, Chrysiptera biocellata; ChryUni, Chrysiptera unimaculata; CtenBin, Ctenochaetus binotatus; CtenStri, C. striatus; CtenTru, C. truncatus; LeptVaig, Leptoscarus vaigiensis; NasoBre, Naso brevirostris; ScarFre, Scarus frenatus; SigaSut, Siganus sutor; ZebrSco, Zebrasoma scopas. Ellipses are 95% confidence interval
Detritivores were most abundant on coral reef sites and absent from seagrass habitats, while seagrass and algivores and microphages were more evenly distributed across the seascape (Fig. 5).
a Top panel displays proportion of different functional groups in all samples (underwater visual censuses (UVCs), all habitats and all sites). Bottom panels display abundance of fish from different functional groups in the three habitat types (all sites pooled) from the UVCs (50 m2); coral reef sites, macroalgal habitats and seagrass meadows (Thalassodendron ciliatum), b seagrass and algivores, c detritivores and d microphages. Each dot in figures represents a sample (1 UVC), boxes show median (thick line), 25th and 75th percentile. Error bars are 95% confidence interval. CR, coral reef; MA, macroalgae; SG, seagrass
Overall, seagrass and algivores and microphages were the most abundant functional groups (Fig. 5a), the former consisting of acanthurids (mainly subadult Naso brevirostris), one Chaetodontid (Chaetodon klenii), one Pomacanthid (Centropyge multispinis) the scarid labrids Calotomus carolinus and Leptoscarus vaigiensis, and the siganid Siganus sutor, while the latter group exclusively consisted of juvenile and initial phases of scarid labrids (Scarus spp. and Chlorurus sordidus).
No difference was found in the total abundance of seagrass and algivores among habitat types (Mixed linear model, F (2,40) = 16.756, P = 0.160, Fig. 5b) or for microphages (Mixed linear model, F (2,40) = 0.256, P = 0.775, Fig. 5d). Detritivores were absent from seagrass habitats and significantly more abundant in coral reef habitats than in macroalgal habitats, (Mixed linear model, F (1,29)=16.756, P = 0.0003), and were composed of acanthurids; a few Acanthurus xanthopterus and dominated by several Ctenochaetus species (Electronic Supplementary Material, Table 6S).
Abundances of focal functional groups of fishes of different size classes differed between habitats (Fig. 6). Seagrass habitats held lower abundance of juveniles (> 5 cm) than the 2 other habitats (Mixed linear model, F (2,26)= 7.744, P = 0.0008), but there was no significant difference between coral reefs and macroalgal habitats (mean value 5.13 ± 1.50 ind. transect−1 in coral reef habitats, 6.60 ± 1.64 ind. transect−1 in macroalgal areas and 3.0 ± 1.39 ind. transect−1 in seagrass meadows). Coral reefs harbored higher abundances of adult fishes than macroalgal (Mixed linear model, F (2,40) = 6.76, P = 0.0025) and seagrass habitats (Mixed linear model, F (2,40) = 6,76, P = 0.0034). Further, macroalgal habitats held higher abundances of microphagous juveniles than seagrass habitats (ANOVA, F (2,45) = 3.248, P = 0.039), and slightly higher than in the coral reef sites, although this was not significant.
Boxplots showing abundance of size classes a fish > 5 cm and b fish < 5 cm of (all functional groups pooled) fishes in different habitat categories (coral reefs, macroalgal habitats and seagrass meadows) from the UVCs (50 m2). Each dot in figures represents a sample (1 UVC), boxes show median (thick line), 25th and 75th percentile. Error bars are 95% confidence intervals. One outlier (a UVC containing 125 adult fish) was removed from the coral reef habitat in the left figure
RUVs revealed a higher diversity of seagrass and algivorous species than the UVCs in all habitat types. For example, N. elegans which were observed feeding on macroalgae in the tethering videos were never observed in the UVCs. Furthermore, few Siganus sutor were observed in UVCs but were common in RUVs (Electronic Supplementary Material; Table 6S).
Environmental variables
Environmental variables identified as food resources targeted by the focal functional groups of fishes (macroalgae, seagrass, EAM, and calcareous rubble) were unevenly distributed between the habitat types (Electronic Supplementary Materials; Table 7S). Calcareous rubble was the most abundant food resource in both coral and macroalgal areas (50.1 ± 5.0 and 52.8 ± 4.6%, respectively). Seagrass habitats had low cover of all food resources except for seagrass (94.1%).
Environmental variables explaining fish abundances differed among the different habitat types (Table 3, Fig. 7, Electronic Supplementary Materials; Fig. 10S). In the coral reef sites, live coral cover and CCA cover had a positive influence on the abundance of all fish (herbivores, detritivores, and microphages pooled) (Table 3, Fig. 7, Electronic Supplementary materials; Fig. 10S). Likewise, EAM cover had a positive influence on fish abundance in coral reef habitats, both independently and in combination with CCA cover. Within the macroalgal beds, there were significant positive relationships between fish abundances and macroalgal height, macroalgal cover and rugosity (Table 3, Electronic Supplementary Materials; Fig. 10S). There was also a significant interaction between macroalgal height and rugosity (Linear regression, r2 = 0.43, F (3,16)=5.703, P = 0.019). In seagrass habitats, there were no significant relationships between fish abundances and any of the measured environmental variables.
Conceptualized illustration of benthic variables influencing abundance of herbivorous fish in a coral reef habitats and b macroalgal habitats. Black arrows denote variables having an effect on the total roving herbivorous/detritivorous fish community and grey arrows are variables only affecting a single functional group. Positive or negative relationships are indicated with (+) or (–), respectively. Symbols courtesy partly of IAN library, University of Maryland
When separating fish into functional groups, slightly different linear models explained abundances in different habitat types (Table 4, Electronic Supplementary Materials; Fig. 11S). In coral reef habitats, no model was found for seagrass and algivores and detritivores, and for microphages the best model included macroalgal cover, live coral cover, predatory fish abundance and CCA cover (Table 4). In the macroalgal areas, the most parsimonious model for explaining abundance of seagrass and algivores was predatory fish abundance and for microphages, macroalgal cover and macrophyte height (Table 4). In the seagrass habitats, there were no significant models.
Browser assays
Biomass loss of tethered algae was significantly higher in the coral reef habitats than in the two macrophyte habitats (mixed linear model, F = 25.454, P < 0.0001, Table 5), with biomass loss being slightly higher in the macroalgal habitats compared to the seagrass meadows. There was no difference in biomass loss between the two macroalgal species.
Macrophyte cover (both seagrass and fleshy macroalgae) in the surrounding habitat was the single significant factor explaining biomass loss of tethered macroalgae (linear regression, r2 = 0.436, F (1,16)=12.371, P =0.0028) (Fig. 8).
Very few fish were observed feeding on the tethered macroalgae in the RUVs, and all observations were from the coral reef sites (Chawe Kubwa, Kitutia and Milimani). Acanthurids were the most common fish observed; small (~ 25 cm) Naso brevirostris (n = 4), adult N. elegans (n = 2), N. vlamingii (n = 1) and one siganid species; Siganus sutor (n = 1). All observed fish targeted E. denticulatum, but bites were recorded on Sargassum aquifolium at the termination of the experiment.
Grazing scar inventory studies
Coral reef sites had significantly higher number and sizes of grazing scars compared to what was found in macroalgal habitats (Mixed linear model, F (1,110) = 16.397, P = 0.0001 and F (1,110) = 21.710, P < 0.0001, respectively) (Fig. 9).
a Number of grazing scars in coral reef habitats and macroalgal areas, b sizes of grazing scars (cm) in different habitats. Due to an unforeseen weather event, the sample size in macroalgae areas is smaller than in the coral reef habitats; ncoral = 78, nmacroalgae = 39. Illustrations from IAN library, University of Maryland
Discussion
To our knowledge, this is the first study examining habitat associations, distribution, and foraging patterns of seagrass and algivorous, detritivorous, and microphagous fishes from varying life stages across several shallow-water habitat types (coral reefs, macroalgae beds, and seagrass meadows) within a tropical seascape. As hypothesized, densities of focal functional groups of fishes and foraging patterns were not equally distributed within the seascape, but varied with habitats. Likewise, species composition of nominally herbivorous and detritivorous fishes also changed significantly with habitat, suggesting that fish communities in this study are subjected to, and to a certain extent, driven by bottom-up processes, which was most obvious for the microphagous parrotfish.
Life stages of microphagous parrotfish also varied with habitat; smaller individuals (< 5 cm) were more abundant in macroalgal habitats and larger individuals more common in coral reef and seagrass habitats. This was mirrored in the inventory of grazing scars, which were significantly smaller in macroalgal habitats compared to coral reef sites, likely reflecting the distribution patterns of ontogenetic stages of this functional group. Lower numbers of grazing scars in the macroalgal habitats might be the result of fishes feeding more intensely on epiphytes than on hard substrate surfaces. However, that would not explain the significant differences in sizes of grazing scars. Whether or not it was the same species using different habitats during distinct life stages was impossible to tell, as a majority of the juveniles were seldom identified to species level. Tano et al. (2017) also observed large numbers of juvenile parrotfish in macroalgal areas in Zanzibar, Tanzania, compared to neighboring habitats, suggesting that shallow areas in the Western Indian Ocean (WIO) dominated by canopy-forming macroalgae may serve as nurseries for this particular group of fishes.
Habitat features benefiting certain functional groups of fishes in one habitat might not be the same in another since different environmental variables explained fish abundances in different habitat types. “Live coral cover”, “CCA cover” and “CCA cover” in combination with “EAM cover”, had a positive effect on the abundance of fish (all functional groups pooled) in coral reef habitats, which is similar to results from other studies (Friedlander et al. 2003; Osuka et al. 2018). In the macroalgal beds, habitat quality variables such as “macrophyte height”, “macrophyte cover” and “rugosity”, had a positive effect, patterns that are consistent with studies of microhabitat selection of labrid fishes in both tropical (Lim et al. 2016) and temperate macroalgal areas (Fulton et al. 2016; van Lier et al. 2017). “Calcareous rubble”, which has been shown to have a strong positive effect on abundances of acanthurids and parrotfish (Russ et al. 2015, 2018), did not have any effects on abundance in the present study. This is probably due to the rather high cover of calcareous rubble in coral and macroalgal habitats (50.3 and 52.8%, respectively), implying that this is currently not a food-limiting resource on Mafia Island.
The positive relationship between “live coral cover” and nominally herbivorous fish abundances is probably due to the numerous feeding surfaces in the form of dead hard substrata this benthic category is associated with. Note that “live coral cover” was almost never the dominating substrate in UVCs (48.465 ± 5.26%), not even in the coral reef habitats, which all had a high degree of calcareous rubble. Although not a food source for the majority of the fishes in the study, CCA cover might indicate areas with low sedimentation, which are more attractive feeding grounds for many herbivorous fishes (Bellwood and Fulton 2008).
The lack of significant explanatory variables in the seagrass habitats is likely a consequence of the limited sample size and small value range within measured variables. Factors such as spatial arrangement within the seascape and distance to other habitats or deeper waters might be more important for structuring fish assemblages in these habitats (Gullström et al. 2008; Henderson et al. 2017).
Fish likely respond differently to habitat characteristics and environmental variables depending on ontogenetic stage (Macpherson 1998; Almany 2004). The differing bottom–up variables explaining fish abundance in different habitat types in the present study are probably reflecting shifts in habitat preferences for different life stages of fishes. Abundance of microphagous parrotfish was negatively associated with macroalgal cover in coral reef habitats, while in macroalgal areas the relationship was reversed and there was instead a positive effect of macroalgal cover and canopy height on parrotfish abundance. This is likely an effect of smaller/younger fish seeking shelter from predators in the form of structural complexity, provided by height and cover of macroalgae, and by hard underlying structure (rugosity) (van Lier et al. 2018). The negative relationship with macroalgal cover is in line with results from previous studies from the Great Barrier Reef, Australia, where dense macroalgal stands of Sargassum spp. in coral reef environments were found to induce avoidance behavior of roving herbivores (Hoey and Bellwood 2011). There is likely a trade-off between food and shelter changing throughout ontogeny in these fishes, but also between different types of shelter, provided by live coral or by microhabitat topography in the macroalgal areas. Shelter from predators is an important resource in coral reef environments (Kerry and Bellwood 2016), and can be size-specific, as inter-structural spaces which are smaller than the potential predator width will act as shelter for a fish that fit within the space provided (Bartholomew et al. 2000; Gullström et al. 2011). Macroalgal areas with both soft and hard complexity in the form of fronds and coral rubble might therefore provide sufficient shelter for smaller individuals, while it is advantageous for larger fish to keep to areas with larger structures, as provided by live coral. Consequently, macroalgal areas in shallow areas with tall canopies and high macrophyte cover might be advantageous for the survival and recruitment of parrotfish and hence contribute to the replenishment of important herbivores and microphages on coral reefs. This is strengthened by recent studies showing that tropical macroalgal habitats host high numbers of juvenile and subadult parrotfish, suggesting that they are important nursery grounds for these species and that linkages between habitats should be highlighted in coral reef management (Tano et al. 2017; Eggertsen et al. 2017). However, we would like to stress that deteriorated coral reefs with high cover of fleshy macroalgae is not what we define as “natural macroalgal areas”, and that nursery habitat qualities might depend on where in the seascape a habitat is located. This is, to some extent, illustrated in the present study as high macroalgal cover in the coral reef habitat had a negative effect on the abundance of parrotfish while it was positive in another habitat type.
Algal consumption, measured as the removal of macroalgae from assays, was the highest within coral reef sites, followed by macroalgal beds, and seagrass meadows. However, it does not necessarily mean that grazing intensity per se was the highest in coral reef habitats in the present study. Since biomass loss of tethered macroalgae was negatively related to macroalgal cover in the surrounding habitat, it suggests a density-dependent process, where grazing pressure might be “smoothed out” in areas with high macrophyte cover. Macroalgal feeding fish might therefore not be able to eradicate dense macroalgal stands or “blooms” on coral reefs, if they are already established. Solitary fronds may, however, be targeted and limited by browsing fish since the survival and growth of newly settled Sargassum spp. have been shown to be strongly suppressed by top–down control (Diaz-Pulido and McCook 2003).
Understanding how habitats structure fish assemblages in spatially heterogeneous environments is imperative because it will improve habitat-based approaches of management and conservation. As tropical natural macroalgal habitats have been shown to host high numbers of juvenile and subadult scarine labrids, these linkages between reefs and macroalgal beds should be considered in seascape management (Tano et al. 2017; Eggertsen et al. 2017; Fulton et al. 2019), even though these habitats have only been recently acknowledged. Furthermore, the availability of nursery grounds and connectivity to coral reefs by nominally herbivorous fishes have been found to enhance herbivory (Adam et al. 2011; Olds et al. 2012, Harborne et al. 2016). Therefore, the protection of important ecological functions urges the preservation of seascapes comprising multiple habitats with a high degree of connectedness. This is particularly important in the WIO area, where a large part of the population is living within the coastal region and is heavily dependent on the local marine resources.
Conclusion
The current study illustrates that multiple habitat types within the seascape matrix are needed to host a diverse community of herbivorous and detritivorous fishes. Further, the study suggests that in the absence of overfishing, the herbivorous community may be driven by bottom–up mechanisms, with emphasis on microphagous parrotfish. The study also highlights the importance of habitat quality in terms of cover and complexity for maintaining certain ecological functions. These features might vary across habitats, and affect life stages of nominally herbivorous fishes differently. A habitat feature (e.g., macroalgal cover on coral reefs) that is detrimental for a certain age class of a functional group of fishes (e.g., adult microphages) might simultaneously be important in an adjacent habitat (e.g., natural macroalgal beds) for a different life stage of a fish (juvenile) of the same functional group. This highlights the need of a holistic view of the shallow tropical seascape for efficient management and conservation.
References
Adam T, Schmitt RJ, Holbrook SJ, Brooks AJ, Edmunds PJ, Carpenter RC, Bernardi G (2011) Herbivoty, connectivity and ecosystem resilience: response of a coral reef to a large-scale perturbation. PLoS One 6(8):e23717. https://doi.org/10.1371/journal.pone.0023717
Adam T, Burkepile D, Ruttenberg B, Paddack M (2015) Herbivory and the resilience of Caribbean coral reefs: knowledge gaps and implications for management. Mar Ecol Prog Ser 520:1–20. https://doi.org/10.3354/meps11170
Almany GR (2004) Differential effects of habitat complexity, predators and competitors on abundance of juvenile and adult coral reef fishes. Oecologia 141:105–113. https://doi.org/10.1007/s00442-004-1617-0
Alonso Aller E, Gullström M, Eveleens Maarse FKJ, Gren M, Nordlund LM, Jiddawi N, Eklöf JS (2014) Single and joint effects of regional- and local-scale variables on tropical seagrass fish assemblages. Mar Biol 161:2395–2405. https://doi.org/10.1007/s00227-014-2514-7
Bartholomew A, Diaz R, Cicchetti G (2000) New dimensionless indices of structural habitat complexity: predicted and actual effects on a predator’s foraging success. Mar Ecol Prog Ser 206:45–58. https://doi.org/10.3354/meps206045
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Bell J, Galzin R (1984) Influence of live coral cover on coral-reef fish communities. Mar Ecol Prog Ser 15:265–274. https://doi.org/10.3354/meps015265
Bellwood DR, Fulton CJ (2008) Sediment-mediated suppression of herbivory on coral reefs: decreasing resilience to rising sea-levels and climate change? Limnol Oceanogr 53:2695–2701
Bellwood DR, Hughes TP, Folke C, Nystrom M (2004) Confronting the coral reef crisis. Nature 429:827
Bellwood DR, Goatley CHR, Brandl SJ, Bellwood O (2014) Fifty million years of herbivory on coral reefs: fossils, fish and functional innovations. Proc R Soc B Biol Sci 281:20133046–20133046. https://doi.org/10.1098/rspb.2013.3046
Berkström C, Gullström M, Lindborg R, Mwandya AW, Yahya SAS, Kautsky N, Nyström M (2012) Exploring ‘knowns’ and ‘unknowns’ in tropical seascape connectivity with insights from East African coral reefs. Estuar Coast Shelf Sci 107:1–21. https://doi.org/10.1016/j.ecss.2012.03.020
Berkström C, Jörgensen T, Hellström M (2013) Ecological connectivity and niche differentiation between two closely related fish species in the mangrove-seagrass-coral reef continuum. Mar Ecol Prog Ser 477:201–215. https://doi.org/10.3354/meps10171
Bonaldo R, Bellwood D (2008) Size-dependent variation in the functional role of the parrotfish Scarus rivulatus on the Great Barrier Reef, Australia. Mar Ecol Prog Ser 360:237–244. https://doi.org/10.3354/meps07413
Bonaldo RM, Bellwood DR (2009) Dynamics of parrotfish grazing scars. Mar Biol 156:771–777. https://doi.org/10.1007/s00227-009-1129-x
Bonaldo R, Krajewski J, Bellwood D (2011) Relative impact of parrotfish grazing scars on massive Porites corals at Lizard Island, Great Barrier Reef. Mar Ecol Prog Ser 423:223–233. https://doi.org/10.3354/meps08946
Bonaldo R, Hoey A, Bellwood D (2014) The Ecosystem roles of parrotfishes on tropical reefs. In: Hughes R, Hughes D, Smith I (eds) Oceanography and marine biology. CRC Press, Boca Raton, pp 81–132
Chabanet P, Ralambondrainy H, Amanieu M, Faure G, Galzin R (1997) Relationships between coral reef substrata and fish. Coral Reefs 16:93–102. https://doi.org/10.1007/s003380050063
Choat JH, Clements K, Robbins W (2002) The trophic status of herbivorous fishes on coral reefs. Mar Biol 140:613–623. https://doi.org/10.1007/s00227-001-0715-3
Clarke KR (1993) Non-parametric multivariate analyses of changes in community structure. Austral Ecol 18:117–143. https://doi.org/10.1111/j.1442-9993.1993.tb00438.x
Clements KD, Choat JH (2018) Nutritional Ecology of parrotfishes (Scarinae, Labridae). In: Hoey AS, Bonaldo R (eds) Biology of parrotfishes. CRC Press, Taylor & Francis Group, Boca Raton, pp 42–68 (ISBN 9781482224016 )
Clements KD, German DP, Piché J, Tribollet A, Choat JH (2017) Integrating ecological roles and trophic diversification on coral reefs: multiple lines of evidence identify parrotfishes as microphages. Biol J Linn Soc 120:729–751
Dahlgren CP, Eggleston DB (2000) Ecological processes underlying ontogenetic habitat shifts in a coral reef fish. Ecology 81:2227–2240. https://doi.org/10.1890/0012-9658(2000)081%5b2227:EPUOHS%5d2.0.CO;2
Diaz-Pulido G, McCook LJ (2003) Relative roles of herbivory and nutrients in the recruitment of coral-reef seaweeds. Ecology 84:2026–2033
Eggertsen L, Ferreira CEL, Fontoura L, Kautsky N, Gullström M, Berkström C (2017) Seaweed beds support more juvenile reef fish than seagrass beds: carrying capacity in a south-western Atlantic tropical seascape. Estuar Coast Shelf Sci. https://doi.org/10.1016/j.ecss.2017.06.041
Evans RD, Wilson SK, Field SN, Moore J (2014) Importance of macroalgal fields as coral reef fish nursery habitat in north-west Australia. Mar Biol 161:599–607. https://doi.org/10.1007/s00227-013-2362-x
Fox RJ, Bellwood DR (2008a) Direct versus indirect methods of quantifying herbivore grazing impact on a coral reef. Mar Biol 154:325–334. https://doi.org/10.1007/s00227-008-0927-x
Fox RJ, Bellwood DR (2008b) Remote video bioassays reveal the potential feeding impact of the rabbitfish Siganus canaliculatus (f: siganidae) on an inner-shelf reef of the Great Barrier Reef. Coral Reefs 27:605–615. https://doi.org/10.1007/s00338-008-0359-6
Friedlander AM, Parrish JD (1998) Habitat characteristics affecting fish assemblages on a Hawaiian coral reef. J Exp Mar Biol Ecol 224:1–30. https://doi.org/10.1016/S0022-0981(97)00164-0
Friedlander AM, Brown EK, Jokiel PL, Smith WR, Rodgers KS (2003) Effects of habitat, wave exposure, and marine protected area status on coral reef fish assemblages in the Hawaiian archipelago. Coral Reefs 22:291–305. https://doi.org/10.1007/s00338-003-0317-2
Froese R, Pauly D (eds) (2017) FishBase. World Wide Web electronic publication. https://www.fishbase.org, version 2017
Fulton CJ, Depczynski M, Holmes TH, Noble MM, Radford B, Wernberg T, Wilson SK (2014) Sea temperature shapes seasonal fluctuations in seaweed biomass within the Ningaloo coral reef ecosystem. Limnol Oceanogr 59:156–166. https://doi.org/10.4319/lo.2014.59.1.0156
Fulton CJ, Noble MN, Radford B, Gallen C, Harasti D (2016) Microhabitat selectivity underpins regional indicators of fish abundance and replenishment. Ecol Indic 70:222–231. https://doi.org/10.1016/j.ecolind.2016.06.032
Fulton CJ, Abesamis RA, Berkström C, Depczynski M, Graham NAJ, Holmes TH, Kulbicki M, Noble MM, Radford BT, Tano S, Tinkler P, Wernberg T, Wilson SK (2019) Form and function of tropical macroalgal reefs in the Anthropocene. Funct Ecol. https://doi.org/10.1111/1365-2435.13282
Ganesan M, Thiruppathi S, Sahu N, Rengarajan N, Veeragurunathan V, Jha B (2006) In situ observations on preferential grazing of seaweeds by some herbivores. Current Science 91(9):1256–1260
Garpe KC, Öhman MC (2003) Coral and fish distribution patterns in Mafia Island Marine Park, Tanzania: fish–habitat interactions. Hydrobiologia 498:191–211
Gaspare L, Bryceson I, Kulindwa K (2015) Complementarity of fishers’ traditional ecological knowledge and conventional science: contributions to the management of groupers (Epinephelinae) fisheries around Mafia Island, Tanzania. Ocean Coast Manag 114:88–101. https://doi.org/10.1016/j.ocecoaman.2015.06.011
Gillespie RD, Critchley AT (1999a) Phenology of Sargassum spp. (Sargassaceae, Phaeophyta) from Reunion Rocks, KwaZulu-Natal, South Africa. In: Kain JM, Brown MT, Lahaye M (eds) Sixteenth International Seaweed Symposium. Springer, Dordrecht, pp 201–210
Gillespie RD, Critchley AT (1999b) Phenology of Sargassum spp. (Sargassaceae, Phaeophyta) from Reunion Rocks, KwaZulu-Natal, South Africa. In: Sixteenth International Seaweed Symposium. Springer, pp 201–210
Goatley C, Bellwood D (2010) Biologically mediated sediment fluxes on coral reefs: sediment removal and off-reef transportation by the surgeonfish Ctenochaetus striatus. Mar Ecol Prog Ser 415:237–245. https://doi.org/10.3354/meps08761
Graham NAJ, Nash KL (2013) The importance of structural complexity in coral reef ecosystems. Coral Reefs 32:315–326. https://doi.org/10.1007/s00338-012-0984-y
Gratwicke B, Speight MR (2005) The relationship between fish species richness, abundance and habitat complexity in a range of shallow tropical marine habitats. J Fish Biol 66:650–667
Gullström M, Bodin M, Nilsson P, Öhman M (2008) Seagrass structural complexity and landscape configuration as determinants of tropical fish assemblage composition. Mar Ecol Prog Ser 363:241–255. https://doi.org/10.3354/meps07427
Gullström M, Berkström C, Öhman MC, Bodin M, Dahlberg M (2011) Scale-dependent patterns of variability of a grazing parrotfish (Leptoscarus vaigiensis) in a tropical seagrass-dominated seascape. Mar Biol 158:1483–1495. https://doi.org/10.1007/s00227-011-1665-z
Harborne AR, Nagelkerken I, Wolff NH, Bozec Y-M, Dorenbosch M, Grol MGG, Mumby PJ (2016) Direct and indirect effects of nursery habitats on coral-reef fish assemblages, grazing pressure and benthic dynamics. Oikos 125(7):957–967
Hay ME (1981) Herbivory, algal distribution, and the maintenance of between-habitat diversity on a tropical fringing reef. Am Nat 118:520–540
Henderson C, Olds A, Lee S, Gilby B, Maxwell P, Connolly R, Stevens T (2017) Marine reserves and seascape context shape fish assemblages in seagrass ecosystems. Mar Ecol Prog Ser 566:135–144. https://doi.org/10.3354/meps12048
Hoey AS, Bellwood DR (2011) Suppression of herbivory by macroalgal density: a critical feedback on coral reefs? Macroalgal density suppresses herbivory. Ecol Lett 14:267–273. https://doi.org/10.1111/j.1461-0248.2010.01581.x
Horrill JC, Darwall WR, Ngoile M (1996) Development of a marine protected area: Mafia Island, Tanzania. Ambio, pp 50–57
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biom J 50(3):346–363
Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, Moltschaniwskyj N, Pratchett MS, Steneck RS, Willis B (2007) Phase Shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17:360–365. https://doi.org/10.1016/j.cub.2006.12.049
Jackson EJ, Donovan M, Cramer K, Lam V (2014) Status and trends of caribbean coral reefs: 1970–2012, p 306
Kerry JT, Bellwood DR (2016) Competition for shelter in a high-diversity system: structure use by large reef fishes. Coral Reefs 35:245–252. https://doi.org/10.1007/s00338-015-1362-3
Kimirei IA, Nagelkerken I, Griffioen B, Wagner C, Mgaya YD (2011) Ontogenetic habitat use by mangrove/seagrass-associated coral reef fishes shows flexibility in time and space. Estuar Coast Shelf Sci 92:47–58. https://doi.org/10.1016/j.ecss.2010.12.016
Lim IE, Wilson SK, Holmes TH, Noble MM, Fulton CJ (2016) Specialization within a shifting habitat mosaic underpins the seasonal abundance of a tropical fish. Ecosphere. https://doi.org/10.1002/ecs2.1212
Longo GO, Floeter SR (2012) Comparison of remote video and diver’s direct observations to quantify reef fishes feeding on benthos in coral and rocky reefs. J Fish Biol 81:1773–1780. https://doi.org/10.1111/j.1095-8649.2012.03441.x
Lugendo BR, Nagelkerken I, Jiddawi N, Mgaya YD, Van Der Velde G (2007) Fish community composition of a tropical nonestuarine embayment in Zanzibar, Tanzania. Fish Sci 73:1213–1223
Macpherson E (1998) Ontogenetic shifts in habitat use and aggregation in juvenile sparid fishes. J Exp Mar Biol Ecol 220:127–150. https://doi.org/10.1016/S0022-0981(97)00086-5
McClanahan TR, Arthur R (2001) The effect of marine reserves and habitat on populations of East African coral reef. Ecol Appl 11:559–569. https://doi.org/10.1890/1051-0761(2001)011%5b0559:TEOMRA%5d2.0.CO;2
McClanahan TR, Hendrick V, Rodrigues MJ, Polunin NVC (1999) Varying responses of herbivorous and invertebrate-feeding fishes to macroalgal reduction on a coral reef. Coral Reefs 18:195–203
McClanahan T, Muthiga N, Mangi S (2001) Coral and algal changes after the 1998 coral bleaching: interaction with reef management and herbivores on Kenyan reefs. Coral Reefs 19:380–391. https://doi.org/10.1007/s003380000133
McClanahan TR, Ateweberhan M, Muhando CA, Maina J, Mohammed MS (2007) Effects of climate and seawater temperature variation on coral bleaching and mortality. Ecol Monogr 77:503–525
McClanahan TR, Cinner J, Kamukuru AT, Abunge C, Ndagala J (2008) Management preferences, perceived benefits and conflicts among resource users and managers in the Mafia Island Marine Park, Tanzania. Environ Conserv 35:340. https://doi.org/10.1017/S0376892908005250
Messmer V, Jones GP, Munday PL, Holbrook SJ, Schmitt RJ, Brooks AJ (2011) Habitat biodiversity as a determinant of fish community structure on coral reefs. Ecology 92:2285–2298. https://doi.org/10.1890/11-0037.1
Montgomery LW, Myrberg AA Jr, Fishelson L (1989) Feeding ecology of surgeonfishes (Acanthuridae) in the northern Red Sea, with particular reference to Acanthurus nigrofuscus (Forsskål). J Exp Mar Biol Ecol 132:179–207
Mörk E, Sjöö GL, Kautsky N, McClanahan TR (2009) Top–down and bottom–up regulation of macroalgal community structure on a Kenyan reef. Estuar Coast Shelf Sci 84:331–336. https://doi.org/10.1016/j.ecss.2009.03.033
Mumby PJ (2006) Fishing, trophic cascades, and the process of grazing on coral reefs. Science 311:98–101. https://doi.org/10.1126/science.1121129
Nagelkerken I, van der Velde G, Gorissen MW, Meijer GJ, Van’t Hof T, den Hartog C (2000) Importance of mangroves, seagrass beds and the shallow coral reef as a nursery for important coral reef fishes, using a visual census technique. Estuar Coast Shelf Sci 51:31–44. https://doi.org/10.1006/ecss.2000.0617
Oksanen J, Blanchet FG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHM, Szoecs E, Wagner H (2017) Vegan: Community Ecology Package Package ‘Vegan’. R package version 2.4-5. https://CRAN.R-project.org/package=vegan
Olds A, Pitt KA, Maxwell PS, Connolly RM (2012) Synergistic effects of reserves and connectivity on ecological resilience. J Appl Ecol 49:1195–1203. https://doi.org/10.1111/jpe.12002
Osuka K, Kochzius M, Vanreusel A, Obura D, Samoilys M (2018) Linkage between fish functional groups and coral reef benthic habitat composition in the Western Indian Ocean. J Mar Biol Assoc UK 98(2):387–400
Robbins BD, Bell SS (2000) Dynamics of a subtidal seagrass landscape: seasonal and annual change in relation to water depth. Ecology 81:1193–1205. https://doi.org/10.1890/0012-9658(2000)081%5b1193:DOASSL%5d2.0.CO;2
Roff G, Mumby PJ (2012) Global disparity in the resilience of coral reefs. Trends Ecol Evol 27(7):404–413. https://doi.org/10.1016/j.tree.2012.04.007
Russ GR, Questel S-LA, Rizzari JR, Alcala AC (2015) The parrotfish–coral relationship: refuting the ubiquity of a prevailing paradigm. Mar Biol 162:2029–2045. https://doi.org/10.1007/s00227-015-2728-3
Russ GR, Payne CS, Bergseth BJ, Rizzari JR, Abesamis RA, Alcala AC (2018) Decadal-scale response of detritivorous surgeonfishes (family Acanthuridae) to no-take marine reserve protection and changes in benthic habitat. J Fish Biol 93:887–900. https://doi.org/10.1111/jfb.13809
Sabater MG, Tofaeono SP (2007) Scale and benthic composition effects on biomass and trophic group distribution of reef fishes in american samoa 1. Pac Sci 61:503–520. https://doi.org/10.2984/1534-6188(2007)61%5b503:SABCEO%5d2.0.CO;2
Samoilys M, Roche R, Koldewey H, Turner J (2018) Patterns in reef fish assemblages: insights from the Chagos Archipelago. PLoS One 13:e0191448. https://doi.org/10.1371/journal.pone.0191448
Steneck RS, Bellwood DR, Hay ME (2017) Herbivory in the marine realm. Curr Biol 27:R484–R489. https://doi.org/10.1016/j.cub.2017.04.021
Tano S, Eggertsen M, Wikström SA, Berkström C, Buriyo AS, Halling C (2016) Tropical seaweed beds are important habitats for mobile invertebrate epifauna. Estuar Coast Shelf Sci 183:1–12. https://doi.org/10.1016/j.ecss.2016.10.010
Tano SA, Eggertsen M, Wikström SA, Berkström C, Buriyo AS, Halling C (2017) Tropical seaweed beds as important habitats for juvenile fish. Mar Freshw Res. https://doi.org/10.1071/mf16153
Taylor DI, Schiel DR (2010) Algal populations controlled by fish herbivory across a wave exposure gradient on southern temperate shores. Ecology 91:201–211
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Tebbett SB, Goatley CHR, Bellwood DR (2017) Clarifying functional roles: algal removal by the surgeonfishes Ctenochaetus striatus and Acanthurus nigrofuscus. Coral Reefs 36:803–813. https://doi.org/10.1007/s00338-017-1571-z
Tootell JS, Steele MA (2016) Distribution, behavior, and condition of herbivorous fishes on coral reefs track algal resources. Oecologia 181:13–24. https://doi.org/10.1007/s00442-015-3418-z
Unsworth RKF, Taylor JD, Powell A, Bell JJ, Smith DJ (2007) The contribution of scarid herbivory to seagrass ecosystem dynamics in the Indo-Pacific. Estuar Coast Shelf Sci 74:53–62. https://doi.org/10.1016/j.ecss.2007.04.001
van Lier JR, Harasti D, Laird R, Noble MM, Fulton CJ (2017) Importance of soft canopy structure for labrid fish communities in estuarine mesohabitats. Mar Biol. https://doi.org/10.1007/s00227-017-3068-2
van Lier JR, Wilson SK, Depczynski M, Wenger LN, Fulton CJ (2018) Habitat connectivity and complexity underpin fish community structure across a seascape of tropical macroalgae meadows. Landsc Ecol. https://doi.org/10.1007/s10980-018-0682-4
van Montfrans J, Wetzel RL, Orth RJ (1984) Epiphyte-grazer relationships in seagrass meadows: consequences for seagrass growth and production. Estuaries 7:289. https://doi.org/10.2307/1351615
Vergés A, Alcoverro T, Ballesteros E (2009) Role of fish herbivory in structuring the vertical distribution of canopy algae Cystoseira spp. in the Mediterranean Sea. Mar Ecol Prog Ser 375:1–11. https://doi.org/10.3354/meps07778
Wenger L, van Lier J, Fulton C (2018) Microhabitat selectivity shapes the seascape ecology of a carnivorous macroalgae-associated tropical fish. Mar Ecol Prog Ser 590:187–200. https://doi.org/10.3354/meps12473
Wilson SK, Depczynski M, Fisher R, Holmes TH, O’Leary RA, Tinkler P (2010) Habitat associations of juvenile fish at ningaloo reef, Western Australia: the importance of coral and algae. PLoS One 5:e15185. https://doi.org/10.1371/journal.pone.0015185
Wulff J (2017) Bottom-up and top-down controls on coral reef sponges: disentangling within-habitat and between-habitat processes. Ecology 98:1130–1139. https://doi.org/10.1002/ecy.1754
Zuur AF, Ieno EN, Elphick CS (2010) A protocol for data exploration to avoid common statistical problems: data exploration. Methods Ecol Evol 1:3–14. https://doi.org/10.1111/j.2041-210X.2009.00001.x
Acknowledgements
We wish to thank the two anonymous reviewers who contributed with valuable and constructive input to the manuscript. We also wish to thank Dr. Amelia Buriyo at the University of Dar es Salaam, Karlina See Kee, Yessenia Rojas, the staff at the Mafia Island Marine Park (MIMP), Big Blu Mafia Island Dive Centre and Mafia Island Diving for their support during field work in Mafia Island. Last but not least, we want to thank our Tanzanian boat captain, Mr. Nahoda Salamala for his invaluable help and local knowledge. The study in Mafia was supported by the Swedish Research Council (Grant nos. 2015-05848, 2015-01257, E0344801) and the National Science Foundation Graduate Research Fellowship and the Graduate Research Opportunities Worldwide (Grant no. 1144244).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. Funding was provided by the Swedish Research Council, the National Science Foundation Graduate Research Fellowship and the Graduate Research Opportunities Worldwide.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals and sampling were followed in the current study. All necessary approvals have been obtained. Field sampling in Tanzania was performed with permission from Tanzania Commission for Science and Technology (COSTECH), research permits no 2016-291-NA-2016-217 (M. Eggertsen), no 2016-294-NA-2016-217 (D. Chacin) and no 2016-293-NA-2016-217 (C. Åkerlund).
Additional information
Responsible Editor: K.D. Clements.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by Undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Eggertsen, M., Chacin, D.H., Åkerlund, C. et al. Contrasting distribution and foraging patterns of herbivorous and detritivorous fishes across multiple habitats in a tropical seascape. Mar Biol 166, 51 (2019). https://doi.org/10.1007/s00227-019-3498-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-019-3498-0