Abstract
Purpose
Haloperidol and clonidine are commonly used to treat agitation in delirious intensive care unit (ICU) patients, but it is unclear whether these agents may shorten the duration of delirium. The objective of this study was to determine whether haloperidol, clonidine, or their combined administration to delirious ICU patients results in delirium resolution.
Methods
This was a cohort study on a mixed ICU, excluding patients with a primary neurological disorder. The main outcome was the probability of delirium resolution, using propensity score matching and Markov multinomial logistic regression models for daily transitions. Secondary outcomes were delirium duration, number of delirium days, ventilation days, length of stay in the ICU and hospital, and ICU mortality.
Results
A total of 3614 patients were included (1165 delirious [32%]; 2449 non-delirious [68%]). Delirium occurred on 4708 (18.9%) of 24,906 days. The probability of delirium resolution was lower in delirious patients who received haloperidol (OR 0.47, 95% CI 0.39–0.57), clonidine (OR 0.78, 95% CI 0.63–0.97), or both (OR 0.45, 95% CI 0.36–0.56) compared to untreated delirious patients. Delirious patients who received haloperidol, clonidine, or both had generally longer delirium duration, more delirium and ventilation days, and spent more time in the ICU and in hospital than untreated delirious patients. These agents had no effect on ICU mortality.
Conclusion
Haloperidol and clonidine use in delirious ICU patients may be associated with reduced probability of delirium resolution. This finding, however, merits further investigation given inherent limitations of this observational analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Delirious ICU patients who are treated with haloperidol, clonidine, or both, were found to have reduced probabilities of delirium resolution and worse short-term clinical outcomes, such as longer delirium duration, ICU and hospital stay, and length of mechanical ventilation. However, due to the observational nature of this analysis, the results should be regarded as hypothesis generating. |
Introduction
Delirium is a clinical manifestation of acute encephalopathy and a frequent complication in critically ill patients [1,2,3,4]. Patients who experience delirium during intensive care unit (ICU) admission are more likely to have a longer stay in the ICU and hospital, and are more likely to incur higher healthcare costs than patients without delirium [5, 6]. Delirium is further associated with an increased risk of long-term cognitive impairment [7, 8]. However, managing delirium remains a challenge for ICU physicians as an established treatment is lacking [9].
Pharmacological management of delirium is currently based on inferences of presumed pathophysiology. A widely used drug for treating delirium in a critical care setting is haloperidol, a dopamine D2 antagonist [10]. However, little evidence is available to support its use in treatment of all patients with delirium [9]. Two randomized trials have studied the efficacy of haloperidol versus placebo as treatment of ICU delirium. One was a pilot trial conducted in mechanically ventilated patients admitted to a medical or surgical ICU [11], and the other was a therapeutic trial in patients with acute respiratory failure or shock [12]. These trials have shown that haloperidol does not deliver a difference of more than two delirium- and coma-free days when compared to placebo. However, a smaller difference could still be clinically relevant.
In addition to an excess of dopaminergic activity, it has been hypothesized that delirium results from an excess of norepinephrine activity [13]. The release of norepinephrine is inhibited by dexmedetomidine, an alpha-2 agonist, which has recently gained popularity for treating agitated delirium in mechanically ventilated patients [14, 15]. However, it is only available as intravenous preparation and, due to its costs, may not be the preferred agent for all critically ill patients [16]. Clonidine is another alpha-2 agonist that is less expensive and may therefore be an alternative to dexmedetomidine. Clonidine is available in both intravenous and enteral formulations, and is regularly administered to treat agitation in both mechanically ventilated and non-ventilated ICU patients [17]. A randomized controlled pilot trial in cardiothoracic surgery patients showed promising results regarding delirium severity [18]. Yet, to our knowledge no studies have been conducted on the efficacy of clonidine for treatment of delirium in a mixed ICU [19].
We hypothesized that treatment with haloperidol and/or clonidine would increase the likelihood of resolving delirium. The aim of this cohort study was therefore to determine whether administration of haloperidol, clonidine, or their combined use was associated with resolution of delirium in critically ill patients.
Methods
Study design and participants
This study was based on a prospectively enrolled cohort of patients admitted to the mixed ICU of the University Medical Center Utrecht the Netherlands between August 2011–June 2013 and June 2015–March 2019 [20, 21]. In between these two periods no data have been collected, due to lack of available research staff. Details on the patients in the first cohort have been published [22]. For the current analysis, exclusion criteria were ICU admission < 24 h, readmissions, transfers from another ICU, or admission with a primary acute neurological or neurosurgical disorder confounding the delirium diagnosis [23], or another condition that could hamper the assessment of delirium, such as mental retardation, anoxic brain injury after cardiopulmonary resuscitation, or inability to speak Dutch or English. ICU physicians adhered to a sedation protocol aiming for light sedation (target Richmond Agitation and Sedation Scale [RASS] score − 2 to 0), without using dexmedetomidine. Because of the non-interventional nature of this investigation, the ethics committee of the University Medical Center Utrecht waived the need for informed consent (protocol #19-768/c). This cohort study was reported using the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement [24].
Data collection and definitions
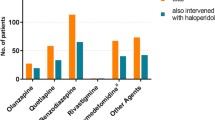
All data have been collected prospectively. Age, sex, ICU admission type, Acute Physiology and Chronic Health Evaluation (APACHE) IV [25], and ICU and hospital length of stay were stored in the electronic patient data management system. On a daily level (one day was defined as a 24-h epoch from 10AM to 09.59AM), the following characteristics were collected: modified Sequential Organ Failure Assessment (SOFA) score (without the central nervous system component) [26], presence of metabolic acidosis (defined as base excess < − 3 in arterial blood gas analysis), mechanical ventilation status, mortality, and administration of antipsychotics (haloperidol, quetiapine, olanzapine, clozapine, risperidone), sedatives (benzodiazepines, propofol, clonidine) and opioids.
Patients were prospectively classified per 24-h epoch as awake without delirium, delirious, unarousable, discharged or dead. The daily mental status was classified according to a delirium recognition algorithm based on three items [22]: (1) the RASS and Confusion Assessment Method-Intensive Care Unit (CAM-ICU) scores, assessed by bedside nurses, (2) evaluation of medical charts for signs of delirium i.e. altered consciousness and > 1 symptoms of delirium, and (3) additional delirium assessments by trained researchers. If in a 24-h period at least one of these items was positive for delirium, patients were classified as delirious. In this current cohort study, the initiation of haloperidol or quetiapine was not used to classify patients as delirious. Sedation was assessed every three hours with the RASS [27]. On a daily level, an unarousable state was defined as all RASS scores on that day being < − 3 or − 3 combined with sedative use—impeding delirium assessment [28]. Patients were classified as awake without delirium if they had at least one RASS score > − 4, or > − 3 if in combination with sedative use, in a 24-h period, with all delirium assessments in that period being negative. With regards to delirium during ICU stay, patients were classified as delirious if they had at least one day with a positive delirium assessment according to the before mentioned algorithm.
Outcomes
The primary outcome was transitioning from delirium on any given day (day X) to an awake state without delirium on the following day (day X + 1). Secondary outcomes were delirium duration (in days), number of delirium and ventilation days, length of stay in the ICU and hospital (in days), and ICU mortality. Delirium duration was the number of consecutive days that a patient was classified as delirious or unarousable during the first delirium episode in the ICU. As delirium may fluctuate over time and as patients may transition between delirium and unarousable states during a delirium episode, we defined a delirium episode as ended if the patient was awake without delirium for at least two consecutive days.
Statistical analysis
Continuous data were summarized with medians and interquartile ranges (IQR) or means with standard deviations, depending on distribution. Categorical variables were shown as proportions with percentages. To describe differences between patients who were ever delirious and patients who were never delirious, continuous variables were compared using Mann–Whitney U tests or independent t tests, and categorical outcomes with Chi square tests. For differences in baseline medication exposures, we used logistic and linear regression analyses.
For the primary analysis the delirious observation days were used. We studied whether the administration of haloperidol, clonidine, or both haloperidol and clonidine on any given delirium day (day X) was associated with a transition to an awake state without delirium on the following day (day X + 1), compared to the probability of either remaining delirious or transitioning to unarousable state on day X + 1, with mortality and discharge on day X + 1 being competing events (Online Resource 1). The combination of delirium and unarousable state on the following day was chosen as a comparator, as we specifically aimed to assess delirium resolution as transition to an awake state without delirium and to minimize confounding of the treatment to the delirium assessment due to sedative side-effects of haloperidol [29] and clonidine [30]. For the primary analysis we used a first-order Markov multinomial logistic regression model. Model 1 involved administration of haloperidol only (yes/no), clonidine only (yes/no), or both haloperidol and clonidine (yes/no) on day X. Additionally, to examine dose-dependency, we modeled two main predictors (Model 2): (1) dosage of intravenously administered haloperidol in milligrams on day X, with a conversion of 0.6 per milligram enterally administered haloperidol based on bioavailability, and (2) dosage of intravenously administered clonidine in micrograms on day X. To control for confounding, we included the following covariates to both Markov models: age, APACHE IV score, and admission type (acute surgery, elective surgery, medicine). Additionally, we included the following daily covariates: patients’ mental status on day X, modified SOFA score, metabolic acidosis (yes/no), use of mechanical ventilation (yes/no), and administration of any antipsychotic other than haloperidol (yes/no), benzodiazepines or propofol (yes/no), and opioids (yes/no). These covariates were associated with delirium development, or were suspected to confound the studied association [31].
Additionally, we used propensity score matching for the primary analysis to match delirium days without medication exposure with delirium days on which haloperidol, clonidine, or both was administered. The match tolerance was set to 0.001. We adjusted for confounding by including the same covariates as in the Markov models.
Three sensitivity analyses were performed. First, as both haloperidol and clonidine may have mild sedative effects [29, 30], they may influence the CAM-ICU assessment. Likewise, agitated delirious patients may have received more haloperidol and/or clonidine than patients with hypoactive delirium. Hence, our first sensitivity analysis stratified outcomes by delirium motor subtypes, comparing “hyperactive delirium days” (all RASS scores on that delirium day > 0), “hypoactive delirium days” (all RASS scores on that delirium day ≤ 0) and “mixed delirium days” (RASS scores of both > 0 and ≤ 0 on that delirium day). Second, we assessed whether the implementation of the 2013 Pain, Agitation and Delirium guideline [32] and general changes in ICU delirium management over the years affected the resolution of delirium by assessing the effect of the inclusion period (2011–2013 versus 2015–2019). Third, as the clinical effects of haloperidol and clonidine may become apparent 24 h after administration, we examined whether there was an association of the administration of haloperidol and/or clonidine on delirium day X with transition to an awake state without delirium on day X + 2, using the same analysis as for X + 1.
Secondary analyses were performed on patient level in delirious patients instead of the level of observation-days to assess whether the administration of haloperidol, clonidine or both haloperidol and clonidine versus none of these agents during ICU stay was associated with the secondary outcomes. Linear regression models were used to analyze delirium duration, number of delirium and ventilation days, and ICU and hospital length of stay. ICU mortality was analyzed with a logistic regression model. These models were adjusted for age, APACHE IV score, admission type, highest modified SOFA score during ICU stay, presence of metabolic acidosis during ICU stay (yes/no), ventilation during ICU stay (yes/no), delirium duration (in days), and administration of any antipsychotic other than haloperidol (yes/no), benzodiazepines or propofol (yes/no) and opioids (yes/no) during ICU stay. If needed, log transformation of the variables was performed.
Analyses were performed in SPSS, version 25, and Markov multinomial logistic regression analyses were performed in R, version 3.5.1. To evaluate the goodness of fit, likelihood ratio tests, F values and R2 values were used. Observations were excluded if delirium classification was missing. A two-sided p value less than 0.05 was considered statistically significant.
Results
Study population and observation days
During the study periods, 12,380 patients were admitted to the ICU. After applying inclusion and exclusion criteria, we included 3614 patients (details outlined in Online Resource 2). Among these, 1165 (32%) experienced delirium during ICU stay and were used for the analyses. The median age was 63 years (IQR 52–72) and 2286 (63.3%) were male (Table 1). Patients with delirium were significantly older, had a higher APACHE IV score, and were more often admitted with a medical or an acute surgical diagnosis than patients without delirium.
See Online Resource 3, Table 1 for the characteristics of the observations days. Delirium occurred on 4708 (18.9%) of 24,906 observation days, which were used for the primary analysis. Haloperidol and clonidine were more frequently administered on delirium days than on days in which patients were unarousable or awake without delirium, and more often on mixed/hyperactive delirium days than hypoactive delirium days (Online Resource 3, Table 2).
No values were missing for the predictors. For the covariates, values were missing for ICU admission type (0.1%), APACHE IV score (5.3%), modified SOFA score (3.9%) and patients’ mental status on day X (14.2%).
Daily transitions and administration of haloperidol and clonidine
From the 4708 delirium days, 1101 (23.4%) patients transitioned to an awake state without delirium the following day, while 2712 (57.6%) remained delirious and 269 (5.7%) transitioned to an unarousable state. Additionally, 509 (10.8%) were discharged or died, and from 117 (2.5%) the mental status on the following day was missing. The transition flow including data on the receipt of haloperidol and clonidine is shown in Fig. 1. Patients in whom delirium resolved the following day were administered haloperidol and/or clonidine less frequently and in lower dosages than patients who remained delirious or transitioned to an unarousable state.
Daily transitions of mental status including administration of haloperidol and clonidine on delirium day X with median dosages. CI confidence interval, IQR interquartile range, Mcg microgram, Mg milligram, OR Odds ratio. a509 (10.8%) patients were deceased or discharged on day X + 1 and for 117 (2.5%) patients the mental status on the following day was missing. bSignificant (p < 0.001) difference in frequency of administration as compared to delirium or unarousable on day X + 1, tested with logistic regression models, adjusted for age, APACHE IV score, admission type (acute surgery, elective surgery, medical reason), patients’ mental status on day X, modified SOFA score, metabolic acidosis, use of ventilation, and administration of any antipsychotic other than haloperidol, benzodiazepines, propofol and opioids. Adjusted OR (95%CI) for haloperidol only was 0.66 (0.55–0.78) and for both haloperidol and clonidine 0.59 (0.48–0.73). cSignificant (p < 0.05) difference in dose of administered medication as compared to delirium or unarousable on day X + 1, tested with linear regression models, adjusted for age, APACHE IV score, admission type (acute surgery, elective surgery, medical reason), patients’ mental status on day X, modified SOFA score, metabolic acidosis, use of ventilation, and administration of any antipsychotic other than haloperidol, benzodiazepines, propofol and opioids. Adjusted OR (95% CI) for haloperidol only was 0.96 (0.93–0.98), for clonidine only 0.88 (0.83–0.93), for haloperidol in case of both haloperidol and clonidine administration 0.95 (0.92–0.98) and for clonidine in case of both haloperidol and clonidine administration 0.9 (0.85–0.95)
Markov modeling and propensity score matching of haloperidol and clonidine administration and delirium resolution
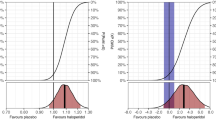
As shown in Table 2, the administration of haloperidol on a delirium day was associated with significant lower odds of delirium resolution on the following day (OR 0.47; 95% CI 0.39–0.57), as was administration of clonidine (OR 0.78; 95%CI 0.63–0.97) and the use of both haloperidol and clonidine (OR 0.45; 95% CI 0.36–0.56). Propensity score matching yielded similar results as our primary analyses (Online Resource 4).
Further, the odds ratio of delirium resolution was 0.84 (95% CI 0.81–0.88) for every 1 mg administered haloperidol, and 0.98 (95% CI 0.97–1) per 100 µg clonidine (Model 2, Table 3).
The results of the three sensitivity analyses are reported in Online Resource 5. First, due to the low number of hyperactive delirium days, we analyzed these days together with mixed delirium days. The use of haloperidol or both haloperidol and clonidine on a hyperactive/mixed or hypoactive delirium day was associated with significantly lower odds on delirium resolution, while clonidine was not. Second, similar findings were obtained for the inclusion periods 2011–2013 and 2015–2019, with administration of clonidine only being associated with lower odds of delirium resolution for inclusion period 2015–2019 only. Last, lower probabilities of delirium resolution on day X + 2 were found for haloperidol only and both haloperidol and clonidine, but not for clonidine only.
Secondary outcomes
As shown in Table 4, the administration of haloperidol, clonidine, or both haloperidol and clonidine in delirious patients was associated with increased delirium duration, more delirium days, and a longer stay in the ICU. These patients also spent more time in the hospital than untreated delirious patients, with exception for patients who were administered clonidine only. Additionally, the number of ventilation days was higher in patients who were administered both haloperidol and clonidine. There was no difference in ICU mortality.
Discussion
In summary, our study suggests that treatment of ICU delirium with haloperidol, clonidine, or both haloperidol and clonidine might reduce the probability of delirium resolution. These associations were dose-dependent, and consistent with almost all secondary outcomes.
Our findings with regards to haloperidol as delirium treatment are in line with previous observational studies, in which higher delirium rates were found with haloperidol use [33]. In non-intubated patients, each additional milligram of haloperidol was associated with 5% higher odds of delirium on the following day [34]. Another retrospective study found that treatment of delirious patients with antipsychotics (quetiapine, olanzapine, risperidone or haloperidol) was associated with a longer delirium duration and ICU stay [35]. However, our observations that haloperidol might be associated with reduced probability of delirium resolution and increased delirium duration are partly in contrast with two recent randomized controlled trials [12, 36]. These trials found that haloperidol is not associated with prevention or resolution of delirium, and the number of delirium days. These differences may be explained by our time-dependent Markov analysis focusing on daily transitions, instead of the number of delirium days in a certain (limited) study period. We found that the use of haloperidol and clonidine on delirium days was associated with reduced probabilities of delirium resolution within 48 h after administration. It might be that these agents have negative short-term effects on delirium resolution, but that these effects will disappear after 48 h or more. Second, our results may differ due to differences in study populations and dosage regimens. We had a high percentage of surgical patients (66.1%) as compared to 28% in the study by Girard et al. [12]. Finally, the optimal dosage of haloperidol and/or clonidine as delirium treatment remains unknown. Despite the dose-dependent association shown in our cohort, the dosages might have been too low to deliver a positive effect on delirium resolution.
The efficacy of clonidine, alone or in combination with haloperidol, as ICU delirium treatment has been studied less extensively, possibly because intravenous clonidine is not available in the United States. A small placebo-controlled prevention trial in patients who underwent surgical correction of acute type-A aortic dissection showed no beneficial effect of intravenous clonidine on delirium incidence, but did report lower delirium severity [18]. Our results may challenge clonidine as treatment in all patients with delirium. However, confirmation in a randomized trial or another cohort study is warranted.
While we had expected that treatment with haloperidol and/or clonidine would increase the likelihood of resolving delirium, our results do not support this. An explanation may be that haloperidol and clonidine induce hypoactive symptoms, and thereby interfere with delirium assessment. However, haloperidol and clonidine dosages in our cohort were relatively low. Subsequently, the probability of false positive delirium assessments due to sedative effects of these drugs is likely to be low. In addition, our primary outcome comparator involved a combination of delirium and unarousable state and not exclusively delirium, and the validated delirium algorithm that was used to classify delirium was based on a comprehensive assessment of several other symptoms of delirium [22].
A few other limitations should be considered. First, this was a single-center observational study in patients who were mostly mechanically ventilated, which may limit generalizability. Second, classifying a patients’ mental status as being either awake without delirium, delirium or unarousable on a daily basis may be a simplification, as mental status may not be categorizable and delirium may fluctuate over the course of the day. Similarly, some delirious patients may have experienced rapidly reversible, sedation-related delirium, which seem to have a prognosis similar to that seen in non-delirious patients [37, 38]. Third, we did not examine whether haloperidol and clonidine were associated with reduction of psychotic features and agitation. Hence, we are not able to make any recommendations regarding treatment of such features. A last important methodological concern is that daily transitions of mental states were assumed to be independent of the patient history beyond the prior day. While the covariables were derived from a systematic review [31], it is possible that unmeasured confounding influenced the results. Additionally, despite using propensity scoring to match groups, more patients with severe delirium, and thus a low risk of delirium resolution, may have received haloperidol or clonidine than patients who had lower delirium severity, which can be regarded as confounding by indication. The sensitivity analysis with respect to delirium subtypes may have dealt with any possible confounding by delirium motor subtypes or delirium severity. It would be important to study the treatment effects of haloperidol and clonidine in a future randomized clinical trial.
A key strength is that this study is the first to investigate haloperidol and clonidine as delirium treatment, both separately as well as in combination, in the same study population. We made use of a validated delirium-recognition algorithm that advocated frequent CAM-ICU assessment and incorporated additional criteria to define delirium [22]. Another strength is the large sample size, allowing us to adjust for numerous confounders. Additionally, we could investigate temporal relationships between administration of medication and transitions of mental states, which allowed us to take account of the factors that varied over time during ICU admission.
Conclusion
In conclusion, this large cohort study suggests that administration of haloperidol, clonidine, or both, in delirious ICU patients may be associated with reduced probabilities of short-term delirium resolution and worse short-term clinical outcomes, such as longer delirium episodes and longer ICU stay. However, although adjusted for known confounders, these results should be regarded as hypothesis generating and future studies are needed to inform clinical practice. We suggest that in such studies symptomatic treatment of psychotic features or agitation should be differentiated from delirium diagnosis as a syndrome.
Availability of data and materials
Data are available upon request.
Code availability
Not applicable.
References
Ely EW, Girard TD, Shintani AK, Jackson JC et al (2007) Apolipoprotein E4 polymorphism as a genetic predisposition to delirium in critically ill patients. Crit Care Med 35(1):112–117
van den Boogaard M, Schoonhoven L, van der Hoeven JG, van Achterberg T et al (2012) Incidence and short-term consequences of delirium in critically ill patients: a prospective observational cohort study. Int J Nurs Stud 49(7):775–783
Mehta S, Cook D, Devlin JW, Skrobik Y et al (2015) Prevalence, risk factors, and outcomes of delirium in mechanically ventilated adults. Crit Care Med 43(3):557–566
Slooter AJC, Otte WM, Devlin JW, Arora RC et al (2020) Updated nomenclature of delirium and acute encephalopathy: statement of ten Societies. Intensive Care Med. 46:1020–1022
Ely EW, Gautam S, Margolin R, Francis J et al (2001) The impact of delirium in the intensive care unit on hospital length of stay. Intensive Care Med 27(12):1892–1900
Milbrandt EB, Deppen S, Harrison PL, Shintani AK et al (2004) Costs associated with delirium in mechanically ventilated patients. Crit Care Med 32(4):955–962
van den Boogaard M, Schoonhoven L, Evers AW, van der Hoeven JG et al (2012) Delirium in critically ill patients: impact on long-term health-related quality of life and cognitive functioning. Crit Care Med 40(1):112–118
Pandharipande PP, Girard TD, Jackson JC, Morandi A et al (2013) Long-term cognitive impairment after critical illness. N Engl J Med 369(14):1306–1316
Devlin JW, Skrobik Y, Gélinas C, Needham DM et al (2018) Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med 46(9):e825–e873
Collet MO, Caballero J, Sonneville R, Bozza FA et al (2018) Prevalence and risk factors related to haloperidol use for delirium in adult intensive care patients: the multinational AID-ICU inception cohort study. Intensive Care Med 44(7):1081–1089
Girard TD, Pandharipande PP, Carson SS, Schmidt GA et al (2010) Feasibility, efficacy, and safety of antipsychotics for intensive care unit delirium: the MIND randomized, placebo-controlled trial. Crit Care Med 38(2):428–437
Girard TD, Exline MC, Carson SS, Hough CL et al (2018) Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 379:2506–2516
Maldonado JR (2013) Neuropathogenesis of delirium: review of current etiologic theories and common pathways. Am J Geriatr Psychiatry 21(12):1190–1222
Reade MC, Eastwood GM, Bellomo R, Bailey M et al (2016) Effect of dexmedetomidine added to standard care on ventilator-free time in patients with agitated delirium: a randomized clinical trial. JAMA 315(14):1460–1468
Reade MC, O’Sullivan K, Bates S, Goldsmith D et al (2009) Dexmedetomidine vs. haloperidol in delirious, agitated, intubated patients: a randomised open-label trial. Crit Care. 13(3):R75
Gagnon DJ, Riker RR, Glisic EK, Kelner A et al (2015) Transition from dexmedetomidine to enteral clonidine for ICU sedation: an observational pilot study. Pharmacotherapy 35(3):251–259
Tonner PH, Weiler N, Paris A, Scholz J (2003) Sedation and analgesia in the intensive care unit. Curr Opin Anaesthesiol 16(2):113–121
Rubino AS, Onorati F, Caroleo S, Galato E et al (2010) Impact of clonidine administration on delirium and related respiratory weaning after surgical correction of acute type-A aortic dissection: results of a pilot study. Interact Cardiovasc Thorac Surg 10(1):58–62
Burry L, Hutton B, Williamson DR, Mehta S et al (2019) Pharmacological interventions for the treatment of delirium in critically ill adults. Cochrane Database Syst Rev 9:C011749
Klein Klouwenberg PM, Zaal IJ, Spitoni C, Ong DS et al (2014) The attributable mortality of delirium in critically ill patients: prospective cohort study. BMJ 349:g6652
Wolters AE, Zaal IJ, Veldhuijzen DS, Cremer OL et al (2015) Anticholinergic medication use and transition to delirium in critically ill patients: a prospective cohort study. Crit Care Med 43(9):1846–1852
Zaal IJ, Tekatli H, van der Kooi AW, Klijn FA et al (2015) Classification of daily mental status in critically ill patients for research purposes. J Crit Care 30(2):375–380
van Eijk MM, van den Boogaard M, van Marum RJ, Benner P et al (2011) Routine use of the confusion assessment method for the intensive care unit a multicenter study. Am J Respir Crit Care Med 184(3):340–344
von Elm E, Altman DG, Egger M, Pocock SJ et al (2007) The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 370(9596):1453–1457
Zimmerman JE, Kramer AA, McNair DS, Malila FM (2006) Acute Physiology and Chronic Health Evaluation (APACHE) IV: hospital mortality assessment for today’s critically ill patients. Crit Care Med 34(5):1297–1310
Vincent JL, Moreno R, Takala J, Willatts S et al (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. Intensive Care Med 22(7):707–710
Sessler CN, Gosnell MS, Grap MJ, Brophy GM et al (2002) The Richmond Agitation-Sedation Scale: validity and reliability in adult intensive care unit patients. Am J Respir Crit Care Med 166(10):1338–1344
Ely EW, Inouye SK, Bernard GR, Gordon S et al (2001) Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA 286(21):2703–2710
Page VJ, Ely EW, Gates S, Zhao XB et al (2013) Effect of intravenous haloperidol on the duration of delirium and coma in critically ill patients (Hope-ICU): a randomised, double-blind, placebo-controlled trial. Lancet Respir Med 1(7):515–523
Pichot C, Ghignone M, Quintin L (2012) Dexmedetomidine and clonidine: from second- to first-line sedative agents in the critical care setting? J Intensive Care Med 27(4):219–237
Zaal IJ, Devlin JW, Peelen LM, Slooter AJ (2015) A systematic review of risk factors for delirium in the ICU. Crit Care Med 43(1):40–47
Barr J, Fraser GL, Puntillo K, Ely EW et al (2013) Clinical practice guidelines for the management of pain, agitation, and delirium in adult patients in the intensive care unit. Crit Care Med 41(1):263–306
Pisani MA, Murphy TE, Araujo KL, Slattum P et al (2009) Benzodiazepine and opioid use and the duration of intensive care unit delirium in an older population. Crit Care Med 37(1):177–183
Pisani MA, Araujo KL, Murphy TE (2015) Association of cumulative dose of haloperidol with next-day delirium in older medical ICU patients. Crit Care Med 43(5):996–1002
Weaver CB, Kane-Gill SL, Gunn SR, Kirisci L et al (2017) A retrospective analysis of the effectiveness of antipsychotics in the treatment of ICU delirium. J Crit Care 41:234–239
van den Boogaard M, Slooter AJC, Bruggemann RJM, Schoonhoven L et al (2018) Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA 319(7):680–690
Patel SB, Poston JT, Pohlman A, Hall JB et al (2014) Rapidly reversible, sedation-related delirium versus persistent delirium in the intensive care unit. Am J Respir Crit Care Med 189(6):658–665
Kenes MT, Stollings JL, Wang L, Girard TD et al (2017) Persistence of delirium after cessation of sedatives and analgesics and impact on clinical outcomes in critically ill patients. Pharmacotherapy 37(11):1357–1365
Funding
No funding or financial support was received.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation and data collection were performed by IJZ. SD-K and LS performed the data analyses. The first draft of the manuscript was written by LS and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
All authors declare no conflicts of interest.
Ethics approval
The ethics committee of the University Medical Center Utrecht waived the need for IRB approval (IRB #19-768/c).
Consent to participate
No signed informed consent from patient was needed because of the observational design.
Consent for publication
Not applicable.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Smit, L., Dijkstra-Kersten, S.M.A., Zaal, I.J. et al. Haloperidol, clonidine and resolution of delirium in critically ill patients: a prospective cohort study. Intensive Care Med 47, 316–324 (2021). https://doi.org/10.1007/s00134-021-06355-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-021-06355-9