Abstract
Summary
To demonstrate the clinical comparability between RGB-10 (a biosimilar teriparatide) and the originator, a comparative pharmacokinetic trial was conducted. The study was successful in establishing bioequivalence. Marketing authorisation for RGB-10 (Terrosa®) was granted by the European Medicines Agency in 2017.
Introduction
Teriparatide, the first bone anabolic agent, is the biologically active fragment of human parathyroid hormone. The imminent patent expiry of the originator will open the door for biosimilars to enter the osteology market, thereby improving access to a highly effective, yet prohibitively expensive therapy.
Methods
Subsequent to establishing comparability on the quality and non-clinical levels between RGB-10, a biosimilar teriparatide, and its reference product (Forsteo®), a randomised, double-blind, 2-way cross-over comparative study (duration: four days) was conducted in 54 healthy women (ages: 18 to 55 years) to demonstrate the pharmacokinetic/pharmacodynamic (PK/PD) equivalence and comparable safety of these products. Extents of exposure (AUC0-tlast) and peak exposure (Cmax), as measured by means of ELISA, were evaluated as co-primary PK endpoints, and serum calcium levels, as measured using standard automated techniques, were assessed for PD effects. Safety was monitored throughout the study.
Results
The 94.12% CIs for the ratio of the test to the reference treatments, used due to the two-stage design (85.20–98.60% and 85.51–99.52% for AUC0-tlast and Cmax, respectively), fell within the 80.00–125.00% acceptance range. The calcium PD parameters were essentially identical with geometric mean ratios (GMRs) of 99.93% and 99.87% for AUC and Cmax, respectively. Analysis of the safety data did not reveal any differences between RGB-10 and its reference.
Conclusion
Based on the high level of similarity in the preclinical data and the results of this clinical study, marketing authorisation for RGB-10 (Terrosa®) was granted by the European Medicines Agency (EMA) in 2017.
Similar content being viewed by others
Introduction
Recombinant human teriparatide is the biologically active N-terminal 34-amino acid fragment of the 84-amino acid native parathyroid hormone [PTH (1–84)]. Since it is produced in Escherichia coli (E. coli), the introduction of Forsteo® in 2003 marked the beginning of the biologic era in the treatment of osteoporosis.
The skeletal effects of teriparatide depend on the pattern of systemic exposure. While continuous exposure to excessive amounts of parathyroid hormone, as seen in hyperparathyroidism, triggers bone resorption, intermittent elevations result in a bone anabolic response [1]. Two phases may be distinguished in the effect of intermittently administered PTH [2]. The prompt increase in formation markers suggests that the first few months of PTH treatment, often referred to as the anabolic window, are predominantly characterised by an increase in bone formation. However, at a later stage, bone formation becomes more remodelling-dependent [3], with bone formation favoured over bone resorption, thereby resulting in a net gain of bone deposition in each basic multicellular unit [4]. The beneficial effects of teriparatide on bone density, microarchitecture and bone geometry are seen predominantly in the cancellous skeleton [5]. In the cortical bone envelope, intermittent PTH stimulates both endocortical and periosteal bone formation [3]. At cortical skeletal sites, teriparatide increases porosity, thereby potentially decreasing BMD; however, the reduction in BMD is not accompanied by diminished bone strength because the increased porosity occurs only in the inner one third of the bone, where the mechanical effect is minimal. Other positive effects of teriparatide at the cortical bone, namely, changes in bone geometry and microarchitecture, effectively counterbalance any increase in cortical porosity [5]. Clinically, the metabolic effects of teriparatide translate into improved bone mineral density (BMD) at vertebral and femoral skeletal sites as well as a reduced incidence of vertebral and non-vertebral fragility fractures [6]. Based on a meta-analysis by Murad et al. of all anti-osteoporotic agents, teriparatide had the highest probability of being ranked as most effective and had the highest reduction in the risk of vertebral, non-vertebral and hip fractures [7]. Although clinical studies adequate to assess the effect of teriparatide on the risk of hip fractures are lacking and are unlikely to be conducted, there is evidence suggesting that teriparatide is associated with beneficial effects on the hip in patients with osteoporosis [8].
Teriparatide is indicated for the treatment of patients with a high risk for fracture (in particular, men and postmenopausal women with osteoporosis, as well as patients with glucocorticoid-induced osteoporosis).
When the period of market exclusivity protecting the innovator biopharmaceutical has expired, biosimilars can be introduced to the market. Since the reference product is facing patent expiry in the near future, the development of the first biosimilar teriparatide was initiated to offer a less costly treatment alternative for patients afflicted with severe osteoporosis. The regulatory requirements for approving biosimilars have been constantly evolving, and the extensive experience amassed over the last 10 years has helped to shape the present criteria laid down in the scientific guidelines issued by the European Medicines Agency (EMA). This clinical programme was designed to conform to the most recent regulatory requirements.
The objective of the clinical study was to demonstrate the clinical comparability of RGB-10 and reference teriparatide after having established a high level of similarity between them on the quality and non-clinical levels (data on file).
Materials and methods
The study protocol followed all relevant regulatory guidelines, especially the Guideline on similar biological medicinal products [9] and the Guideline on the investigation of bioequivalence [10] and was approved by the Office for Research Ethics Committees Northern Ireland (ORECNI), Lisburn, which is responsible for the study location at Celerion, a centre that specialises in clinical pharmacology studies (Belfast, Northern Ireland). The trial identifiers are EudraCT number 2013-004040-31 and ClinicalTrials.gov identifier NCT02223416. The details of the clinical trial were subject to extensive regulatory discussions. All participants gave their written informed consent prior to the initial study-related assessments. The performance and supervision of this trial followed the principles of Good Clinical Practice (GCP) as laid down in ICH E 6 [11]. The study was conducted in accordance with the ethical requirements referred to in EU directive 2001/20/EC [12] and the ethical principles set forth in the Declaration of Helsinki [13].
Subjects and design
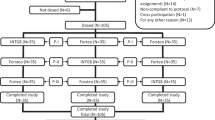
From August 2014 to January 2015, 54 healthy adult female volunteers, 18–55 years of age, were recruited for this randomised, double-blind, two-period, two-sequence cross-over comparative pharmacokinetic (PK) trial. In order to standardise the study subject population and to ensure homogeneity, the study was performed in healthy adult pre-menopausal female subjects. The pharmacokinetics of the reference had been characterised across a broad age range, and no differences were identified. In addition, there are no literature data suggestive of a difference in PTH receptor density between osteoporotic and healthy individuals. Consequently, it was deemed appropriate to extrapolate the data generated in healthy women to the target population of people diagnosed with osteoporosis. A BMI of 18.5–27.0 kg/m2 and the absence of any signs of clinically significant illness constituted the main eligibility criteria. Women of childbearing potential had to use medically acceptable means of birth control (other than hormonal contraceptives) and to agree to continue its use during the study and for at least 28 days after the last dosing. The main exclusion criteria included known osteoporosis, the history or presence of bone diseases (Paget’s disease, bone carcinoma or bone metastases), any significant endocrine (including thyroid and parathyroid gland) disease and hypercalciuria and/or nephrolithiasis, urolithiasis in the past 5 years, serum alkaline phosphatase levels exceeding the upper limit of the normal range, as well as any prior or planned radiation therapy involving the skeleton. Subjects with prior exposure to teriparatide or any other PTH analogue product or with a history of sensitivity to E. coli-derived proteins were also excluded.
Subjects were randomised (1:1, using a computer-generated list) to treatment sequences AB or BA, treatment A corresponding to a single-dose of RGB-10 and treatment B representing a single injection of reference. There were two dosing days: period 1 day 1 and period 2 day 1, which were separated by a washout period of 24 h. In both study periods, the injections were administered into the abdomen universally for all volunteers at hour 0 in the early morning. In each period, blood sampling for PK evaluation was performed before dosing and at pre-defined time points of 10, 20, 25, 30, 35, 40, 50, 60, 70, 80, 90, 120, 180 and 240 min post-dose. Laboratory assessments for serum Ca were performed pre-dose and at 120, 180 and 240 min and 6, 8 and 12 h post-dose in each period. Safety was monitored throughout the study.
Methods
RGB-10 (drug substance manufactured by Richter-Helm, Bovenau, Germany; drug product manufactured by Gedeon Richter Plc., Debrecen, Hungary) was supplied as a fixed 20 μg/80 μL solution for injection in a cartridge inserted in a reusable, multi-dose pen injector (ServoPen Fix®, manufactured by Ypsomed AG, Switzerland). A single RGB-10 cartridge of 2.4 mL contains 600 μg of teriparatide (corresponding to 250 μg per ml). The reference was supplied using the marketed pre-filled pen, Forsteo® (Lilly France S.A.S, Fegersheim, France) containing the same amount and concentration of teriparatide per single injection. The same type of needle, a BD Micro-Fine™ + Pen Needle (0.25 mm (31G), TW, 8 mm), was used for both devices. The two injection devices were comparable in terms of dose accuracy. Since the appearance as well as the handling of the two devices differed, the study drugs were injected by an unblinded study team member to ensure the trial design was double-blind for the investigator and the volunteers.
A commercially available, high-sensitivity human PTH(1-34) ELISA kit from Immutopics, Inc. (San Clemente, California, Cat no. 60-3900) was specially adapted to the references Teriparatide RGB-10 (rhPTH(1-34), Gedeon Richter) and Forsteo® (rhPTH(1-34), Eli Lilly) and GLP validated with respect to the assay parameters required by the applicable regulations. The lower limit of quantification (LLQ) was set at 6.0 pg/mL. The primary PK endpoints were AUC0-tlast and Cmax. The time to reach maximum concentration (tmax), the apparent terminal elimination half-life (t1/2) and the AUC from time zero extrapolated to infinity (AUC0-inf), AUC%extrap, the time to last measurable concentration (tlast), the elimination rate constant (kel), the apparent total plasma clearance (CL/F), and the volume of distribution (Vz/F) were measured as secondary PK parameters.
Serum calcium was measured using Calcium Gen.2, an in vitro test for the quantitative determination of calcium in human serum, and a Cobas 6000 c501 analyser (Roche Diagnostics A/S, Mannheim, Germany). Under alkaline conditions, calcium ions form a complex with 5-nitro-5′-methyl-BAPTA (NM-BAPTA), which reacts in a second step with EDTA. The resulting change in absorbance is directly proportional to the calcium concentration and is measured photometrically. The lower limit of quantitation is 0.8 mg/dl, and the average CV is 1.85%. Total serum calcium values were corrected for albumin (ALB) using the formula: Measured Ca (mmol/L) + ((40-ALB (g/L)) * 0.02).
The safety and local tolerance of RGB-10 were compared to those of the reference product through evaluating the findings from physical examinations, vital sign measurements, electrocardiograms (ECGs), pulse oximetry, the recording of adverse events (AEs), assessing injection site reactions and clinical laboratory tests. Immunogenicity was not investigated in this study, since neither the sample size nor the duration of the trial would have allowed us to collect any clinically relevant data on the immunogenic potential of RGB-10.
Sample size calculation
Due to the limited amount of literature data available on the variability characterising teriparatide pharmacokinetics, a two-stage design was selected. Two-stage designs are applied when the variability is unknown prior to the study and allow the modification of the sample size at an interim analysis with full control of the type I error [14]. If the stage 1 sample size turns out to be smaller than appropriate for the variability estimated based on the stage 1 data, further subjects can then be enrolled in the second stage. The sample size pre-determined for stage 1 was based on an expected test/reference ratio of 0.95–1.05 and an intra-subject CV% value of 27% (for both AUC and Cmax), values that are consistent with the total CV% in the literature [15,16,17]. Allowing for 6 potential drop-outs, a sample size of 56 subjects was defined for stage 1, of which 50 had to be evaluable to achieve a power of at least 90% using the α-level of 0.0294; i.e. a 94.12% confidence interval (CI) instead of the usual 90% CI, due to the type I error control for the two-stage design. The standard bioequivalence (BE) acceptance range of 80.00–125.00% was applied. This approach allowed for stopping for equivalence at interim and for sample size reassessment [10].
Statistical analyses
The primary endpoints were the 94.12% confidence intervals of the ratios of the geometric means (test/reference) derived from the analyses on the natural log (ln)-transformed PK parameters AUC0-tlast and Cmax of the test and reference formulations of teriparatide. ANOVA was performed on the ln-transformed values of AUC0-tlast, AUC0-inf, Cmax and t1/2. Since the subjects were dosed in groups, the ANOVA model included sequence, group, period nested within group, treatment and treatment*group interaction as fixed effects, and subject nested within group*sequence as a random effect. If the treatment*group interaction was not statistically significant at the 5% level, the interaction term was dropped from the model. If a statistically significant interaction was found, results for those PK parameters that showed interactions were to be presented by group as well as combined. Each ANOVA included calculation of the least square means (LSMs), the difference between treatment LSMs and the standard error associated with this difference.
A further type of ANOVA analysis without the group factor was also performed for robustness reasons.
Safety variables were summarised by treatment arm, and no inferential statistics were analysed. The appropriate non-compartmental PK parameters were calculated from the plasma teriparatide concentration-time data using Phoenix® WinNonlin® version 6.3. All descriptive statistical analyses were performed using SAS 9.3 software.
Results
Although 56 subjects were predefined for stage 1 due to small cohort sizes (thus potentially resulting in a significant treatment-by-group interaction and thereby compromising the primary PK analysis), the last cohort, which would have consisted of two subjects only, was not enrolled. Consequently, a total of 54 subjects were randomised to study treatments and analysed for safety. All subjects satisfied all inclusion criteria and fulfilled none of the exclusion criteria. The demographic data for all 54 patients showed a mean age of 29.2 ± 8.3 years, a height of 164.2 ± 6.0 cm, a weight of 62.1 ± 7.1 kg and a BMI of 23.0 ± 2.0 kg/m2. One subject withdrew prematurely after period 1 for personal reasons. Two further subjects were excluded from the PK analysis as a result of events compromising the evaluation of their PK profiles; one had measurable pre-dose concentrations greater than 5% of the Cmax in both periods (an exclusion criterion predefined in the statistical analysis plan), and the other lacked several PK samples in the absorption phase. Therefore, the final PK population for the primary endpoints of AUC0-tlast and Cmax comprised 51 subjects.
After reviewing the results from the interim analyses, it was not necessary to proceed to stage 2 because the data variability was less than anticipated based on the literature, and concluding on equivalence was possible using only stage 1 data. If the study had continued to stage 2, due to obtaining a power of less than 90%, the sample size would have been re-estimated based on stage 1 intrasubject CV PK results, an expected power of at least 90%, and the initially assumed ratio of 0.95–1.05 using an alpha level of 0.0294 (i.e. using a 94.12% CI). A maximum of 58 subjects (between 1 and 58 subjects) would have been enrolled in stage 2, and the same procedures would have been followed for stage 2 subjects as those followed for subjects in stage 1. Bioequivalence would have been evaluated for the second time after the completion of stage 2 based on data from both stages at an alpha level of 0.0294 (using a 94.12% CI).
Pharmacokinetics
Teriparatide was rapidly absorbed following subcutaneous (s.c.) administration, and measurable concentrations were observed as early as 10 min after dosing in all subjects. A summary of the PK parameters for RGB-10 and the reference drug is presented in Table 1, and the arithmetic mean teriparatide serum concentration–time profiles are displayed in Fig. 1.
Throughout most of the sampling interval, mean concentrations were slightly lower for RGB-10 than for the reference. In more than half of the subjects, individual plasma teriparatide concentration–time profiles showed multiple absorption peaks for either RGB-10 or the reference product, or following both treatments.
Statistical comparisons of plasma teriparatide PK parameters are summarised in Table 2.
Bioavailability and mean concentrations after the administration of RGB-10 appeared to be slightly lower than those for the reference. Based on the geometric mean ratios (GMR), the extent, total extent and peak teriparatide exposure in plasma (as measured by AUC0-tlast, AUC0-inf and Cmax) were 8 to 10% lower for RGB-10 than for the reference. Nevertheless, the 94.12% CIs for the ratio of the test to the reference treatments fell entirely within the 80.00–125.00% acceptance range for BE. The data derived from the second ANOVA analysis that did not include the group factor virtually coincided with those of the ANOVA model that did include the group effect, thus confirming that the group factor had no impact on the statistical results.
Regarding the secondary PK endpoints, no clinically significant differences were detected between the treatments. The half-life was 9% shorter for RGB-10 than for the reference, and there was a small (4.5 min) difference in plasma teriparatide median tmax values. The mean teriparatide CL/F value was approximately 13% higher for RGB-10 than for the reference product, whereas the mean Vz/F values were similar between the two treatments.
In conclusion, the 94.12% CIs for the GMRs (test/reference) of primary PK endpoints AUC0–last (85.20–98.60%) and Cmax (85.51–99.52%) were contained within the standard BE acceptance range of 80–125%. Based on these statistical results, RGB-10 can be considered as bioequivalent to its reference.
Pharmacodynamics
The serum calcium concentration–time curves for RGB-10 and the reference were characterised by computing the pharmacodynamic (PD) parameters AUC0-tlast, Cmax and tmax. Descriptive statistics of the parameters are presented in Table 3.
Statistical analysis of all serum calcium PD parameters showed close similarity between RGB-10 and the reference with GMRs of 99.93 and 99.87% for AUC and Cmax, respectively, and a median difference of 0.001 for tmax.
The relationship between the pharmacokinetics and the pharmacodynamics of RGB-10 and the reference is displayed in Fig. 2.
Serum calcium concentrationsc and plasma teriparatide concentrations following single 20 μg/80 μL s.c. doses of RGB-10 and the reference teriparatide. a Formula used for calcium correction: Measured Ca (mmol/L) + ((40-ALB (g/L)) * 0.02). b Baseline samples for serum calcium were collected within 2.5 h prior to the administration of single 20 μg/80 μL subcutaneous injections of RGB-10 and the reference teriparatide. c Serum calcium concentrations (mmol/L) are given as the means ± SE
PK and PD profiles indicate that the concentration–effect relationships for the two treatments are not different and that equivalent exposure results in an equivalent response, thus supporting the biosimilarity.
Safety
All subjects who received at least 1 dose of the study medication (i.e., a total of 54 subjects) were included in the safety analysis. There were no deaths, serious adverse events (SAEs) or subject discontinuations due to AEs. Overall, a total of 127 treatment emergent adverse events (TEAEs) were experienced by 43 (80%) subjects participating in this study. The most common events reported during the study were nausea (20 [37%] subjects) followed by dizziness (15 [28%] subjects), headache (13 [24%] subjects) and injection site erythema (12 [22%] subjects). There were no remarkable or new and unexpected findings from the safety assessments, and RGB-10 and the reference had similar safety profiles and exhibited no significant differences in the type or incidence of AEs recorded. The most commonly reported (≥ 10%) TEAEs for RGB-10 and the reference are presented in Table 4.
It can be concluded that single 20 μg/80 μL s.c. injections of RGB-10 are safe, and the nature and incidence of adverse events coincided with those recorded for the reference. No new safety concerns emerged from this study.
Discussion
Here, we report the clinical comparability of two teriparatide products based on a comparative single-dose, two-way cross-over study in healthy female pre-menopausal adults following single s.c. administrations of RGB-10 and the reference drug.
No product-specific regulatory guidelines explicitly define the requirements pertaining to the development of a biosimilar teriparatide. Moreover, no prior development programmes exist that could have been followed since RGB-10 is the first biosimilar teriparatide that has been granted marketing authorisation. Only some overarching regulatory guidance was available at the time when RGB-10 development was planned.
In 2004, a dedicated route was introduced for biosimilars, thus establishing a solid legal framework for their approval in the EU. The more than 10 years of expertise acquired since then has allowed EU regulators to integrate experience-based knowledge with the initial science-driven concept shaping current requirements for approval [18]. The basic principle underlying the development of a biosimilar product is its comparability with the reference product; this is assessed using a comparability exercise, which is the comparative testing performed at all stages of the biosimilar development (i.e., on quality, non-clinical and clinical levels) [19]. Based on the EMA requirements, comparability testing is conceived as a step-wise process, which is tailored to each product; that is, knowledge from the initial quality comparability studies is used to determine the extent and type of non-clinical and clinical studies that are required in the next step of the development [18]. Consistently, the design of the clinical development programme considers the nature and characteristics of the medicine and its intended use and how comparable the profile of the biosimilar medicine is to that of the reference product. Consequently, the closer the profiles of the biosimilar and reference products and the higher the similarity (as demonstrated through appropriate studies; e.g., comparative quality, biological and receptor-binding assays as well as in vitro studies) are, the more a tailored clinical trial programme can be accepted by the EMA [19]. For simpler molecules with a well-established action (e.g., filgrastim) and where comparative quality and non-clinical data are solid, it may be sufficient to compare the effect of the biosimilar and the reference medicine based on PK and PD studies in healthy volunteers [18].
Due to its low molecular weight and structural simplicity, teriparatide qualifies as a very simple molecule. In this case, the quality and non-clinical profiles of the biosimilar and reference products were close enough to lend legitimacy to the application of a more tailored clinical trial programme. This new development approach is reflected in the clinical study, which was designed to establish clinical comparability between RGB-10 and its reference.
The results of this study showed that the PK profile of RGB-10 is consistent with that of the originator drug. The statistical analysis of the PK data formally established BE between the two drugs, with the 94.12% CIs for the primary endpoints (AUC0-tlast and Cmax) falling entirely within the predefined equivalence margins of 80–125%.
The multiple-peak phenomenon detected in this study was also observed by Liu et al. [20], who described double peaks in two thirds of their study subjects following single and multiple s.c. doses of teriparatide; the first peak appeared within 5 to 20 min, and the second appeared within 30 to 60 min post-dose. A double-peak profile was also reported following the s.c. administration of recombinant human PTH(1–84). Plasma and serum concentration time profiles of recombinant human PTH(1-84) and its synthetic N-terminal fragment teriparatide showed similar double-peak profiles, with no temporal difference in the appearance of both peaks [21, 22]. It was suggested that the double-peak phenomenon was related to the fast and slow release rates of exogenous PTH into the systemic circulation following s.c. administration [20, 22].
Serum calcium was assessed not only as a safety endpoint but also as a PD parameter to support the biosimilarity claim of RGB-10. Teriparatide is known to cause transient increases in serum calcium after each dose through increased intestinal absorption and increased tubular reabsorption of calcium. This is observed in healthy volunteers and osteoporotic patients and can therefore be seen as a surrogate marker for the PD effects of teriparatide. The results for RGB-10 and the reference were highly similar, with 95% CIs of the GMR of the RGB-10/reference correcting calcium within 99–101%. The comparable calcium response for RGB-10 and the reference provides evidence supporting the clinical comparability of these drugs.
The safety profile of RGB-10 was similar to that of the originator drug with respect to AEs, clinical laboratory measurements, vital signs, electrocardiographic and local tolerance assessments. The safety results were comparable to the data published for teriparatide [23,24,25], and no differences were observed between the two treatment groups. There were no new safety signals reported during this trial, and RGB-10 proved to be as safe as the reference, with no SAEs observed. Similarly, the incidence and severity of injection site reactions associated with the use of teriparatide were similar to those seen in previous studies.
A limitation of this study is that immunogenicity could not be investigated. Establishing pharmacokinetic equivalence was the primary objective of the trial; consequently, the requirements pertaining to the study design, duration and sample size laid down in the Guideline on the investigation of bioequivalence [10] were adhered to. Although satisfying all the criteria described in the Guideline on the investigation of bioequivalence [10], the cross-over design, the short study duration due to the rapid elimination half-life of teriparatide and the relatively low sample size needed to demonstrate bioequivalence all precluded the assessment of immunogenicity. Nevertheless, the general immunogenic potential of teriparatide is expected to be low because it is a low molecular weight, fully human molecule of microbial origin with no glycosylation or any other posttranslational modifications. The immunogenic potential of the reference drug proved negligible in the clinical studies for registration purposes in that anti-teriparatide antibodies were detected after at least 12 months of treatment in only 2.8% of the patients receiving 20 μg daily s.c. doses of teriparatide [26]; the lack of literature data on the immunogenicity of the reference product over the 15 years that it has been available on the market seems to confirm this. Consequently, assessment of the immunogenic potential of RGB-10 was not among the objectives of the bioequivalence study.
The strength of the development programme designed for RGB-10 is that it sets a notable example of the tailored approach recently adopted by the EMA, in which, for simple molecules whose mechanism of action is well-established and where comparative quality and non-clinical data are solid, comparative PK/PD studies in healthy volunteers may be sufficient to conclude therapeutic equivalence [18].
Given the high level of similarity between RGB-10 and its reference as demonstrated in the in-depth quality and non-clinical comparability exercise (data on file) and the BE and PD comparability established in our clinical study, marketing authorisation for RGB-10 has been granted for all indications by the European Medicines Agency (EMA).
References
Mitlak BH (2002) Parathyroid hormone as a therapeutic agent. Curr Opin Pharmacol 2:694–699
Gonzalez MJ, Olmos MJM (2010) Physiopathology of osteoporosis and the action mechanism of PTH. Rev Osteoporos Metab Miner 2(S2):S5–S17
Dobnig H (2004) A review of teriparatide and its clinical efficacy in the treatment of osteoporosis. Expert Opin Pharmacother 5(5):1153–1162
Gagnon C, Li V, Ebeling PR (2008) Osteoporosis in men: its pathophysiology and the role of teriparatide in its treatment. Clin Interv Aging 3(4):635–645
Bilezikian JP (2008) Combination anabolic and antiresorptive therapy for osteoporosis: opening the anabolic window. Curr Osteoporos Rep 6:24–30
Canalis E, Giustina A, Bilezikian JP (2007) Mechanisms of anabolic therapies for osteoporosis. N Engl J Med 357:905–916
Murad MH, Drake MT, Mullan RJ, Mauck KF, Stuart LM, Lane MA, Abu Elnour NO, Erwin PJ, Hazem A, Puhan MA, Li T, Montori VM (2012) Comparative effectiveness of drug treatments to prevent fragility fractures: a systematic review and network meta-analysis. J Clin Endocrinol Metab 97(6):1871–1880
Eriksen EF, Keaveny TM, Gallagher ER, Krege JH (2014) Literature review: the effects of teriparatide therapy at the hip in patients with osteoporosis. Bone 67:246–256
Guideline on similar biological medicinal products (2014) CHMP/437/04 rev. 1
Guideline on the investigation of bioequivalence (2010) CPMP/EWP/QWP/1401/98 rev. 1/Corr**
ICH (1996) Guideline for good clinical practice E6 (R1)
Clinical trials - Directive 2001/20/EC
World Medical Association (2013) WMA declaration of Helsinki: ethical principles for medical research involving human subjects. Retrieved from http://www.wma.net/en/30publications/10policies/b3/
Reflection paper on methodological issues in confirmatory clinical trials planned with an adaptive design (2007) CHMP/EWP/2459/02
Potvin D, DiLiberti CE, Hauck WW et al (2008) Sequential design approaches for bioequivalence studies with crossover designs. Pharm Stat 7(4):245–262
Chu NN, Li XN, Chen WL, Xu HR (2007) Pharmacokinetics and safety of recombinant human parathyroid hormone (1-34) (teriparatide) after single ascending doses in Chinese healthy volunteers. Pharmazie 62(11):869–871
Shiraki M, Sugimoto T, Nakamura T (2013) Effects of a single injection of teriparatide on bone turnover markers in postmenopausal women. Osteoporos Int 24(1):219–226
European Medicines Agency and the European Commission (2017) Biosimilars in the EU Information guide for healthcare professionals
Medicines for Europe (2016) Biosimilar medicines handbook. 3rd edn
Liu Y, Yang C, Li Z, Zhou J, Lv Y, Zhang Y, Zeng F, Shi S (2014) Safety, tolerability, pharmacokinetics, and pharmacodynamics of recombinant human parathyroid hormone (1-34) in healthy Chinese subjects. Clin Ther 36(6):940–952
Liu Y, Shi S, Wu J, Li Z, Zhou X, Zeng F (2012) Safety, tolerability, pharmacokinetics and pharmacodynamics of recombinant human parathyroid hormone after single- and multiple-dose subcutaneous administration in healthy Chinese volunteers. Basic Clin Pharmacol Toxicol 110(2):154–161
Schwietert HR, Groen EW, Sollie FA, Jonkman JH (1997) Single-dose subcutaneous administration of recombinant human parathyroid hormone [rhPTH(1-84)] in healthy postmenopausal volunteers. Clin Pharmacol Ther 61(3):360–376
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, Hodsman AB, Eriksen EF, Ish-Shalom S, Genant HK, Wang O, Mellström D, Oefjord ES, Marcinowska-Suchowierska E, Salmi J, Mulder H, Halse J, Sawicki AZ, Mitlak BH (2001) Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 344:1434–1441
Prince R, Sipos A, Hossain A, Syversen U, Ish-Shalom S, Marcinowska E, Halse J, Lindsay R, Dalsky GP, Mitlak BH (2005) Sustained nonvertebral fragility fracture risk reduction after discontinuation of teriparatide treatment. Bone Miner Res 20:1507–1513
Andrews EB, Gilsenan AW, Midkiff K, Sherrill B, Wu Y, Mann BH, Masica D (2012) The US postmarketing surveillance study of adult osteosarcoma and teriparatide: study design and findings from the first 7 years. J Bone Miner Res 27(12):2429–2437
Forsteo® EPAR European Medicines Agency. European Public Assessment Report—Scientific Discussion (Committee for Medicinal Products for Human Use [CHMP]); October 2005
Acknowledgments
The study group is indebted to all of the study personnel who made this research possible and to all participating volunteers. Dr. Wilfried Hauke is deeply acknowledged for his insight and contributions to the development of this work.
Funding
The study was funded by Gedeon Richter Plc.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All procedures in studies involving human participants were performed in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all participants included in the study.
Conflict of interest
István Takács is a consultant and advisory board member at Gedeon Richter Plc. and has received payment from Gedeon Richter for a lecture. Enikő Jókai, Dóra Eszter Kováts, Ildikó Aradi are employees at Gedeon Richter Plc.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution-NonCommercial 4.0 International License (http://creativecommons.org/licenses/by-nc/4.0/), which permits any noncommercial use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Takács, I., Jókai, E., Kováts, D.E. et al. The first biosimilar approved for the treatment of osteoporosis: results of a comparative pharmacokinetic/pharmacodynamic study. Osteoporos Int 30, 675–683 (2019). https://doi.org/10.1007/s00198-018-4741-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-018-4741-0