Abstract
Violative chemical residues in animal-derived food products affect food safety globally and have impact on the trade of international agricultural products. The Food Animal Residue Avoidance Databank program has been developing scientific tools to provide appropriate withdrawal interval (WDI) estimations after extralabel drug use in food animals for the past three decades. One of the tools is physiologically based pharmacokinetic (PBPK) modeling, which is a mechanistic-based approach that can be used to predict tissue residues and WDIs. However, PBPK models are complicated and difficult to use by non-modelers. Therefore, a user-friendly PBPK modeling framework is needed to move this field forward. Flunixin was one of the top five violative drug residues identified in the United States from 2010 to 2016. The objective of this study was to establish a web-based user-friendly framework for the development of new PBPK models for drugs administered to food animals. Specifically, a new PBPK model for both cattle and swine after administration of flunixin meglumine was developed. Population analysis using Monte Carlo simulations was incorporated into the model to predict WDIs following extralabel administration of flunixin meglumine. The population PBPK model was converted to a web-based interactive PBPK (iPBPK) framework to facilitate its application. This iPBPK framework serves as a proof-of-concept for further improvements in the future and it can be applied to develop new models for other drugs in other food animal species, thereby facilitating the application of PBPK modeling in WDI estimation and food safety assessment.




Similar content being viewed by others
References
Anissimov YG, Roberts MS (2014) Mathematical models for topical and transdermal drug products. In: Shah VP, Maibach HI, Jenner J (eds) Topical drug bioavailability, bioequivalence and penetration, 2nd edn. Springer, New York, pp 249–298
Baron KT, Hindmarsh AC, Petzold LR, Gillespie B, Margossian C, Metrum Research Group LLC (2018) mrgsolve: simulation from ODE-based population PK/PD and systems pharmacology models. R package version 0.8.12. https://metrumrg.com/try-open-source-tools/. Accessed 14 Nov 2018
Baynes R, Riviere JE (2014) Strategies for reducing drug and chemical residues in food animals: international approaches to residue avoidance, management, and testing. Wiley, Hoboken
Bois FY (2010) Physiologically based modelling and prediction of drug interactions. Basic Clin Pharmacol Toxicol 106(3):154–161
Bon C, Toutain PL, Concordet D, Gehring R, Martin-Jimenez T, Smith J, Pelligand L, Martinez M, Whittem T, Riviere JE, Mochel JP (2018) Mathematical modeling and simulation in animal health. Part III: using nonlinear mixed-effects to characterize and quantify variability in drug pharmacokinetics. J Vet Pharmacol Ther 41(2):171–183
Brown RP, Delp MD, Lindstedt SL, Rhomberg LR, Beliles RP (1997) Physiological parameter values for physiologically based pharmacokinetic models. Toxicol Ind Health 13(4):407–484
Buur JL, Baynes RE, Craigmill AL, Riviere JE (2005) Development of a physiologic-based pharmacokinetic model for estimating sulfamethazine concentrations in swine and application to prediction of violative residues in edible tissues. Am J Vet Res 66(10):1686–1693
Buur JL, Baynes RE, Smith GW, Riviere JE (2006a) Pharmacokinetics of flunixin meglumine in swine after intravenous dosing. J Vet Pharmacol Ther 29(5):437–440
Buur JL, Baynes RE, Smith GW, Riviere JE (2006b) Use of probabilistic modeling within a physiologically based pharmacokinetic model to predict sulfamethazine residue withdrawal times in edible tissues in swine. Antimicrob Agents Chemother 50(7):2344–2351
Carpenter B, Gelman A, Hoffman M, Lee D, Goodrich B, Betancourt M, Brubaker M, Guo J, Li P, Riddell A (2017) Stan: a probabilistic programming language. J Stat Softw 76(1):1–32
Cheng Y-H, Lin Y-J, You S-H, Yang Y-F, How C-M, Tseng Y-T, Chen W-Y, Liao C-M (2016) Assessing exposure risks for freshwater tilapia species posed by mercury and methylmercury. Ecotoxicology 25(6):1181–1193
Chittenden JT, Riviere JE (2016) Assessment of penetrant and vehicle mixture properties on transdermal permeability using a mixed effect pharmacokinetic model of ex vivo porcine skin. Biopharm Drug Dispos 37(7):387–396
Chou WC, Lin Z (2019) Bayesian evaluation of a physiologically based pharmacokinetic (PBPK) model for perfluorooctane sulfonate (PFOS) to characterize the interspecies uncertainty between mice, rats, monkeys, and humans: development and performance verification. Environ Int (in press)
Clewell RA, Clewell HJ (2008) Development and specification of physiologically based pharmacokinetic models for use in risk assessment. Regul Toxicol Pharmacol 50:129e143
Clewell RA, Gearhart JM (2002) Pharmacokinetics of toxic chemicals in breast milk: use of PBPK models to predict infant exposure. Environ Health Perspect 110(6):A333–A337
Cohen Hubal EA, Wetmore BA, Wambaugh JF, El-Masri H, Sobus JR, Bahadori T (2018) Advancing internal exposure and physiologically-based toxicokinetic modeling for 21st-century risk assessments. J Expo Sci Environ Epidemiol 29:11–20
Covington TR, Robinan Gentry P, Van Landingham CB, Andersen ME, Kester JE, Clewell HJ (2007) The use of Markov chain Monte Carlo uncertainty analysis to support a Public Health Goal for perchloroethylene. Regul Toxicol Pharmacol 47(1):1–18
Craigmill A (2003) A physiologically based pharmacokinetic model for oxytetracycline residues in sheep. J Vet Pharmacol Ther 26(1):55–63
Craigmill AL, Riviere JE, Webb AI (2006) Tabulation of FARAD comparative and veterinary pharmacokinetic data. Blackwell Pub, Ames
Edginton AN, Schmitt W, Willmann S (2006) Development and evaluation of a generic physiologically based pharmacokinetic model for children. Clin Pharmacokinet 45(10):1013–1034
El-Masri H, Kleinstreuer N, Hines RN, Adams L, Tal T, Isaacs K, Wetmore BA, Tan YM (2016) Integration of life-stage physiologically based pharmacokinetic models with adverse outcome pathways and environmental exposure models to screen for environmental hazards. Toxicol Sci 152(1):230–243
Elwell-Cuddy T, Li M, KuKanich B, Lin Z (2018) The construction and application of a population physiologically based pharmacokinetic model for methadone in Beagles and Greyhounds. J Vet Pharmacol Ther 41(5):670–683
EMEA (1999) Committee for veterinary medicinal products flunixin summary reports (1). http://www.ema.europa.eu/docs/en_GB/document_library/Maximum_Residue_Limits_-_Report/2009/11/WC500014324.pdf. Accessed 14 Nov 2018
EPA (2006) Approaches for the application of physiologically based pharmacokinetic (PBPK) models and supporting data in risk assessment. Final Report EPA/600/R-05/043A, Washington, DC, USA, 2006. http://ofmpub.epa.gov/eims/eimscomm.getfile?p_download_id=458188. Accessed 25 Feb 2019
FDA (1998) Freedom of information. NADA 101-479. BANAMINE (flunixin meglumine) Injectable Solution. US Food and Drug Administration, Silver Spring, MD. https://wayback.archive-it.org/7993/20170406083138/https://www.fda.gov/downloads/AnimalVeterinary/Products/ApprovedAnimalDrugProducts/FOIADrugSummaries/ucm064905.pdf. Accessed 14 Nov 2018
FDA (2005) Freedom of information. NADA 101-479. BANAMINE-S (flunixin meglumine) Injectable Solution. U.S. Food and Drug Administration, Silver Spring, MD. https://animaldrugsatfda.fda.gov/adafda/app/search/public/document/downloadFoi/356. Accessed 14 Nov 2018
FDA (2012) Regulation of carcinogenic compounds used in food-producing animals. Code of federal regulations title 21, Parts 500.82. https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=500.82. Accessed 14 Nov 2018
FDA (2018) Physiologically based pharmacokinetic analyses—format and content. Guidance for Industry. https://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM531207.pdf. Accessed 25 Feb 2019
Henri J, Carrez R, Meda B, Laurentie M, Sanders P (2017) A physiologically based pharmacokinetic model for chickens exposed to feed supplemented with monensin during their lifetime. J Vet Pharmacol Ther 40(4):370–382
Horii Y, Ikenaga M, Shimoda M, Kokue E (2004) Pharmacokinetics of flunixin in the cat: enterohepatic circulation and active transport mechanism in the liver. J Vet Pharmacol Ther 27(2):65–69
Howard JT, Baynes RE, Brooks JD, Yeatts JL, Bellis B, Ashwell MS, Routh P, O’Nan AT, Maltecca C (2014) The effect of breed and sex on sulfamethazine, enrofloxacin, fenbendazole and flunixin meglumine pharmacokinetic parameters in swine. J Vet Pharmacol Ther 37(6):531–541
Hsieh NH, Reisfeld B, Bois FY, Chiu WA (2018) Applying a global sensitivity analysis workflow to improve the computational efficiencies in physiologically-based pharmacokinetic modeling. Front Pharmacol 9:588
Jaroszewski J, Jedziniak P, Markiewicz W, Grabowski T, Chrostowska M, Szprengier-Juszkiewicz T (2008) Pharmacokinetics of flunixin in mature heifers following multiple intravenous administration. Pol J Vet Sci 11(3):199–203
Kim SJ, Shin H, Lee YB, Cho HY (2018) Sex-specific risk assessment of PFHxS using a physiologically based pharmacokinetic model. Arch Toxicol 92(3):1113–1131
Kissell LW, Smith GW, Leavens TL, Baynes RE, Wu H, Riviere JE (2012) Plasma pharmacokinetics and milk residues of flunixin and 5-hydroxy flunixin following different routes of administration in dairy cattle. J Dairy Sci 95(12):7151–7157
Kissell LW, Brinson PD, Gehring R, Tell LA, Wetzlich SE, Baynes RE, Smith GW (2016) Pharmacokinetics and tissue elimination of flunixin in veal calves. Am J Vet Res 77(6):634–640
Kleinhenz MD, Van Engen NK, Gorden PJ, KuKanich B, Rajewski SM, Walsh P, Coetzee JF (2016) The pharmacokinetics of transdermal flunixin meglumine in Holstein calves. J Vet Pharmacol Ther 39(6):612–615
Konigsson K, Torneke K, Engeland IV, Odensvik K, Kindahl H (2003) Pharmacokinetics and pharmacodynamic effects of flunixin after intravenous, intramuscular and oral administration to dairy goats. Acta Vet Scand 44(3–4):153–159
KuKanich B, Gehring R, Webb AI, Craigmill AL, Riviere JE (2005) Effect of formulation and route of administration on tissue residues and withdrawal times. J Am Vet Med Assoc 227(10):1574–1577
Leavens TL, Tell LA, Kissell LW, Smith GW, Smith DJ, Wagner SA, Shelver WL, Wu H, Baynes RE, Riviere JE (2014) Development of a physiologically based pharmacokinetic model for flunixin in cattle (Bos taurus). Food Addit Contam Part A Chem Anal Control Expo Risk Assess 31(9):1506–1521
Li M, Gehring R, Lin Z, Riviere J (2015a) A framework for meta-analysis of veterinary drug pharmacokinetic data using mixed effect modeling. J Pharm Sci 104(4):1230–1239
Li R, Barton HA, Maurer TS (2015b) A mechanistic pharmacokinetic model for liver transporter substrates under liver cirrhosis conditions. CPT Pharmacomet Syst Pharmacol 4(6):338–349
Li M, Gehring R, Riviere JE, Lin Z (2017) Development and application of a population physiologically based pharmacokinetic model for penicillin G in swine and cattle for food safety assessment. Food Chem Toxicol 107:74–87
Li M, Gehring R, Riviere JE, Lin Z (2018) Probabilistic physiologically based pharmacokinetic model for penicillin G in milk from dairy cows following intramammary or intramuscular administrations. Toxicol Sci 164(1):85–100
Lin Z, Fisher JW, Ross MK, Filipov NM (2011) A physiologically based pharmacokinetic model for atrazine and its main metabolites in the adult male C57BL/6 mouse. Toxicol Appl Pharmacol 251(1):16–31
Lin Z, Li M, Gehring R, Riviere JE (2015) Development and application of a multiroute physiologically based pharmacokinetic model for oxytetracycline in dogs and humans. J Pharm Sci 104(1):233–243
Lin Z, Gehring R, Mochel JP, Lave T, Riviere JE (2016a) Mathematical modeling and simulation in animal health—part II: principles, methods, applications, and value of physiologically based pharmacokinetic modeling in veterinary medicine and food safety assessment. J Vet Pharmacol Ther 39(5):421–438
Lin Z, Vahl CI, Riviere JE (2016b) Human food safety implications of variation in food animal drug metabolism. Sci Rep 6:27907
Lin Z, Jaberi-Douraki M, He C, Jin S, Yang RS, Fisher JW, Riviere JE (2017) Performance assessment and translation of physiologically based pharmacokinetic models from acslX to Berkeley Madonna, MATLAB, and R language: oxytetracycline and gold nanoparticles as case examples. Toxicol Sci 158(1):23–35
Malik MY, Jaiswal S, Sharma A, Shukla M, Lal J (2016) Role of enterohepatic recirculation in drug disposition: cooperation and complications. Drug Metab Rev 48(2):281–327
Martin-Jimenez T, Riviere JE (1998) Population pharmacokinetics in veterinary medicine: potential use for therapeutic drug monitoring and prediction of tissue residues. J Vet Pharmacol Ther 21(3):167–189
McNally K, Cotton R, Loizou GD (2011) A workflow for global sensitivity analysis of PBPK models. Front Pharmacol 2:31
NRC (1999a) Drug Residues and microbial contamination in food: monitoring and enforcement. National Academy Press, Washington, DC
NRC (1999b) The use of drugs in food animals. In: Council NR (ed) The use of drugs in food animals: benefits and risks. National Academy Press, Washington, DC, pp 110–141
Odensvik K (1995) Pharmacokinetics of flunixin and its effect on prostaglandin F2 alpha metabolite concentrations after oral and intravenous administration in heifers. J Vet Pharmacol Ther 18(4):254–259
Odensvik K, Johansson IM (1995) High-performance liquid chromatography method for determination of flunixin in bovine plasma and pharmacokinetics after single and repeated doses of the drug. Am J Vet Res 56(4):489–495
Paini A, Leonard JA, Kliment T, Tan YM, Worth A (2017) Investigating the state of physiologically based kinetic modelling practices and challenges associated with gaining regulatory acceptance of model applications. Regul Toxicol Pharmacol 90:104–115
Pairis-Garcia MD, Karriker LA, Johnson AK, Kukanich B, Wulf L, Sander S, Stalder KJ, Coetzee JF (2013) Pharmacokinetics of flunixin meglumine in mature swine after intravenous, intramuscular and oral administration. BMC Vet Res 9(1):165
Pearce RG, Setzer RW, Strope CL, Wambaugh JF, Sipes NS (2017) Httk: R package for high-throughput toxicokinetics. J Stat Softw 79(4):1
Pujol G, Iooss B, Boumhaout AJ with contributions from Veiga SD, Delage T, Fruth J, et al (2017) Sensitivity: global sensitivity analysis of model outputs. https://cran.r-project.org/web/packages/sensitivity/index.html. Accessed 4 Feb 2019
Rasool MF, Khalil F, Läer S (2015) A physiologically based pharmacokinetic drug-disease model to predict carvedilol exposure in adult and paediatric heart failure patients by incorporating pathophysiological changes in hepatic and renal blood flows. Clin Pharmacokinet 54(9):943–962
Riviere JE, Tell LA, Baynes RE, Vickroy TW, Gehring R (2017) Guide to FARAD resources: historical and future perspectives. J Am Vet Med Assoc 250(10):1131–1139
Rowland M, Peck C, Tucker G (2011) Physiologically-based pharmacokinetics in drug development and regulatory science. Annu Rev Pharmacol Toxicol 51:45–73
Schlender JF, Meyer M, Thelen K, Krauss M, Willmann S, Eissing T, Jaehde U (2016) Development of a whole-body physiologically based pharmacokinetic approach to assess the pharmacokinetics of drugs in elderly individuals. Clin Pharmacokinet 55(12):1573–1589
Shebley M, Sandhu P, Emami Riedmaier A et al (2018) Physiologically based pharmacokinetic model qualification and reporting procedures for regulatory submissions: a consortium perspective. Clin Pharmacol Ther 104(1):88–110
Shelver WL, Tell LA, Wagner S, Wetzlich SE, Baynes RE, Riviere JE, Smith DJ (2013) Comparison of ELISA and LC–MS/MS for the measurement of flunixin plasma concentrations in beef cattle after intravenous and subcutaneous administration. J Agric Food Chem 61(11):2679–2686
Sidhu PK, Gehring R, Mzyk DA, Marmulak T, Tell LA, Baynes RE, Vickroy TW, Riviere JE (2017) Avoiding violative flunixin meglumine residues in cattle and swine. J Am Vet Med Assoc 250(2):182–189
Skidmore A, Roder J, Van De Ven J, Cloet P, Montgomery A (2008) Flunixin meglumine (Finadyne®, Banamine®) in pain management of cattle: pharmacology and applications, proceedings XXV World Buiatrics Congress, July 6–11, 2008, Budapest, Hungary, p 293
Sundlof SF, Cooper J (1996) Human health risks associated with drug residues in animal-derived foods veterinary drug residues, vol 636. American Chemical Society, Washington, pp 5–17
Tan YM, Liao KH, Conolly RB, Blount BC, Mason AM, Clewell HJ (2006) Use of a physiologically based pharmacokinetic model to identify exposures consistent with human biomonitoring data for chloroform. J Toxicol Environ Health Part A 69(18):1727–1756
Tan YM, Worley RR, Leonard JA, Fisher JW (2018) Challenges associated with applying physiologically based pharmacokinetic modeling for public health decision-making. Toxicol Sci 162(2):341–348
Tan ML, Zhao P, Zhang L, Ho YF, Varma MVS, Neuhoff S, Nolin TD, Galetin A, Huang SM (2019) Use of physiologically-based pharmacokinetic (PBPK) modeling to evaluate the effect of chronic kidney disease on the disposition of hepatic CYP2C8 and OATP1B drug substrates. Clin Pharmacol Ther 105(3):719–729
Templeton IE, Jones NS, Musib L (2018) Pediatric dose selection and utility of PBPK in determining dose. AAPS J 20(2):31
Thiry J, Fournier R, Roy O, Catala M (2017) Evaluation of flunixin meglumine pour-on administration on prostaglandin E2 concentration in inflammatory exudate after induction of inflammation in cattle. Res Vet Sci 114:294–296
USDA (2018) US National Residue Program: residue sample results—"Red Book”. https://www.fsis.usda.gov/wps/portal/fsis/topics/data-collection-and-reports/chemistry/red-books/red-book. Accessed 14 Nov 2018
Weijs L, Yang RS, Covaci A, Das K, Blust R (2010) Physiologically based pharmacokinetic (PBPK) models for lifetime exposure to PCB 153 in male and female harbor porpoises (Phocoena phocoena): model development and evaluation. Environ Sci Technol 44(18):7023–7030
WHO (2010) Characterization and application of physiologically based pharmacokinetic models in risk assessment. World Health Organization, International Programme on Chemical Safety (WHO/IPCS), Geneva, Switzerland. https://apps.who.int/iris/bitstream/handle/10665/44495/9789241500906_eng.pdf. Accessed 22 Apr 2019
Wojciechowski J, Hopkins AM, Upton RN (2015) Interactive pharmacometric applications using r and the shiny package. CPT Pharmacomet Syst Pharmacol 4(3):146–159
Wu H, Baynes RE, Leavens T, Tell LA, Riviere JE (2013) Use of population pharmacokinetic modeling and Monte Carlo simulation to capture individual animal variability in the prediction of flunixin withdrawal times in cattle. J Vet Pharmacol Ther 36(3):248–257
Yang F, Liu HW, Li M, Ding HZ, Huang XH, Zeng ZL (2012) Use of a Monte Carlo analysis within a physiologically based pharmacokinetic model to predict doxycycline residue withdrawal time in edible tissues in swine. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 29(1):73–84
Yang X, Doerge DR, Teeguarden JG, Fisher JW (2015) Development of a physiologically based pharmacokinetic model for assessment of human exposure to bisphenol A. Toxicol Appl Pharmacol 289(3):442–456
Yang X, Duan J, Fisher J (2016) Application of physiologically based absorption modeling to characterize the pharmacokinetic profiles of oral extended release methylphenidate products in adults. PLoS One 11(10):e0164641
Yoon M, Nong A, Clewell HJ 3rd, Taylor MD, Dorman DC, Andersen ME (2009) Evaluating placental transfer and tissue concentrations of manganese in the pregnant rat and fetuses after inhalation exposures with a PBPK model. Toxicol Sci 112(1):44–58
Yu Z, Jiang C, Guo Y, Hu Y, Chen D (2007) Pharmacokinetics of flunixin meglumine after intravenous and intramuscular administration in pigs. Agric Sci China 6(11):1396–1401
Zeng D, Lin Z, Fang B, Li M, Gehring R, Riviere JE, Zeng Z (2017) Pharmacokinetics of mequindox and its marker residue 1, 4-bisdesoxymequindox in swine following multiple oral gavage and intramuscular administration: an experimental study coupled with population physiologically based pharmacokinetic modeling. J Agric Food Chem 65(28):5768–5777
Zurlinden TJ, Reisfeld B (2017) Characterizing the effects of race/ethnicity on acetaminophen pharmacokinetics using physiologically based pharmacokinetic modeling. Eur J Drug Metab Pharmacokinet 42(1):143–153
Acknowledgements
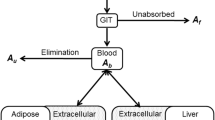
The authors would like to acknowledge the help from Mal Hoover, the medical illustrator affiliated with College of Veterinary Medicine of Kansas State University, for creating Fig. 1. This work was supported by the United States Department of Agriculture (USDA) National Institute of Food and Agriculture (NIFA) for the Food Animal Residue Avoidance Databank (FARAD) Program (Award No. 2015-41480-23972, 2016-41480-25729, 2017-41480-27310, and 2018-41480-28805).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Li, M., Cheng, YH., Chittenden, J.T. et al. Integration of Food Animal Residue Avoidance Databank (FARAD) empirical methods for drug withdrawal interval determination with a mechanistic population-based interactive physiologically based pharmacokinetic (iPBPK) modeling platform: example for flunixin meglumine administration. Arch Toxicol 93, 1865–1880 (2019). https://doi.org/10.1007/s00204-019-02464-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-019-02464-z