Abstract
Purpose
To determine clinical predictors associated with corticosteroid administration and its association with ICU mortality in critically ill patients with severe influenza pneumonia.
Methods
Secondary analysis of a prospective cohort study of critically ill patients with confirmed influenza pneumonia admitted to 148 ICUs in Spain between June 2009 and April 2014. Patients who received corticosteroid treatment for causes other than viral pneumonia (e.g., refractory septic shock and asthma or chronic obstructive pulmonary disease [COPD] exacerbation) were excluded. Patients with corticosteroid therapy were compared with those without corticosteroid therapy. We use a propensity score (PS) matching analysis to reduce confounding factors. The primary outcome was ICU mortality. Cox proportional hazards and competing risks analysis was performed to assess the impact of corticosteroids on ICU mortality.
Results
A total of 1846 patients with primary influenza pneumonia were enrolled. Corticosteroids were administered in 604 (32.7%) patients, with methylprednisolone the most frequently used corticosteroid (578/604 [95.7%]). The median daily dose was equivalent to 80 mg of methylprednisolone (IQR 60–120) for a median duration of 7 days (IQR 5–10). Asthma, COPD, hematological disease, and the need for mechanical ventilation were independently associated with corticosteroid use. Crude ICU mortality was higher in patients who received corticosteroids (27.5%) than in patients who did not receive corticosteroids (18.8%, p < 0.001). After PS matching, corticosteroid use was associated with ICU mortality in the Cox (HR = 1.32 [95% CI 1.08–1.60], p < 0.006) and competing risks analysis (SHR = 1.37 [95% CI 1.12–1.68], p = 0.001).
Conclusion
Administration of corticosteroids in patients with severe influenza pneumonia is associated with increased ICU mortality, and these agents should not be used as co-adjuvant therapy.
Similar content being viewed by others
Systemic corticosteroids have been widely used as co-adjuvant therapy in patients ARF/ARDS due to influenza pneumonia to modulate lung inflammation, despite controversy on clinical outcomes. Our findings provide solid evidence to support the association of corticosteroids administration with increased ICU mortality in critically ill patients with influenza pneumonia. |
Introduction
Pneumonia caused by the influenza A(H1N1)pdm09 virus infection may lead to life-threatening acute respiratory failure (ARF) and acute respiratory distress syndrome (ARDS). Antiviral therapy is the cornerstone of treatment for influenza pneumonia [1,2,3]; in addition, intravenous corticosteroids have been used as co-adjuvant therapy in patients with ARF/ARDS to modulate lung inflammation and improve clinical outcomes [4,5,6,7,8]. However, no randomized clinical trials have investigated the potential benefit or harm of corticosteroid therapy for ARF/ARDS due to acute influenza pneumonia.
During the 2009 H1N1 pandemic, corticosteroids were widely used despite contradictory [9, 10], unfavorable [7, 9,10,11], or inconclusive [12, 13] available data. A recent Cochrane review [14] concluded that co-adjuvant corticosteroid therapy was associated with increased mortality in patients with influenza pneumonia. However, the data were derived from observational studies of very low quality and with several methodological limitations, including other clinical indications of corticosteroids as a major potential concern. Thus, it is impossible to be sure that patients who were treated with corticosteroids did not have other corticosteroid indications or were not more severely ill in the first place. We have previously reported that corticosteroid therapy does not improve survival in patients with primary viral pneumonia [12]. However, in that observational study, we assessed the effects of corticosteroid therapy on survival between patients who were and were not treated, but we did not apply a statistical method that would have balanced all the variables between the two groups. Therefore, the aim of the present study was to identify the factors associated with corticosteroid use and its impact on intensive care unit (ICU) mortality using propensity score (PS) matching analysis in ICU patients with influenza pneumonia. Preliminary results of this analysis were presented in the 38th International Symposium on Intensive Care and Emergency Medicine [15].
Materials and methods
Study participants
This was a secondary analysis of prospective and observational cohorts of critically ill subjects admitted to 148 ICUs in Spain (which represents approximately 50% of the country's ICUs) between June 2009 and April 2014. Data were obtained from a voluntary registry created by SEMICYUC (Sociedad Española de Medicina Intensiva, Crítica y Unidades Coronarias). All consecutive cases admitted to the ICU were collected.
The study was approved by the Joan XXIII University Hospital Ethics Committee (IRB#11809). Patient identity remained anonymous, and the requirement for informed consent was waived due to the observational nature of the study, as reported elsewhere [3, 16,17,18,19,20].
Inclusion criteria Participants included patients admitted with fever (> 38 °C); respiratory symptoms consistent with cough, sore throat, myalgia, or influenza-like illness; acute respiratory failure requiring ICU admission; and microbiological confirmation of viral A, B, or C infection identified by reverse transcription polymerase chain reaction (rt-PCR) at ICU admission. Data were reported by the attending physician reviewing medical charts and radiological and laboratory records. The attending physician ordered all tests and procedures related to patient care.
Exclusion criteria Patients receiving corticosteroids as rescue therapy (due to shock) or due to chronic obstructive pulmonary disease (COPD)/asthma exacerbation were excluded (see definition below). Children < 15 years old were not enrolled in the study. Patients with non-pulmonary influenza infection and those with healthcare-associated pneumonia were also excluded.
The following variables were recorded at ICU admission: demographic data, comorbidities, time from illness onset to hospital admission, time to first dose of antiviral delivery, microbiological findings, and laboratory and chest radiological findings at ICU admission (all the collected variables are reported in e-Table 1 of the supplementary material). To determine illness severity, the Acute Physiology and Chronic Health Evaluation (APACHE) II score [21] was estimated for all patients within 24 h of ICU admission. Organ failure was assessed using the Sequential Organ Failure Assessment (SOFA) scoring system [22], also at ICU admission. The indication of corticosteroid treatment was clearly reported in the case report form and was confirmed by the medical records.
Study definitions
Community-acquired pneumonia (CAP) was defined in accordance with current American Thoracic Society and Infectious Diseases Society of America guidelines (ATS/IDSA) [23].
The rt-PCR test for influenza was carried out in accordance with the guidelines of the Centers for Disease Control and Prevention (CDC) [24].
Primary viral pneumonia was defined as acute respiratory failure and unequivocal alveolar opacities involving two or more lobes, with negative respiratory and blood bacterial cultures during the acute phase of influenza virus infection at ICU admission [5].
COPD “exacerbation” was defined according to COPD exacerbation guidelines of the European Respiratory Society/ATS [25] as increased respiratory symptoms, particularly dyspnea, cough, and increased sputum purulence without pulmonary infiltrates in chest X-ray. COPD patients with pulmonary infiltrates in chest X-ray were considered as CAP and were included in the present analysis.
Asthma exacerbation was defined as acute or subacute episodes characterized by a progressive increase in one or more typical asthmatic symptoms (dyspnea, coughing, wheezing, and tightness of the chest [26] without infiltrates in the chest X-ray. Asthmatic patients with pulmonary infiltrates in chest X-ray were considered as CAP and were included in the present analysis.
Community-acquired respiratory co-infection (CARC) was considered in patients with confirmation of influenza virus infection showing recurrence of fever, increase in cough and production of purulent sputum plus positive bacterial/fungal respiratory or blood cultures at ICU admission [27, 28].
Refractory septic shock was defined in accordance with the Surviving Sepsis Campaign guidelines [29]; that is, patients in whom adequate fluid resuscitation and vasopressor therapy are unable to restore hemodynamic stability.
Ventilator-associated pneumonia was defined according to the new ATS/IDSA guidelines [30] among ICU patients who developed a new pneumonic event while mechanically ventilated for at least 48 h after clinical presentation.
Corticosteroid treatment: we considered the primary indication recorded by the treating physician as co-adjuvant treatment for viral pneumonia. Corticosteroid therapy was defined as corticosteroid administration at ICU admission (within the first 24 h). Patients receiving corticosteroids as rescue therapy (due to shock) or due to COPD/asthma exacerbation were excluded (see exclusion criteria).
Obese patients were defined as those with a body mass index (BMI) of > 30 kg/m2.
The ICU admission criteria and treatment decisions for all patients, including the decision to intubate and type of antibiotic, antiviral, or corticosteroid therapy administered, were not standardized between centers and were left to the discretion of the attending physician, according to the Spanish Society of Intensive Care recommendations [31].
Endpoints
Primary To determine whether corticosteroid use was associated with ICU mortality. In addition, the primary outcome was examined in eight pre-specified subgroups defined according to the following baseline characteristics: (1) severity of illness (APACHE score < 15 vs. ≥ 15), (2) intensity of organ dysfunction (SOFA < 5 vs. ≥ 5), (3) presence of shock upon ICU (yes vs. no), (4) need for mechanical ventilation (MV) upon ICU admission (yes vs. no), (5) inflammatory response to C-reactive protein (CRP < 25 vs. ≥ 25 mg/dL), (6) presence of bacterial co-infection (yes vs. no), (7) chronic lung disease such as COPD (yes vs. no), and (8) asthma (yes vs. no). The cut-off for continuous variables was determined according to our population median value.
Secondary To determine risk factors associated with corticosteroid use. ICU length of stay (LOS) and MV days were also examined in survivors between groups receiving and not receiving corticosteroid therapy.
Statistical analysis
Discrete variables were expressed as counts (percentage) and continuous variables as means with standard deviation (SD) or medians and interquartile range 25–75% (IQR). For patient demographics and clinical characteristics, differences between groups were assessed using the chi-squared test and Fisher’s exact test for categorical variables, and the Student t test or the Mann–Whitney U test for continuous variables.
To investigate the association between baseline (ICU admission) variables and corticosteroid use, a multivariate analysis (binary logistic regression) was performed. The multivariate model comprised factors of clinical interest and all significant covariates in the univariate analysis. The results are presented as odds ratios (OR) and 95% confidence intervals (CI). Model integrity was examined using standard diagnostic statistics and plots and goodness of fit for each model for all outcomes, and was assessed with the Hosmer–Lemeshow test.
After this first approach, we generated a full-matching PS analysis in order to minimize the effect of a corticosteroid treatment selection bias and to control for potential confounding factors (additional information about the PS full-matching analysis can be found in the electronic supplementary material) [32]. This allowed us to study two comparable (almost identical) cohorts: (1) the corticosteroid-treated group and (2) the control group, comprising patients who did not receive corticosteroid treatment. PS matching analysis attempts to compare outcomes between patients who have a similar distribution of all the covariates measured. An attractive feature of this approach is that it uses the entire sample. Using the PS methodology, all patients were assigned a weight between 0 and 1; this propensity-matched cohort was generated by choosing the best weight balance. This method optimizes the post-weighting balance of covariates between groups and, in this way, approximates the conditions of random site-of-treatment assignment. To assess our PS adjustment, we checked for adequate overlap in propensity scores between groups with a cross-validation model. To do so, we divided the patients in the database into two subsets: (a) a “training set” with 1466 patients (80%), and (b) a “validation set” with 366 patients (20%).
After the matching, a Kaplan–Meier survival plot was generated to track ICU mortality over time for corticosteroid-treated and untreated patients. In addition, Cox proportional hazards regression models were fitted to assess the impact of corticosteroids on ICU mortality. The results are presented as hazard ratios (HR) and 95% CI and adjusted survival plots. Because Cox hazard survival analysis is not satisfactory for describing ICU patient mortality over time [33], we performed a competing risks analysis to confirm our results. First, we computed the cumulative incidence function (CIF) of death over time. At time t, the CIF defines the probability of dying in the ICU by that time t when the population can be discharged alive. The CIF was estimated from the data using the cmprsk package developed by Gray [34]. We used the Fine and Gray model [35], which extends the Cox model to competing risks data by considering the sub-distribution hazard (for instance, the hazard function associated with the CIF). The strength of the association between each variable and the outcome was assessed using the sub-hazard ratio (SHR), which is the ratio of hazards associated with the CIF in the presence of and in the absence of a prognostic factor.
In order to avoid spurious associations, the variables that we entered in the regression models were those with a relationship in the univariate analysis (p < 0.05) or a plausible relationship with the dependent variable. Data analysis was performed using SPSS for Windows version 22.0 (IBM Corp., Armonk, NY, USA). Mixed-effects models were performed with R (cran.r-project.org).
Results
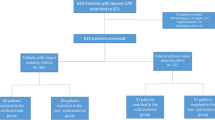
A total of 2684 patients with confirmed influenza pneumonia were enrolled at 148 ICUs in Spain during the study period (2009–2014). Of these, 1846 (68.7%) met the inclusion criteria and were included in the study (Fig. 1).
Comparison between subjects with and without corticosteroid therapy
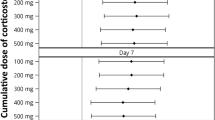
Among 604 patients with corticosteroid therapy, 578 (95.7%) received methylprednisolone, 23 (3.8%) prednisolone, and three (0.5%) dexamethasone. For all patients who received therapy with corticosteroids due to pneumonia, this was initiated within the first 24 h of ICU admission. Patients received a median (interquartile range [IQR]) daily dose equivalent to 80 (60–120) mg of methylprednisolone, and the median duration of corticosteroid treatment was 7 (5–10) days. The frequency of corticosteroid treatment by study period was 34.9% in 2009, 39.6% in 2010, 29% in 2013, and 31.4% in 2014. Considering the 2009 period as baseline, we observed that only in 2013 was there a significant reduction (p = 0.02) in the indication of corticosteroid treatment as co-adjuvant therapy for pneumonia. No differences in the rate of ventilator-associated pneumonia were observed between patients with (n = 46, 7.6%) and without (n = 80, 6.4%) corticosteroid therapy.
Clinical characteristics of patients and their distribution in the two groups are shown in Table 1. Patients who received corticosteroid therapy were sicker according to the APACHE II score, more obese, and more likely to have asthma, COPD, and hematological diseases than those who did not receive treatment. MV use, serum procalcitonin concentrations, and ICU mortality rate were higher in patients who received corticosteroids. There were no significant differences between groups regarding ICU LOS or MV days. No other differences were found between the groups. Overall mortality was 21.6% (400/1846).
Factors for corticosteroid use in subjects with influenza pneumonia infection
To determine factors associated with corticosteroid use, a stepwise logistic regression model was performed. APACHE II score, asthma, COPD, obesity, hematological disease, and MV were the independent variables included in the model. As shown in Table 2, MV (OR = 1.78), asthma (OR = 2.38), COPD (OR = 2.10), and hematological disease (OR = 2.51) were independently associated with corticosteroid use.
Mortality analysis
In all, 166 of 604 patients (27.5%) who received corticosteroid therapy died in the ICU, compared with 234 of 1242 (18.8%) patients who did not receive corticosteroids (OR = 1.6 [95% CI 1.3–2.0], p < 0.001). There were significant between-group differences in the rate of ICU death (Fig. 2), with the exception of patients with SOFA scores < 5 and CRP < 25 mg/dL, non-ventilated patients, patients with bacterial co-infection, and patients with asthma. Three hundred eighty-seven patients (26%) had CARC at ICU admission, and 111 (28.6%) of them died. The most frequently isolated microorganism was Streptococcus pneumoniae (n = 190; 49.1%), followed by Pseudomonas aeruginosa (n = 39; 10.1%), methicillin-sensitive Staphylococcus aureus (n = 29; 7.5%), Aspergillus spp. (n = 21; 5.5%), and Streptococcus pyogenes (n = 15; 3.9%).
Corticosteroid use was independently associated with ICU mortality (OR = 1.37 [95% CI 1.01–1.87], p = 0.001; e-Table 2, supplementary material). Corticosteroid therapy was associated with higher mortality in both A(H1N1)pdm09 virus (27.1% vs. 18.8%, p = 0.001) and non A(H1N1)pdm09 (29.8% vs. 19.5%; e-Table 3, supplementary material).
PS matching was applied, and 1242 control and 604 treated patients were matched. The summaries of balance for unmatched and matched data are shown in Table 3 (and e-Fig. 1 in the supplementary material). The APACHE II score, SOFA score, delay at ICU admission, number of quadrants infiltrated in chest X-ray, serum lactate dehydrogenase (LDH), white blood cell (WBC) count, continuous renal replacement therapy (CRRT), serum CRP, MV, shock, chronic heart disease, human immunodeficiency virus (HIV/AIDS), primary viral pneumonia, bacterial co-infection, and corticosteroid use were the variables included in the logistic regression analysis of the PS model.
The discriminatory power of the model (e-Fig. 2, supplementary material) was good, with an area under the receiver operating characteristic curve of 0.82 (95% CI 0.77–0.87, p < 0.01). The accuracy of the predictive model (training set) with respect to the validation set was 0.82. e-Figure 3 (supplementary material) shows the Kaplan–Meier estimates of the mortality rate during ICU admission, differentiating between patients with and without corticosteroid use. The cumulative survival was lower in patients with corticosteroid therapy than in untreated patients (log-rank test 560.6, p < 0.001). When we excluded patients with CARC, the results were similar (log-rank test 5.175, p = 0.02; e-Fig. 4, supplementary material). However, in patients with CARC, only a trend towards higher mortality related to corticosteroid treatment was observed (log-rank test 0.249, p = 0.61; e-Fig. 5, supplementary material).
Finally, to determine the impact of corticosteroid use on ICU mortality, a Cox regression analysis adjusted for APACHE II and potential confounding factors (see e-Fig. 6 in supplementary material) was performed. The survival plot (Fig. 3) showed that the use of corticosteroids was significantly associated with a higher ICU mortality rate (HR 1.32 [95% CI 1.08–1.60], p < 0.006). When a multivariate Fine and Gray regression model was used (Fig. 4 and e-Table 4 in the supplementary material), corticosteroid use remained as a factor associated with mortality (SHR = 1.37 [95% CI 1.12–1.68], p < 0.001).
Discussion
Our results strongly suggest that administration of corticosteroids as co-adjuvant therapy to standard antiviral treatment in critically ill patients with severe influenza pneumonia is associated with increased ICU mortality. This negative effect was evident in all subgroups considered and after careful adjustments, including a PS matching analysis.
To assess the potential effects of corticosteroids on these severely ill patients, we limited our analysis to a well-defined cohort of ICU patients with severe influenza pneumonia, and excluded those with other indications for corticosteroid use. The effect analysis of corticosteroids was restricted to early administration (within the first 24 h of ICU admission) in order to avoid the inclusion of patients receiving rescue therapy and to reduce the effects of time-dependent confounders. We found that MV, asthma, COPD, and hematological disease were independently associated with corticosteroid use.
Severe acute lung injury following influenza infection is characterized by uncontrolled local and systemic inflammation [36,37,38]. This damage is caused by an excessive host innate response with exaggerated migration of macrophages, neutrophils, and pro-inflammatory cytokines, leading to classic exudative diffuse alveolar damage, severe necrotizing bronchiolitis with predominantly neutrophilic inflammation, and intense alveolar hemorrhage [4]. Corticosteroids have several anti-inflammatory, immunomodulatory, and vascular properties, including inhibition of pro-inflammatory cytokines, reduction of leukocyte trafficking, stimulation of apoptosis in T-lymphocytes, and maintenance of endothelial integrity and vascular permeability. Therefore, they may represent an option for adjunctive therapy; however, although they are frequently prescribed in critically ill patients with influenza pneumonia, their potential benefits and harms are controversial [4, 7, 9, 39, 40].
Three recent systematic reviews and meta-analyses [41,42,43] concluded that corticosteroid therapy is significantly associated with mortality, even in the subgroup of patients with influenza hospitalized in or outside the ICU. These systematic reviews recognize similar limitations such as the heterogeneity of the studies, lack of sufficient data on indication for corticosteroids, dosage, therapy timing, type of corticosteroid use, and severity of illness. A recent Cochrane review [14] reported an association between corticosteroid therapy and increased mortality. However, all studies included were observational (only seven studies included patients admitted to the ICU) and of very low quality due to confounding by indication. Therefore, it was impossible to determine whether additional corticosteroid therapy is indeed harmful in patients with influenza infection.
Several observational studies have evaluated the impact of corticosteroids on mortality in patients with influenza infection [6,7,8,9, 11, 14, 40, 44,45,46], and have offered conflicting perspectives. Observational studies are potentially susceptible to bias and do not provide robust results. Despite these weaknesses, however, observational data are representative of current clinical practice, and applying modern methods such as PS matching may help in evaluating the effects of certain interventions in clinical settings and may help to guide decision-making.
To the best of our knowledge, only one study has used an analysis similar to ours in patients with influenza infection. In 245 critically ill patients, Kim et al. [11] analyzed the effect of corticosteroid treatment on 90-day mortality with a similar methodology to ours, applying multivariate adjustment (controlling for variables that differed between the two groups and incorporating the PS) and PS matching (1:1). Sixty-five pairs were generated, and the 90-day mortality rate was higher in the corticosteroid group (54% vs. 31%, p = 0.004). These data are in concordance with our results; however, the mortality rate in our patients was substantially lower. This discrepancy might be due to several factors, including differences in severity of illness, endpoint observational period (ICU mortality vs. 90-day mortality), and early recognition vs. standard of care. Interestingly, Kim et al. reported that half of the patients treated with corticosteroids received hydrocortisone, a non-standard co-adjuvant treatment of pneumonia. The authors did not report the treatment indication for corticosteroid therapy; thus many patients in this cohort may have received corticosteroids for a reason other than influenza-induced acute lung injury. In contrast, our population comprised only patients treated with corticosteroids as an co-adjuvant therapy for severe viral pneumonia, excluding patients with other indications for corticosteroids (such as shock). Therefore, with a homogeneous group of critically ill patients, and after carefully controlling for important confounders through a PS matching analysis and competing risks analysis, we provide robust evidence to support the association between corticosteroid administration and increased mortality.
Interestingly, the subgroup analysis showed that, in contrast to patients with asthma, COPD patients treated with corticosteroids had a higher risk of ICU mortality than those without corticosteroid therapy. We are not able to explain this finding using our database because we did not collect data on the degree of COPD severity. COPD patients may be at an advanced stage of disease. This condition, and other uncontrolled confounding factors, may explain the higher mortality among COPD patients even after excluding patients with COPD exacerbation.
The main strengths of this study are the homogeneous and uniform population, the high number of critically ill patients included in our multicenter study, data regarding the kind/indication of corticosteroid treatment, and the carefully executed analysis to resolve confounding factors including the presence of competing risks. However, we recognize some limitations. First, our results were obtained in a homogeneous population of patients with influenza pneumonia and cannot be extrapolated to other populations. Second, we did not review the duration of viral shedding or appearance of drug-resistant virus in either group. Third, PS matching analysis may also be a limitation, because this method may not reflect the possible biases in observational studies, and some residual confounding may persist. However, as PS matching analysis can balance the population and reduces observational bias, it is the best evidence available for physicians. Fourth, data on MV of patients were not recorded. Lung-protective ventilation is the standard of care for patients with acute lung injury/ARDS because of the evidence that it decreases mortality. Although we did not provide data regarding ventilation of patients, it is broadly accepted in this country that applying protective ventilation improves results and is one of the national quality indicators. Finally, we did not record data about muscle weakness or metabolic alterations related to corticosteroid treatment.
Conclusion
In a homogeneous group of critically ill patients with severe influenza pneumonia, after adequate adjustment by PS matching and competing risks, co-adjuvant corticosteroid therapy was significantly associated with increased ICU mortality. Our data strongly suggest that corticosteroids should not be used as co-adjuvant therapy in patients with influenza pneumonia.
References
Muthuri SG, Venkatesan S, Myles PR, Leonardi-Bee J, Al Khuwaitir TSA, Al Mamun A et al (2014) Effectiveness of neuraminidase inhibitors in reducing mortality in patients admitted to hospital with influenza A H1N1pdm09 virus infection: a meta-analysis of individual participant data. Lancet Respir Med 2:395–404
Muthuri SG, Venkatesan S, Myles PR, Leonardi-Bee J, Lim WS, Al Mamun A et al (2016) Impact of neuraminidase inhibitors on influenza A(H1N1)pdm09-related pneumonia: an IPD meta-analysis. Influenza Other Respir Viruses 10:192–204
Rodríguez A, Díaz E, Martín-Loeches I, Sandiumenge A, Canadell L, Díaz JJ et al (2011) Impact of early oseltamivir treatment on outcome in critically ill patients with 2009 pandemic influenza A. J Antimicrob Chemother 66:1140–1149
Pro Annane D (2011) The illegitimate crusade against corticosteroids for severe H1N1 pneumonia. Am J Respir Crit Care Med 183:1125–1128
Bin Cao MD, Gao Hainv, Zhou Boping, Deng Xilong, Chengping Hu, Deng Chaosheng et al (2016) Adjuvant corticosteroid treatment in adults with influenza A (H7N9) viral pneumonia*. Crit Care Med 44:e318–e328
Boudreault AA, Xie H, Leisenring W, Englund J, Corey LBM (2011) Impact of corticosteroid treatment and antiviral therapy on clinical outcomes in hematopoietic cell transplant patients infected with influenza virus. Biol Blood Marrow Transpl 17:979–986
Brun-Buisson Christian, Richard Jean-Christophe M, Alain Mercat ACMT, Laurent Brochard for the REVA-SRLF A/H1N1v 2009 Registry Group* (2011) Early corticosteroids in severe influenza A/H1N1 pneumonia and acute respiratory distress syndrome. Am J Respir Crit Care Med 183:1200–1206
Confalonieri M, Cifaldi R, Dreas L, Viviani M, Biolo M (2010) Methylprednisolone infusion for life-threatening H1N1-virus infection. Ther Adv Respir Dis 4:233–237
Linko R, Pettilä V, Ruokonen E, Varpula T, Karlsson S, Tenhunen J et al (2011) Corticosteroid therapy in intensive care unit patients with PCR-confirmed influenza A (H1N1) infection in Finland. Acta Anaesthesiol Scand 55:971–979
Quispe-laime AM, Bracco JD, Barberio PA, Campagne CG, Rolfo VE, Umberger RMG (2010) H1N1 influenza A virus-associated acute lung injury: response to combination oseltamivir and prolonged corticosteroid treatment. Intensive Care Med 36:34–41
Kim S, Hong S, Yun S, Choi W, Ahn J, Lee YJ et al (2011) Corticosteroid treatment in critically ill patients with pandemic influenza A/H1N1 2009 infection analytic strategy using propensity scores. Am J Respir Crit Care Med 183:1207–1214
Diaz E, Martin-Loeches I, Canadell L, Vidaur L, Suarez D, Socias L et al (2012) Corticosteroid therapy in patients with primary viral pneumonia due to pandemic (H1N1) 2009 influenza. J Infect 64:311–318
Martin-Loeches I, Lisboa T, Rhodes A, Moreno RP, Silva E, Sprung C et al (2011) Use of early corticosteroid therapy on ICU admission in patients affected by severe pandemic (H1N1)v influenza A infection. Intensive Care Med 37:272–283
Rodrigo C, Ws L, Rodrigo C, Leonardi-bee J, Nguyen-van-tam J, Lim WS (2016) Corticosteroids as adjunctive therapy in the treatment of influenza (Review). Cochrane Database Syst Rev 3:10–12
Moreno G, Rodriguez A, Reyes LF, Sole-Violan J, Díaz E, Bodí M et al (2018) Corticosteroid treatment in patients with severe influenza pneumonia: a propensity score matching analysis. Crit Care 22(Suppl 1):P082
Alvarez-Lerma F, Marrín-Corral J, Vilá C, Masclans JR, Loeches IM, Barbadillo S et al (2017) Characteristics of patients with hospital-acquired influenza A (H1N1)pdm09 virus admitted to the intensive care unit. J Hosp Infect 95:200–206
Díaz E, Rodríguez A, Martin-Loeches I, Lorente L, Del Mar Martín M, Pozo JC et al (2011) Impact of obesity in patients infected with 2009 influenza A(H1N1). Chest 139:382–386
Marin-Corral J, Climent C, Muñoz R, Samper M, Dot I, Vilà C et al (2018) Patients with influenza A (H1N1)pdm09 admitted to the ICU. Impact of the recommendations of the SEMICYUC. Med Intensiva. https://doi.org/10.1016/j.medin.2018.02.002
Martin-Loeches I, Díaz E, Vidaur L, Torres A, Laborda C, Granada R et al (2011) Pandemic and post-pandemic Influenza A (H1N1) infection in critically ill patients. Crit Care 15:R286
Martin-Loeches I, Rodriguez A, Bonastre J, Zaragoza R, Sierra R, Marques A et al (2011) Severe pandemic (H1N1)v influenza A infection: report on the first deaths in Spain. Respirology 16:78–85
Knaus WA, Draper EA, Wagner DPZJ (1985) APACHE II: a severity of disease classification system. Crit Care Med 13:818–829
Vincent JL, Moreno R, Takala J, Willatts S, De Mendonça A, Bruining H, Reinhart CK, Suter PMTL (1996) The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. On behalf of the working group on Sepsis-related problems of the European Society of Intensive Care Medicine. Intensive Care Med 22:707–710
Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC et al (2007) Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44:S27–S72
CDC Protocol of realtime RT-PCR for influenza A (H1N1) (2009) World Heal Organization. http://www.who.int/csr/resources/publications/swineflu/CDCRealtimeRTPCR_SwineH1Assay-2009_20090430.pdf. Accessed 15 Dec 2017
Wedzicha JA, Miravitlles M, Hurst JR, Calverley PMA, Albert RK, Anzueto A et al (2017) Management of COPD exacerbations: a European Respiratory Society/American Thoracic Society guideline. Eur Respir J 49:1–16
Jackson DJ, Sykes A, Mallia P, Johnston SL (2011) Asthma exacerbations: origin, effect, and prevention. J Allergy Clin Immunol 128:1165–1174
Martin-Loeches I, Schultz MJ, Vincent JL, Alvarez-Lerma F, Bos LD, Sole-Violan J et al (2017) Increased incidence of co-infection in critically ill patients with influenza. Intensive Care Med 43:48–58
Martín-Loeches I, Sanchez-Corral A, Diaz E, Granada RM, Zaragoza R, Villavicencio C et al (2011) Community-acquired respiratory coinfection in critically III patients with pandemic 2009 influenza A(H1N1) virus. Chest 139:555–562
Dellinger RP, Levy MM, Rhodes A, Annane D, Gerlach H, Opal SM et al (2013) Surviving sepsis campaign: international guidelines for management of severe sepsis and septic shock: 2012. Crit Care Med 41:580–637
Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB et al (2016) Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111
Rodríguez A, álvarez-Rocha L, Sirvent JM, Zaragoza R, Nieto M, Arenzana A et al (2012) Recommendations of the Infectious Diseases Work Group (GTEI) of the Spanish Society of Intensive and Critical Care Medicine and Coronary Units (SEMICYUC) and the Infections in Critically Ill Patients Study Group (GEIPC) of the Spanish Society of Infectiou. Med Intensiva 36:103–137
Austin PC, Stuart AE (2015) Optimal full matching for survival outcomes: a method that merits more widespread use. Stat Med 34:3949–3967
Resche-Rigon M, Azoulay E, Chevret S (2006) Evaluating mortality in intensive care units: contribution of competing risks analyses. Crit Care 10:R5
cmprsk package (2014) https://cran.r-project.org/web/packages/cmprsk/index.html. Accessed 04 June 2018
Fine J, Gray R (1999) A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc 94:496–509
Cornejo R, Llanos O, Fernández C, Carlos J, Cardemil G, Salguero J et al (2010) Organizing pneumonia in patients with severe respiratory failure due to novel A (H1N1) influenza. BMJ Case Rep. https://doi.org/10.1136/bcr.02.2010.2708
Hagau N, Slavcovici A, Gonganau DN, Oltean S, Dirzu DS, Brezoszki ES et al (2010) Clinical aspects and cytokine response in severe H1N1 influenza A virus infection. Crit Care 14:R203
Ramsey C, Kumar A (2011) H1N1: viral pneumonia as a cause of acute respiratory distress syndrome. Curr Opin Crit Care 17:64–71
Ariani F, Liu K, Jing Z, Qu J (2013) Glucocorticosteroid in treatment of severe pneumonia. Mediadors Inflamm 2013:865635
Han K, Ma H, An X, Su Y, Chen J, Lian Z et al (2011) Early use of glucocorticoids was a risk factor for critical disease and death from pH1N1 infection. Clin Infect Dis 53:323–333
Rodrigo C, Leonardi-bee J, Nguyen-van-tam JS, Lim WS (2015) Effect of corticosteroid therapy on influenza-related mortality: a systematic review and meta-analysis. J Infect Dis 212:183–194
Zhang Y, Sun W, Svendsen ER, Tang S, Macintyre RC, Yang P et al (2015) Do corticosteroids reduce the mortality of influenza A (H1N1) infection? A meta-analysis. Crit Care 19:46
Jw Y, Lc F, Xy M, Mao B, Mh L, Hw L et al (2015) Corticosteroids for the treatment of human infection with influenza virus: a systematic review and meta-analysis. Clin Microbiol Infect 21:956–963
Kil H, Lee J, Lee K, Rhim J, Youn Y, Kang J (2011) Early corticosteroid treatment for severe pneumonia caused by 2009 H1N1 influenza virus. Crit Care 15:413
Kudo K, Takasaki J, Manabe T, Uryu H, Yamada R, Kuroda E (2012) Systemic corticosteroids and early administration of antiviral agents for pneumonia with acute wheezing due to influenza A (H1N1) pdm09 in Japan. PLoS One 7:e32280
Li H, Li SY, Yao G, Yan ZX, Zhang W, Wei HJ et al (2017) Effect of low-to-moderate-dose corticosteroids on mortality of hospitalized adolescents and adults with influenza A (H1N1) pdm09 viral pneumonia. Influenza Other Respir Viruses 11:345–354
Acknowledgements
The authors are grateful to Michael Maudsley for language editing and Eudald Correig for the statistical analysis.
GETGAG (Grupo Español de Trabajo Gripe A Grave) Study Group Investigators. Andalucía: Pedro Cobo (Hospital Punta de Europa, Algeciras); Javier Martins (Hospital Santa Ana Motril, Granada); Cecilia Carbayo (Hospital Torrecardenas, Almería); Emilio Robles-Musso, Antonio Cárdenas, Javier Fierro (Hospital del Poniente, Almería); Dolores Ocaña Fernández (Hospital Huercal—Overa, Almería); Rafael Sierra (Hospital Puerta del Mar, Cádiz); Mª Jesús Huertos, Mª Luz Carmona Pérez (Hospital Puerto Real, Cádiz); Juan Carlos Pozo Laderas, R. Guerrero, Juan Carlos Robles, Melissa Echevarría León, Alberto Bermejo Gómez (Hospital Reina Sofía, Córdoba); Enrique Márquez (Hospital Infanta Elena, Huelva); Manuel Rodríguez-Carvajal (Hospital Juan Ramón Jiménez, Huelva); Ángel Estella (Hospital del SAS de Jerez, Jerez de la Frontera); José Pomares, José Luis Ballesteros, Olga Moreno Romero (Hospital Universitario San Cecilio, Granada); Yolanda Fernández, Francisco Lobato, José F. Prieto, José Albofedo-Sánchez (Hospital Costa del Sol, Marbella); Pilar Martínez; María Victoria de la Torre; María Nieto, Estefanía Camara Sola (Hospital Vírgen de la Victoria, Málaga); Miguel Angel Díaz Castellanos, (Hospital Santa Ana de Motril, Granada); Guillermo Sevilla Soler, Carlos Ortiz Leyba (Clínica Sagrado Corazón, Sevilla); José Garnacho-Montero, Rafael Hinojosa, Esteban Fernández, (Hospital Virgen del Rocío, Sevilla); Ana Loza, Cristóbal León, Samuel González López (Hospital Universitario Nuestra Señora de Valme, Sevilla); Angel Arenzana, (Hospital Virgen de la Macarena, Sevilla), Dolores Ocaña (Hospital de la Inmaculada, Sevilla), Inés Navarrete (Hospital Virgen de las Nieves, Granada), Medhi Zaheri Beryanaki (Hospital de Antequera); Ignacio Sánchez, Manuel Pérez Alé (Hospital NISA Sevilla ALJARAFE, Sevilla); Ana Mª Poullet Brea (Hospital Quirón Málaga, Málaga); Juan Francisco Machado Casas (Complejo Hospitalario de Jaén, Jaén). Aragón: Carlos Serón, Manuel Luis Avellanas, Arantxa Lander, S Garrido Ramírez de Arellano, MI Marquina Lacueva (Hospital San Jorge, Huesca); Pilar Luque; Elena Plumed Serrano; Juan Francisco Martín Lázaro, Carlos Sánchez Polo, Isabel Gutiérrez Cia, Belén Jiménez Bartolomé, Carlos López Nuñez (Hospital Lozano Blesa, Zaragoza); Ignacio González, José Ignacio Tomás Marsilla, Clara Jaques Andrés, Pablo Gutiérrez Ibañes, Pilar Araujo Aguilar (Hospital Miquel Servet, Zaragoza); Jose Mª Montón (Hospital Obispo Polanco, Teruel); Paloma Dorado Regil (Hospital Royo Villanova, Zaragoza). Asturias: Lisardo Iglesias, Carmen Pascual González, Brígida Quindós Fernández, Lorena Martín Iglesias, Lucía Viña Soria, Raquel Yano Escudero, Mª del Rosario Mtnez Revuelta (Hospital Universitario Central de Asturias—HUCA-UCI 1, Oviedo); Quiroga (Hospital De Cabueñes, Gijón); Águeda García-Rodríguez (Hospital Valle del Nalón, Langreo); Marta Martín Cuadrado, Ana Luz Balán Mariño (Hospital San Agustín, Avilés). Baleares: Lorenzo Socias, Pedro Ibánez, Marcío Borges-Sa; A. Socias, Del Castillo A (Hospital Son LLatzer, Palma de Mallorca); Ricard Jordà Marcos, Cristina Muñoz (Clínica Rotger, Palma de Mallorca); José M Bonell (USP. Clínica Palmaplanas, Palma de Mallorca); Ignacio Amestarán (Hospital Son Dureta, Palma de Mallorca); M. Angeles González López, Cecilia Vilanova Pàmies (Hospital Mateu Orfila, Palma de Mallorca); José Ma. Bonell Goytisolo, José Antonio Morales Carbonero (Hospital General de Muro, Palma de Mallorca); José Ma. Bonell Goytisolo, José Antonio Morales Carbonero (Clínica Quirón Palmaplanas, Palma de Mallorca), Rossana Pérez Senoff, Marta Generelo López de Medrano (Hospital Comarcal de Inca, Palma de Mallorca). Canarias: ergio Ruiz-Santana, Juan José Díaz, Catalina Sánchez Ramírez (Hospital Dr Negrín, Las Palmas de Gran Canaria); Montse Sisón (Hospital Doctor José Molina, Lanzarote); David Hernández, Ana Trujillo, Luis Regalado, Sonia Rodríguez Fndez (Hospital General la Palma, La Palma); Leonardo Lorente, Judith Cabrera Rivero, Mª Luisa Mora Quintero (Hospital Universitario de Canarias, Tenerife); Mar Martín (Hospital de la Candelaria, Tenerife), Sergio Martínez, J. J. Cáceres, Manuel Sanchez Palacio, Marcos (Hospital Insular de Gran Canaria); D. García Rodríguez, María Ripoll Leria (Hospital General de Fuerteventura, Fuerteventura). Cantabria: Borja Suberviola, P. Ugarte (Hospital Universitario Marqués de Valdecilla, Santander). Castilla La Mancha: Ernando García-López, Rafael Sánchez Iniesta (Hospital General, Albacete); Angel Álvaro Alonso, Antonio Padilla, Basi Martínez Palacios (Hospital General La Mancha Centro, Alcázar de San Juan); Mª Luisa Gómez Grande, Ma. Carmen Martín Rodríguez, Hasania Adbel-Hadi Álvarez, Alfonso Ambros Checa, Higinio Martín Hernández (Hospital General de Ciudad Real, Ciudad Real); Antonio Albaya, Alberto Silva Obregón, Carlos Marian Crespo, Carlos Armendariz Estrella, Carmen Benito Puncel, Eduardo Quirós Oyargue (Hospital Universitario de Guadalajara, Guadalajara); Alfonso Canabal, Luis Marina, Ismael López de Toro (Hospital Virgen de la Salud, Toledo); Almudena Simón (Hospital Nuestra Señora del Prado, Toledo); José María Añón (Hospital Virgen de la Luz, Cuenca). Castilla y León: Juan B López Messa, (Complejo Asistencial de Palencia, Palencia), Mª Jesús López Pueyo, Ortíz María del valle, Sergio Ossa Echeverri (Hospital General Yagüe, Burgos); Zulema Ferreras, Juan C Ballesteros Herraez (Hospital Universitario de Salamanca, Salamanca); Santiago Macias, (Hospital General de Segovia, Segovia); José Ángel Berezo, Jesús Blanco Varela, Pablo Blanco Schweizer, Angela González Salamanca, Luis Tamayo Lomas (Hospital Universitario Río Hortega, Valladolid), Andaluz Ojeda Anzález, Ramón Cicuéndez Avila, Francisco Javier Pérez G (Hospital Universitario, Valladolid); Antonio Álvarez Terrero (Hospital Virgen de la Concha, Zamora), Fabiola Tena Ezpeleta, Christian Sala, Oliverio López (Hospital Santa Bárbara, Soria); Zulema Paez; Álvaro García (Hospital Virgen Vega, Salamanca), Demetrio Carriedo Ule, Miriam Riesco Crespo (Complejo Asistencial Universitario de León-CAULE, León); Jesús Pino Rebolledo, Nicolás Hidalgo Andrés (Hospital Campo Grande, Valladolid); Ana Carolina Caballero Zirena (Complejo Asistencial de Zamora, Zamora); Belén Román García, Juan Bautista López Messa (Hospital General Río Carrión, Palencia); María del Valle Ortiz, Sergio Ossa Echeverri (Hospital Universitario de Burgos-HUBU, Burgos). Cataluña: Rosa Mª Catalán (Hospital General de Vic, Vic); Miquel Ferrer, Antoni Torres, Catia Cilloniz (Hospital Clínic, Barcelona); Sandra Barbadillo Ansorregui (Hospital General de Catalunya—CAPIO, Barcelona); Lluís Cabré, Ignacio Baeza (Hospital de Barcelona, Barcelona); Assumpta Rovira (Hospital General de l’Hospitalet, L’Hospitalet); Francisco Álvarez-Lerma, Antonia Vázquez, Joan Nolla ( Hospital Del Mar, Barcelona); Francisco Fernández, Joaquim Ramón Cervelló; Raquel Iglesia (Centro Médico Delfos, Barcelona); Rafael Mañéz, J. Ballús, Rosa Mª Granada (Hospital de Bellvitge, Barcelona); Jordi Vallés, Emili Díaz, Marta Ortíz, C. Guía, Ignacio Martín-Loeches ( Hospital de Sabadell, Sabadell); Joaquim Páez (Hospital Dos De Mayo, Barcelona); Jordi Almirall, Xavier Balanzo, Estel Güell, Juan Carlos Yebenes (Hospital de Mataró, Mataró); Elena Arnau, Marcos Pérez; César Laborda; Jesica Souto, Leonel Lagunes (Hospital Vall d’Hebron, Barcelona); Iñaki Catalán (Hospital Sant Joan de Déu, Manresa); Josep Mª Sirvent, Nerea López de Arbina, Anna Baró Serra, Adriana Sánchez, Silvia M. Cuenca (Hospital Josep Trueta, Girona); Mariona Badía, Begonia Baseda-Garrido, Montserrat Valverdú-Vidal, Fernando Barcenilla, Mercedes Palomar, Xavier Nuvials (Hospital Arnau de Vilanova, Lleida); Pedro Garrido Benedicto (Hospital Sant Joan de Reus, Reus); Ferran Roche-Campo, MF Esteban, José Luna, Gaspar Masdeu Eixarch, Angels Pascual Diago (Hospital Verge de la Cinta, Tortosa); Juan Mª Nava, J González de Molina, Josep Trenado, Ricard Ferrer (Hospital Universitario Mutua de Terrassa, Terrassa); Zoran Josic, Montserrat Casanovas (Hospital de Igualada, Igualada); Francisco Gurri; Paula Rodríguez (Hospital Quirón, Barcelona) Alejandro Rodríguez, Laura Claverias, Sandra Trefler, María Bodí, Mónica Magret, Cristina Ferri (Hospital Universitario Joan XXIII, Tarragona); Rosa María Díaz (Hospital San Camil. Sant Pere de Ribes, Barcelona); Eduard Mesalles, Fernando Arméstar (Hospital Germans Trias i Pujol, Badalona); Diego de Mendoza, Carmen Lomas Fernández, José Julián Berrade (Hospital M. Broggi, Sant Joan Despí); Alfonso Bonet Saris, Marina Pechkova (Clínica Girona, Girona); Cristina Mora Jiménez (Hospital Sagrat Cor, Barcelona); Santiago Picos Gil (Clínica Diagonal). Extremadura: Juliá-Narváez José, Manuel Robles Marcos, Vanessa Farje Mallqui, Mª. Angeles Santiago Triviño, Pablo Martínez García (Hospital Infanta Cristina, Badajoz), Alberto Fernández-Zapata, Teresa Recio, Abilio Arrascaeta, Mª José García-Ramos, Elena Gallego, Esther Saiz Rodrigo (Hospital San Pedro de Alcántara, Cáceres); Fernándo Bueno (Hospital Virgen del Puerto, Plasencia); Mercedes Díaz, Noemí Gil Pérez, David López Hormigo (Hospital de Mérida, Mérida); Juan Diego Jiménez Delgado (Hospital Don Benito, Villanueva de la Serena, Badajoz); Pérez frutos, Rivera Pinna MJ (Hospital Perpetuo Socorro). Galicia: Mª Lourdes Cordero, José A. Pastor, Luis Álvarez-Rocha, Alexandra Ceniceros Barros, Alejandra Virgós Pedreira (CHUAC, A Coruña); Dolores Vila, (Hospital Do Meixoeiro, Vigo); Carmen Fernández González (C.H.U. de Ferrol, Ferrol); Javier Blanco Pérez, M Ortiz Piquer, (Hospital Xeral—Calde, Lugo); Eleuterio Merayo, Victor Jose López-Ciudad, Juan Cortes Cañones, Eva Vilaboy, José Villar Chao, Francisco Savira Cid López, Pablo Vidal Cortés, Marcos A. Pérez Veloso(Complejo Hospitalario de Ourense, Ourense); Eva Maria Saborido, Enrique Alemparte Pardavila, Ana Ortega Montes (Hospital Montecelo, Pontevedra); Raul José González, (H. Miguel Domínguez, Pontevedra); Santiago Freita, Enrique Alemparte; Ana Ortega (Complejo Hospitalario de Pontevedra, Pontevedra); Ana María López; Julio Canabal, Enrique Ferres (Clinica Universitaria Santiago de Compostela, Santiago); Javier Blanco Pérez, M Ortiz Piquer (Hospital Lucus Augusti-HULA, Lugo); Santiago Freitas Ramos, Lucas Lage Cendón, Vanesa Gómez Casal, Sabela Vara Adrio, Eva Menor Fernández, Susana González Prado (H. Xeral—C.H.U. de Vigo, Vigo); Antonio Varela Franco (Hospital Vithas Nª Sª de Fátima, Vigo). La Rioja: José Luis Monzón, Félix Goñi (Hospital San Pedro, Logroño). Madrid: Frutos Del Nogal Sáez, M Blasco Navalpotro, Ricardo Díaz Abad, José Luis Flordelis Lasierra (Hospital Severo Ochoa, Madrid); Mª Carmen García-Torrejón (Hospital Infanta Elena, Madrid); César Pérez-Calvo, Diego López ( Fundación Jiménez Díaz, Madrid); Luis Arnaiz, S. Sánchez- Alonso, Carlos Velayos, (Hospital Fuenlabrada, Madrid); Francisco del Río, Miguel Ángel González, Mercedes Nieto, Carmen Sánchez Cesteros (Hospital Clínico San Carlos, Madrid); María Cruz Martín, José Mª Molina (Hospital Nuestra Señora de América, Madrid); Juan Carlos Montejo, Mercedes Catalán (Hospital Universitario 12 de Octubre, Madrid); Patricia Albert, Ana de Pablo (Hospital del Sureste, Arganda del rey); José Eugenio Guerrero, María Zurita; Jaime Benitez Peyrat, Miriam Díaz Cámara (Hospital Gregorio Marañón, Madrid); Enrique Cerdá, Manuel Alvarez, Carlos Pey, Eva Manteiga Riestra, Concepción Martinez-Fidalgo (Hospital Infanta Cristina, Parla); Montse Rodríguez, Eduardo Palencia (Hospital Infanta Leonor, Madrid); Rafael Caballero, (Hospital de San Rafael, Madrid); Concepción Vaquero, Francisco Mariscal, S. García, Rico Cepeda (Hospital Infanta Sofía, Madrid); Nieves Carrasco, (Hospital Universitario La Princesa, Madrid); Isidro Prieto, A Liétor, R. Ramos, Rosario Cuadra Casas, Cruz Soriano Cuesta, Susana Sánchez Alonso (Hospital Ramón y Cajal, Madrid); Beatriz Galván, Juan C. Figueira, M. Cruz Soriano, Bélen Civantos Martín, Alejgandro Robles Caballero (Hospital La Paz, Madrid); P Galdós, Bárbara Balandin Moreno, Sara Alcántara Carmona (Hospital Puerta de Hierro, Madrid); Fernández del Cabo (Hospital Monte Príncipe, Madrid); Cecilia Hermosa, Federico Gordo (Hospital de Henares, Madrid); Alejandro Algora (Hospital Universitario Fundación Alcorcón, Madrid); Amparo Paredes, Teodoro Grau Carmona (Hospital Sur de Alcorcón, Madrid); JA Cambroner, Esther López Ramos, Yaiza Ortiz de Zárate (Hospital Universitario Príncipe de Asturias, Madrid); Sonia Gómez-Rosado, Margarita Mas Lodo, Nieves Franco Garrobo, Silvia Álvarez Hernández, Teresa Honrubia (Hospital de Móstoles, Madrid); Luis Miguel Prado López (Hospital Sanitas La Zarzuela, Madrid); Esteban A, Lorente JA, Nin N, Carlos Jaramillo Sotomayor (Hospital de Getafe, Madrid); Luis Arnaiz (Sanitas de Moraleja, Madrid); Esperanza Molero Silvero, Eduardo Morales Fdez. de la Reguera (Hospital Central de la Defensa “Gómez Ulla”, Madrid); Rosa Mª de la Casa Monje, Fátima Martín Serrano (Clínica Moncloa, Madrid); Mª Victoria Trasmonte Martínez, M. Cruz Martín Delgado (Hospital de Torrejón, Torrejón de Ardoz). Murcia: Sofía Martínez (Hospital Santa María del Rosell, Murcia); F. Felices Abad, Isabel Cremades Navalon, Martín Vigil Velis (Hospital Universitario Reina Sofía, Murcia); Mariano Martínez, Domingo Martínez Baño, Enriqueta Andreu (Hospital Universitario Virgen de la Arrixaca, Murcia); Sergio Manuel Butí, Bernardo Gil Rueda, Francisco García ( Hospital Morales Messeguer, Murcia); Noemí Llamas Fernández (Hospital General Universitario Rafael Méndez, Lorca); Luis Herrera Para, Alejandro Ortín Freire (Hospital Universitario Santa Lucía, Cartagena); Mª Rosa Nvarro Ruiz, C.R. Hernández Romero (Hospital Los Arcos del Mar Menor, Murcia). Navarra: Enrique Maraví-Poma, I Jimenez Urra, Laura Macaya Redin, A Tellería (Hospital Virgen del Camino, Pamplona); Josu Insansti, (Hospital de Navarra, Pamplona). Noelia Artesero Garcia, Laura Macaya (Complejo Hospitalario Navarra-UCI A); Joaquín Lobo Palanco (Complejo Hospitalario Navarra UCI B). País Vasco: Nagore González, Pilar Marco, Loreto Vidaur, Estibaliz Salas, Ruth Salaberría Udabe (Hospital de Donostia, San Sebastián); B. Santamaría, Tomás Rodríguez (Hospital de Basurto, Bilbao); Juan Carlos Vergara, Jose Ramon Iruretagoyena Amiano, Iratí Garrido Santos (Hospital de Cruces, Bilbao); Alberto Manzano, (Hospital Santiago Apóstol, Vitoria); Carlos Castillo Arenal (Hospital Txagorritxu, Vitoria); Pedro María Olaechea, Higinio Martín Hernández (Hospital Galdakao-Usansolo, Vizcaya); Alejandro Martín López, Fernando Fonseca San Miguel (Hospital Universitario de Alava-Santiago, Vitoria). Valencia: José Blanquer, Nieves Carbonell, José Ferreres Franco (Hospital Clinic Universitari, Valencia); Roberto Reig Valero, A. Belenger, Susana Altaba (Hospital General de Castellón, Castellón); Bernabé Álvarez-Sánchez, José Canovas Robles, Jaime Sánchez Francisco, Mar Ruiz Sánchez (Hospital General de Alicante, Alicante); Santiago Alberto Picos, Abilio Arrascaeta Llanes, Eugenio Herrero Gutiérrez, Alberto Fernández Zapata (Hospital Torrevieja Salud, Alicante); Ángel Sánchez-Miralles, José Luis Antón Pascual (Hospital San Juan, Alicante); Juan Bonastre, M. Palamo, Javier Cebrian, José Cuñat, Mónica Gordón Sahuquillo (Hospital La Fe, Valencia); Belén Romero, Santiago Borrás Pallé, Javier de León Belmar (Hospital de Manises, Valencia); Rafael Zaragoza, Constantino Tormo, Susana Sancho Chinesta (Hospital Dr Peset, Valencia); Virgilio Paricio, (Hospital de Requena, Valencia); Asunción Marques, S. Sánchez-Morcillo, S. Tormo (Hospital de la Ribera, Valencia). J. Latour (H.G Universitario de Elche, Valencia), M Ángel García, Manuel Palomo (Hospital de Sagunto, Castellón), Francisco Tarín Royo, Pedro Manzano Hinojosa (Hospital de Denia, Alicante); Mª Salomé Sánchez Pino (Hospital Vega Baja, Alicante); Concha Maragues Ribes, Rubén González Luis (Hospital de la Plana, Castellón). Andorra: Antoli Ribas (Hospital Nuestra Señora de Meritxell, Andorra).
Funding
This study was supported in part by grants from SEMICYUC (Spanish Society of Critical Care) and the Ricardo Barri Casanovas Foundation. The study sponsors had no role in the study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding authors (AR/GM) had full access to all the data in the study and final responsibility for the decision to submit for publication.
Author information
Authors and Affiliations
Consortia
Contributions
GM, AR, JSV, IML, ED and AT conceived and designed the study. All authors, apart from MR, LFR, JG, and AS, contributed to the acquisition and local preparation of the constituent database. GM, AR, EC, ST, IML, ED, JGM, LS, and JCY contributed to database creation and standardization, design of statistical analyses, and data analysis. GM, AR, LFR, JG, JSV, ED, MB, ST, JG, JCY, AS, JGM, LS, MVO, JMC, MVV, MIR, AT, and IML made important intellectual contributions and actively participated in the interpretation of the data and wrote the paper. All authors contributed to critical examination of the paper for important intellectual content and approval of the final manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
All named authors declare that they have no conflicting interests.
Ethical approval
The institutional review board of Joan XXIII Hospital approved the original study (IRBRef#11809).
Additional information
The GETGAG Study Group Investigators are listed in Acknowledgements.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Moreno, G., Rodríguez, A., Reyes, L.F. et al. Corticosteroid treatment in critically ill patients with severe influenza pneumonia: a propensity score matching study. Intensive Care Med 44, 1470–1482 (2018). https://doi.org/10.1007/s00134-018-5332-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-018-5332-4