Abstract
Purpose
Percutaneous dilational tracheostomy (PDT) is routinely performed in the intensive care unit with bronchoscopy guidance. Recently, ultrasound has emerged as a potentially useful tool to assist PDT and reduce procedure-related complications.
Methods
An open-label, parallel, non-inferiority randomized controlled trial was conducted comparing an ultrasound-guided PDT with a bronchoscopy-guided PDT in mechanically ventilated critically ill patients. The primary outcome was procedure failure, defined as a composite end-point of conversion to a surgical tracheostomy, unplanned associated use of bronchoscopy or ultrasound during PDT, or the occurrence of a major complication.
Results
A total of 4965 patients were assessed for eligibility. Of these, 171 patients were eligible and 118 underwent the procedure, with 60 patients randomly assigned to the ultrasound group and 58 patients to the bronchoscopy group. Procedure failure occurred in one (1.7 %) patient in the ultrasound group and one (1.7 %) patient in the bronchoscopy group, with no absolute risk difference between the groups (90 % confidence interval, −5.57 to 5.85), in the “as treated” analysis, not including the prespecified margin of 6 % for noninferiority. No other patient had any major complication in either group. Procedure-related minor complications occurred in 20 (33.3 %) patients in the ultrasound group and in 12 (20.7 %) patients in the bronchoscopy group (P = 0.122). The median procedure length was 11 [7–19] vs. 13 [8–20] min (P = 0.468), respectively, and the clinical outcomes were also not different between the groups.
Conclusions
Ultrasound-guided PDT is noninferior to bronchoscopy-guided PDT in mechanically ventilated critically ill patients.
Similar content being viewed by others
Introduction
Percutaneous dilatational tracheostomy (PDT) is a widely utilized technique in the intensive care unit (ICU) [1], with a safety profile that favorably compares to surgical tracheostomy [2, 3]. Although overall complication rates are low, severe adverse events, including death, are still reported [4]. Bronchoscopy guidance has traditionally been used as a safety adjunctive tool in order to define the appropriate site for the tracheal puncture, to guide the real-time entrance of the needle into the trachea, avoiding tracheal posterior wall injuries, and confirming the endotracheal tube placement [5, 6]. By contrast, bronchoscopy might not precisely identify the cervical anatomical structures and prevent complications such as vascular lesions or thyroid punctures.
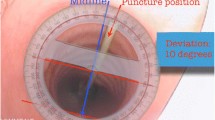
Ultrasound has emerged as a potentially useful tool in assisting PDT. The potential advantages of ultrasound include the ability to identify the cervical vasculature [7], to help identify the most appropriate location for the tracheal puncture site, and to guide the needle insertion into the trachea, similar to the technique used in ultrasound-guided vascular punctures. Several studies have demonstrated the value of preprocedure cervical ultrasound in order to improve the safety of PDT [8–10]. In 1999, the first real-time ultrasound-guided PDT was described [11], followed by the publication of several case series and observational studies suggesting that the method was effective and safe [12–16].
Recently, three randomized controlled trials (RCTs) have demonstrated that the use of real-time ultrasound-guided PDT may significantly improve the rate of first-pass punctures, puncture accuracy, and reduce procedure-related complications, when compared to an anatomical landmark-guided PDT [17, 18] and bronchoscopy-guided PDT [19]. However, in the first two trials bronchoscopy was not evaluated and the last of these trials was underpowered to detect a difference in complication rates.
Therefore, we designed a noninferiority RCT in order to evaluate the safety and the efficacy of real-time ultrasound-guided PDT compared to bronchoscopy-guided PDT in mechanically ventilated patients in the ICU.
Methods
Trial design
The ultrasound-guided versus bronchoscopy-guided percutaneous dilational tracheostomy trial (TRACHUS, NCT02084862) is an open-label, parallel, noninferiority RCT that was conducted at eight ICUs in the Hospital das Clínicas of São Paulo University. The study was conducted in accordance with the amended Declaration of Helsinki. Local institutional review boards approved the protocol (Comissão de Ética para Análise de Projetos de Pesquisa—CAPPesq, reference number 488506), and written informed consent was obtained from all of the patients or their legal surrogates.
Patients
Adult patients, intubated, mechanically ventilated, and indicated for a tracheostomy were considered eligible. Tracheostomy indication was at the discretion of the patient’s attending physician. Patients were excluded if they had an unsuitable anatomy to undergo a PDT as judged by the patient attending physician (i.e., short neck, tracheal deviation, cervical anatomical anomaly, previous cervical surgery, cervical trauma, cervical tumors, or the inability to perform a neck extension) or the inability to get written informed consent.
Study intervention
Enrolled patients were randomized in a 1:1 ratio to ultrasound or bronchoscopy arms in random permuted blocks. None of the investigators or ICU staff members was aware of the randomization list prior to group allocation, as well as blocks numbers or blocks sizes at any moment, as the randomization was performed by using an automated third-party Internet-based service (Sealed Envelope, London, UK) in order to maintain allocation concealment. Treatment assignments could not be blinded to the ICU staff members. The patients were followed until hospital discharge.
Randomization was performed immediately before the procedure, as soon as all of the equipment and the PDT team were available. The procedures were performed according to standardized practices following hospital routines, as previously published [16]. Detailed procedure descriptions are available in supplemental materials.
Outcome measurements
Primary outcome
The primary outcome was procedure failure, defined as a composite end-point of conversion to a surgical tracheostomy, associated use of bronchoscopy in the case of ultrasound-guided PDT, associated use of ultrasound in the case of bronchoscopy-guided PDT, or the occurrence of a major complication.
The decision to proceed conversion to a surgical tracheostomy, associated use of bronchoscopy in the case of ultrasound-guided PDT, or associated use of ultrasound in the case of bronchoscopy-guided PDT was at discretion of the attending intensivist, during the procedure, without any interference of the investigators.
Major complications were defined as follows: procedure-related death, cardiac arrest, tracheal wall injury, false passage cannulation, pneumothorax, pneumomediastinum, tracheostomy cannula obstruction, esophageal injury, tracheoesophageal fistula, conversion to surgical tracheostomy, persistent hypotension (systolic blood pressure below 90 mmHg for more than 5 min and an associate intervention that was used to increase blood pressure using fluids, vasopressors infusion, or repeated vasopressors bolus), persistent acute hypoxemia (oxygen peripheral saturation below 90 % for more than 5 min as measured by a pulse oximeter), major bleeding (stomal, intratracheal, or trachea-vascular fistula causing hypoxemia and/or requiring an emergency transfusion and/or a surgical repair), tracheostomy-related sepsis (stoma infection as the only identifiable source).
Secondary outcomes
The secondary outcomes were the occurrence of a minor complication, procedure length, procedure difficulty, liberation from mechanical ventilation (breathing without ventilator assistance for at least 48 h), alive ventilation-free days at day 60 after hospital admission, days to ICU and hospital discharge, ICU mortality, and hospital mortality. Procedure difficulty was qualified as (1) easy, (2) somewhat difficult, (3) difficult, (4) very difficult, or (5) impossible [14] on the basis of subjective evaluation of the participating intensive care medicine residents about the procedure.
Minor complications were defined as follows: transient hypotension (systolic blood pressure below 90 mmHg for less than 5 min and an associate intervention that was used to increase blood pressure using fluids or vasopressors single bolus), transient acute hypoxemia (oxygen peripheral saturation below 90 % for less than 5 min as measured by a pulse oximeter), atelectasis, inadvertent cuff puncture, accidental decannulation, tracheostomy stoma infection, localized minor bleeding (stomal or intratracheal self-limiting bleeding or bleeding successfully when treated with a local compression or by an instillation of topical vasoconstrictive agents), localized subcutaneous emphysema without any evidence of pneumothorax or pneumomediastinum, and local stomal infections not causing sepsis.
Statistical analysis
On the basis of the findings of both our previous observational study [16] and retrospective cohorts [20, 21], we estimated 1.2 % as being the primary outcome occurrence rate in the bronchoscopy-guided PDT group. Assuming an absolute noninferiority margin (Δ) of 6 %, at a one-sided α level significance of 0.05, we calculated that a sample size of 114 patients (57 per group) would be required to obtain a statistical power (1 − β) of 90 %. To assess for noninferiority we used the two one-sided test (TOST) method [22], where noninferiority is assumed, at the α significance level of 0.05, if the upper limit of a (1 − 2α) × 100 % confidence interval (CI) for the difference in efficacy is below Δ [23].
All analyses were performed on both an intention-to-treat and an “as treated” basis. As a noninferiority trial, data was presented mainly as an “as treated” population, because it is a stricter approach. No interim analysis was planned. For continuous variables, the Shapiro–Wilk test and kernel density estimation plots were used to assess normal distribution. Continuous variables were presented as a mean ± standard deviation or as median and percentiles [25th–75th], according to the distribution, and they were compared by using the Student’s t test or the Wilcoxon–Mann–Whitney test. Categorical variables were presented as occurrences and their respective percentages, and they were compared by using the χ 2 or Fisher exact tests, as appropriate. Unadjusted Kaplan–Meier curves were used to assess the time from the endotracheal intubation to unassisted breathing within 28 days and were compared with a log-rank test, whereas an adjusted analysis was done by using Cox proportional hazards regression. A logistic regression was used to adjust for any significance between the groups on the baseline covariates. Statistical significance was assumed with a P value less than 0.05. In the Shapiro–Wilk test, the null hypothesis that the data are normally distributed was rejected with a P value less than 0.05. The statistical analysis was performed by using SPSS 21.0 software (SPSS Inc, Chicago, IL).
Results
Study patients
Between March 2014 and May 2015, a total of 4965 patients were assessed for eligibility. Of these, 171 patients were eligible and 123 were randomized. After exclusion of five patients before treatment for different causes (death before the procedure, withdrawn by physician, and randomization errors), 118 patients underwent the procedure, with 60 patients randomly assigned to the ultrasound group and 58 patients to the bronchoscopy group (Fig. 1). Baseline characteristics were well balanced between the study groups, except for the Simplified Acute Physiology Score (SAPS) 3 (Table 1).
Procedure
The procedure was described as easy or somewhat difficult 88.3 % of the time in the ultrasound group and with a similar 86.2 % rate in the bronchoscopy group (P = 0.960). The median procedure length was similar in both groups, 11 [7–19] vs. 13 [8–20] minutes, respectively (P = 0.468). The puncture site was changed after the ultrasound procedure in 24 (23.3 %) patients in the ultrasound group (Table 2).
Outcomes
The primary outcome, procedure failure, occurred in one (1.7 %) patient in the ultrasound group and one (1.7 %) patient in the bronchoscopy group, with no absolute risk difference between the groups (90 % CI from −5.57 to 5.85) in the “as treated” analysis. In the intention-to-treat analysis, the absolute risk difference between the groups for the primary outcome was also none (90 % CI from −5.42 to 5.55). These confidence intervals did not include the prespecified margin of 6 %, meaning that the ultrasound-guided PDT met the prespecified criteria for noninferiority when compared to the bronchoscopy-guided PDT (Fig. 2). An adjusted analysis for the primary outcome was not possible because of an insufficient number of events for a multivariate analysis.
Noninferiority plots show the absolute risk differences for a procedure failure associated with the ultrasound group when compared with the bronchoscopy group in the “as treated” (a) and intention-to-treat (b) populations. To allow for one-sided testing of noninferiority, 90 % confidence intervals were calculated (shown in black). Confidence intervals within the gray-shaded area are noninferior. The noninferiority margin is 6 % (shown as Δ)
In the ultrasound group, one patient had the tracheostomy tube inserted too low, between the fifth and sixth tracheal rings, with a subsequent tracheal laceration and mediastinitis. In the bronchoscopy group, one patient suffered from a tracheal anterior wall laceration during the tracheal dilation with a Griggs clamp and this was complicated with pneumomediastinum. No other major complication occurred in either group. Minor procedure-related complications were reported for a total of 32 (27.1 %) patients, 20 (33.3 %) patients in the ultrasound group and 12 (20.7 %) patients in the bronchoscopy group (P = 0.122) (Table 3). These results persisted after an adjusted analysis for the SAPS 3 score (P = 0.148).
Clinical outcomes were also not different between the ultrasound and the bronchoscopy groups. Forty-nine (81.7 %) patients achieved unassisted breathing in the ultrasound group when compared with 44 (75.9 %) patients in the bronchoscopy group (P = 0.440). The ventilation-free days were 38.5 [18–47] days vs. 38 [8–45] days (P = 0.505), and the time from tracheostomy to unassisted breathing was 3 [2–6] days vs. 4 [2.5–7] days (P = 0.231) in the ultrasound and the bronchoscopy groups, respectively. The time from tracheostomy to unassisted breathing within 30 days was also not different between the groups (P = 0.972, Fig. 3). A total of 52 (44 %) patients died during the hospital stay, 26 (44.8 %) patients in the ultrasound group and 26 (46.4 %) patients in the bronchoscopy group (P = 0.864) (Table 3).
Adjusted estimated probability of achieving unassisted breathing from percutaneous dilational tracheostomy (PDT) up to day 30, with no statistically significant difference between the ultrasound and the bronchoscopy groups. Events indicate the total number of unassisted breathing achievements. The blue line represents the ultrasound group and the red line represents the bronchoscopy group. The analysis was adjusted for SAPS 3 by using Cox proportional hazards regression. The lines overlapped after adjusted analysis
Discussion
Ultrasound-guided PDT is noninferior to bronchoscopy-guided PDT in ICU mechanically ventilated patients. Furthermore, the major and minor complication rates were not statistically different between the groups, the procedure length was the same in both groups, and ultrasound-guided PDT was described as being as easy as bronchoscopy-guided PDT.
Several studies evaluated the value of ultrasound in order to assist PDT [24, 25], and practice guidelines [26] have recommended its use to improve the safety of the technique. However, most of the studies were observational, and only three RCTs have been published to date comparing ultrasound-guided PDT to landmark- or bronchoscopy-guided PDT [17–19]. Despite significant methodological differences, all of these trials point out that ultrasound-guided PDT is safe, fast, and might improve procedure efficiency and reduce procedure-related complications when compared to landmark- or bronchoscopy-guided PDT.
Major complication rates are usually low. In the two largest retrospective cohorts previously published on the topic, including a total of 4162 patients in the analyses, the total major complication rates ranged from 0.38 to 1.4 % [4, 27], similar to the 1.7 % major complication rate in our trial.
Minor complications that were procedure related were reported for 20 (33.3 %) patients in the ultrasound group and 12 (20.7 %) patients in the bronchoscopy group. Total minor complications rate was higher in the ultrasound group, which might be related to higher baseline SAPS 3 score in the ultrasound group or less team experience with the new method. Nevertheless, this difference was not statistically significant, remaining nonsignificant after adjusted analysis and with an incidence similar to that previously reported [2, 28]. However, the study was not designed and might be underpowered to detect differences based on secondary outcomes.
The complication rates in previous trials were highly variable, depending on the population and upon which complications were taken into account to elaborate the rate. In a meta-analysis comparing surgical with percutaneous tracheostomy, including 17 randomized trials with a total of 1212 patients, the overall incidence of bleeding in both of the groups was 5.7 % and the infection rate was 6.6 % [2]. In all three RCTs comparing ultrasound-guided PDT with bronchoscopy-guided PDT or landmark-guided PDT published to date, the total minor complication rates ranged from 11.52 to 56.75 % [17–19] and were not different between the groups. Additionally, in our previous retrospective cohort, the total minor complication rate was 30 % in both of the groups [16].
Bronchoscopy was chosen as the active comparator because, in our institution, a bronchoscopy-guided PDT has been the standard method for tracheostomy procedures over the past 15 years [29]. However, no RCTs comparing bronchoscopy-guided PDT to landmark-guided PDT (considered “placebo”) have been published to date. Therefore, the primary outcome occurrence rates and the noninferiority margins were estimated on the basis of retrospective cohorts comparing a bronchoscopy-guided PDT to a landmark-guided PDT [20, 21].
Furthermore, bronchoscopy has been traditionally recommended as an adjunctive tool to assist PDT and to help in preventing complications [5, 30, 31]. International surveys have revealed that 69.2–97.7 % routinely used bronchoscopy guidance during PDT [1, 32], and that of the remaining, 1 % would opt for a bronchoscopy procedure in the presence of a difficult airway [32]. Moreover, several trials have shown the efficacy and the safety of a bronchoscopy-guided PDT, especially when compared to a surgical tracheostomy [2, 33]. Nevertheless, other reports have found no difference in the complication rates when a PDT was performed with or without bronchoscopy guidance, suggesting that its use is not routinely required and should be limited to selected cases [20, 21].
Since the introduction of ultrasound-guided PDT in our institution in 2013, it has become the preferred method for tracheostomy, accounting for more than 80 % of the tracheostomies performed in the ICU, when the patient was out of the clinical trial [16]. This increase could be related to the following: (1) the greater availability of ultrasound equipment than of bronchoscopy equipment in the ICU, (2) the ultrasound equipment cleaning is faster, it is much less labor intensive, it is less costly, and it does not require the removal of the ultrasound equipment from the ICU setting, (3) the need for fewer staff than a bronchoscopy, (4) a steep learning curve of ultrasound-guided PDT, and (5) confidence in the new method over time. However, bronchoscopy and ultrasound are not mutually exclusive and can be used in a combination to improve PDT efficacy and safety [34–36].
All the attending intensivists were previously trained in ultrasound-guided PDT, bronchoscopy-guided PDT, critical care ultrasound, and bronchoscopy. However, all the procedures were performed by inexperienced intensive care medicine residents, assisted by the attending intensivists. Furthermore, the ultrasound-guided PDT procedures were described as easy or somewhat difficult in 88.3 % of time by the participating residents. Although these data suggest that our results might be generalizable, similar results might not be obtained by less experienced teams.
The strengths of our trial include a prospective randomization, a strict maintenance of allocation concealment, a limited exclusion criterion, a high percentage of enrollments of eligible patients, a sample size calculation, and a prespecified noninferiority margin. The clinical outcomes were clinically relevant and the data analysis was blinded.
This study has important limitations. First, this is a single-center investigation, although it included eight ICUs, with a heterogeneous patient’s population, and with many attending physicians with different backgrounds before any critical care training (surgeons, anesthesiologists, and internal medicine). Second, this is a noninferiority trial, and the noninferiority margin of 6 % might be considerably high for the low primary outcome incidence rate. A rational decision about noninferiority margin should be prespecified on the basis of clinical factors and data from previous studies [37, 38]. The TRACHUS noninferiority margin was prespecified on the basis of 95 % CI from previous published observational cohorts on the topic [20, 21], clinically relevant, and similar to noninferiority margins used in recently published noninferiority RCTs evaluating surgical procedures [39, 40]. Third, the patients were followed only until hospital discharge and were not assessed for late complications such as tracheal stenosis, vocal abnormalities, or scar characteristics. Fourth, blinding was not possible because of the nature of the procedures and the outcome assessments were also not blinded. Fifth, the bronchoscopy procedure was performed by trained intensivists and not by the specialized bronchoscopy team. However, this is in line with the routine practice of most of the ICUs worldwide, which could improve external validity.
Conclusions
Our data indicates that ultrasound-guided PDT is noninferior to bronchoscopy-guided PDT in mechanically ventilated patients in the ICU. Therefore, an ultrasound-guided PDT might be effectively and safely used as an alternative to a bronchoscopy-guided PDT.
Abbreviations
- CAPPesq:
-
Comissão de Ética para Análise de Projetos de Pesquisa
- CNS:
-
Central nervous system
- FiO2 :
-
Fraction of inspired oxygen
- ICU:
-
Intensive care unit
- MV:
-
Mechanical ventilation
- NA:
-
Not applicable
- PaO2 :
-
Arterial oxygen pressure
- PDT:
-
Percutaneous dilational tracheostomy
- RCT:
-
Randomized clinical trial
- SAPS 3:
-
Simplified Acute Physiology Score 3
- US:
-
Ultrasound
References
Vargas M, Sutherasan Y, Antonelli M, Brunetti I, Corcione A, Laffey JG, Putensen C, Servillo G, Pelosi P (2015) Tracheostomy procedures in the intensive care unit: an international survey. Crit Care 19:291
Delaney A, Bagshaw SM, Nalos M (2006) Percutaneous dilatational tracheostomy versus surgical tracheostomy in critically ill patients: a systematic review and meta-analysis. Critical Care 10: R55
Putensen C, Theuerkauf N, Guenther U, Vargas M, Pelosi P (2014) Percutaneous and surgical tracheostomy in critically ill adult patients: a meta-analysis. Crit Care 18:544
Dennis BM, Eckert MJ, Gunter OL, Morris JA Jr, May AK (2013) Safety of bedside percutaneous tracheostomy in the critically ill: evaluation of more than 3,000 procedures. J Am Coll Surg 216:858–865 (discussion 865–857)
Hinerman R, Alvarez F, Keller CA (2000) Outcome of bedside percutaneous tracheostomy with bronchoscopic guidance. Intensive Care Med 26:1850–1856
Fernandez L, Norwood S, Roettger R, Gass D, Wilkins H 3rd (1996) Bedside percutaneous tracheostomy with bronchoscopic guidance in critically ill patients. Arch Surg 131:129–132
Flint AC, Midde R, Rao VA, Lasman TE, Ho PT (2009) Bedside ultrasound screening for pretracheal vascular structures may minimize the risks of percutaneous dilatational tracheostomy. Neurocrit Care 11:372–376
Singh M, Chin KJ, Chan VW, Wong DT, Prasad GA, Yu E (2010) Use of sonography for airway assessment: an observational study. J Ultrasound Med 29:79–85
Bonde J, Nørgaard N, Antonsen K (1999) Implementation of percutaneous dilation tracheotomy—value of preincisional ultrasonic examination? Acta Anaesthesiol Scand 43:163–166
Kollig E, Heydenreich U, Roetman B, Hopf F, Muhr G (2000) Ultrasound and bronchoscopic controlled percutaneous tracheostomy on trauma ICU. Injury 31:663–668
Sustic A, Zupan Z, Eskinja N, Dirlic A, Bajek G (1999) Ultrasonographically guided percutaneous dilatational tracheostomy after anterior cervical spine fixation. Acta Anaesthesiol Scand 43:1078–1080
Rajajee V, Fletcher JJ, Rochlen LR, Jacobs TL (2011) Real-time ultrasound-guided percutaneous dilatational tracheostomy: a feasibility study. Crit Care 15:R67
Rezende-Neto JB, Oliveira AJ, Neto MP, Botoni FA, Rizoli SB (2011) A technical modification for percutaneous tracheostomy: prospective case series study on one hundred patients. World J Emerg Surg 6:35
Guinot PG, Zogheib E, Petiot S, Marienne JP, Guerin AM, Monet P, Zaatar R, Dupont H (2012) Ultrasound-guided percutaneous tracheostomy in critically ill obese patients. Crit Care 16:R40
Rajajee V, Williamson CA, West BT (2015) Impact of real-time ultrasound guidance on complications of percutaneous dilatational tracheostomy—a propensity score analysis. Crit Care 19:198
Gobatto AL, Besen BA, Tierno PF, Mendes PV, Cadamuro F, Joelsons D, Melro L, Park M, Malbouisson LM (2015) Comparison between ultrasound- and bronchoscopy-guided percutaneous dilational tracheostomy in critically ill patients: a retrospective cohort study. J Crit Care 30:220.e213–220.e227
Yavuz A, Yilmaz M, Goya C, Alimoglu E, Kabaalioglu A (2014) Advantages of US in percutaneous dilatational tracheostomy: randomized controlled trial and review of the literature. Radiology 273:927–936
Rudas M, Seppelt I, Herkes R, Hislop R, Rajbhandari D, Weisbrodt L (2014) Traditional landmark versus ultrasound guided tracheal puncture during percutaneous dilatational tracheostomy in adult intensive care patients: a randomised controlled trial. Crit Care 18:514
Ravi PR, Vijay MN (2015) Real time ultrasound-guided percutaneous tracheostomy: is it a better option than bronchoscopic guided percutaneous tracheostomy? Med J Armed Forces India 71:158–164
Jackson LS, Davis JW, Kaups KL, Sue LP, Wolfe MM, Bilello JF, Lemaster D (2011) Percutaneous tracheostomy: to bronch or not to bronch–that is the question. J Trauma 71:1553–1556
Abdulla S, Conrad A, Vielhaber S, Eckhardt R, Abdulla W (2013) Should a percutaneous dilational tracheostomy be guided with a bronchoscope? B-ENT 9:227–234
Schuirmann DJ (1987) A comparison of the two one-sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm 15:657–680
Walker E, Nowacki AS (2011) Understanding equivalence and noninferiority testing. J Gen Intern Med 26:192–196
Rudas M, Seppelt I (2012) Safety and efficacy of ultrasonography before and during percutaneous dilatational tracheostomy in adult patients: a systematic review. Crit Care Resusc 14:297–301
Alansari M, Alotair H, Al Aseri Z, Elhoseny MA (2015) Use of ultrasound guidance to improve the safety of percutaneous dilatational tracheostomy: a literature review. Critical Care 19:229
Madsen KR, Guldager H, Rewers M, Weber SO, Kobke-Jacobsen K, White J (2015) Danish guidelines 2015 for percutaneous dilatational tracheostomy in the intensive care unit. Dan Med J 61(3):pii B5042
Kornblith LZ, Burlew CC, Moore EE, Haenel JB, Kashuk JL, Biffl WL, Barnett CC, Johnson JL (2011) One thousand bedside percutaneous tracheostomies in the surgical intensive care unit: time to change the gold standard. J Am Coll Surg 212:163–170
Higgins KM, Punthakee X (2007) Meta-analysis comparison of open versus percutaneous tracheostomy. Laryngoscope 117:447–454
Park M, Brauer L, Sanga RR, Amaral ACK-B, Ladeira JP, Azevedo LCPd, Taniguchi LU, Cruz-Neto LMd (2004) Traqueostomia percutânea no doente crítico: a experiência de uma unidade de terapia intensiva clínica. J Bras Pneumol 30:237–242
Polderman KH, Spijkstra JJ, de Bree R, Christiaans HM, Gelissen HP, Wester JP, Girbes AR (2003) Percutaneous dilatational tracheostomy in the ICU: optimal organization, low complication rates, and description of a new complication. Chest 123:1595–1602
Tomsic JP, Connolly MC, Joe VC, Wong DT (2006) Evaluation of bronchoscopic-assisted percutaneous tracheostomy. Am Surg 72:970–972
Kluge S, Baumann HJ, Maier C, Klose H, Meyer A, Nierhaus A, Kreymann G (2008) Tracheostomy in the intensive care unit: a nationwide survey. Anesth Analg 107:1639–1643
Kost KM (2005) Endoscopic percutaneous dilatational tracheotomy: a prospective evaluation of 500 consecutive cases. Laryngoscope 115:1–30
Dinh VA, Farshidpanah S, Lu S, Stokes P, Chrissian A, Shah H, Giri P, Hecht D, Nguyen HB (2014) Real-time sonographically guided percutaneous dilatational tracheostomy using a long-axis approach compared to the landmark technique. J Ultrasound Med 33:1407–1415
Chacko J, Gagan B, Kumar U, Mundlapudi B (2015) Real-time ultrasound guided percutaneous dilatational tracheostomy with and without bronchoscopic control: an observational study. Minerva Anestesiol 81:166–174
Chacko J, Nikahat J, Gagan B, Umesh K, Ramanathan M (2012) Real-time ultrasound-guided percutaneous dilatational tracheostomy. Intensive Care Med 38:920–921
Siegel JP (2000) Equivalence and noninferiority trials. Am Heart J 139:S166–S170
Snapinn SM (2004) Alternatives for discounting in the analysis of noninferiority trials. J Biopharm Stat 14:263–273
Stevenson AR, Solomon MJ, Lumley JW, Hewett P, Clouston AD, Gebski VJ, Davies L, Wilson K, Hague W, Simes J, ALaCaRT Investigators (2015) Effect of laparoscopic-assisted resection vs open resection on pathological outcomes in rectal cancer: the ALaCaRT randomized clinical trial. JAMA 314:1356–1363
Bonjer HJ, Deijen CL, Haglind E, COLOR II Study Group (2015) A randomized trial of laparoscopic versus open surgery for rectal cancer. N Engl J Med 373:194
Acknowledgments
We would like to thank the nursing staff, the respiratory therapists, the intensive care medicine residents, and all of the attending physicians from the ICU departments for performing treatment for our patients and for their cooperation in the trial. Special acknowledgments are due to Dr. Mino Cestari whose collaboration was indispensable to the trial.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interests
The authors have no relevant conflict of interest to disclose. The study was not financially supported by any funding source. The design, collection, analysis, and the interpretation of data, plus the writing and the publication of the manuscript, were done by the authors without participation or influence from any funding source.
Additional information
Take home massage: Ultrasound-guided percutaneous dilational tracheostomy (PDT) is easy, fast and safe compared with bronchoscopy-guided PDT.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gobatto, A.L.N., Besen, B.A.M.P., Tierno, P.F.G.M.M. et al. Ultrasound-guided percutaneous dilational tracheostomy versus bronchoscopy-guided percutaneous dilational tracheostomy in critically ill patients (TRACHUS): a randomized noninferiority controlled trial. Intensive Care Med 42, 342–351 (2016). https://doi.org/10.1007/s00134-016-4218-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00134-016-4218-6