Abstract
Aims/hypothesis
Berardinelli–Seip congenital lipodystrophy type 2 (BSCL2) is an autosomal recessive disorder characterised by lipodystrophy and insulin resistance. BSCL2 is caused by loss-of-function mutations in the Seipin gene (also known as Bscl2). Deletion of this gene in mice induces insulin resistance, glucose intolerance and a loss of adipose tissue. This study evaluated the effects of genetic deletion of Seipin on islet beta cell function.
Methods
We examined seipin expression in islet cells and measured glucose profiles, insulin synthesis, glucose-stimulated insulin secretion (GSIS), islet expression of peroxisome proliferator-activated receptor γ (PPARγ), levels of Pdx-1, Nkx6.1, Glut2 (also known as Slc2a2) and proinsulin mRNA, nuclear translocation of pancreatic duodenal homeobox 1 (PDX-1), islet numbers, and beta cell mass and proliferation in male and female Seipin-knockout homozygous (Seipin−/−) and heterozygous (Seipin+/−) mice.
Results
Male and female Seipin−/− mice displayed glucose intolerance, insulin resistance, hyperinsulinaemia and a lack of adipose tissue. By contrast, male but not female Seipin+/− mice showed glucose intolerance without adipose tissue loss or insulin resistance. Seipin was highly expressed in islet beta cells in wild-type mice. Expression of islet PPARγ was reduced in male Seipin−/− and Seipin+/− mice but not in female Seipin−/− or Seipin+/− mice. Treatment of male Seipin+/− mice with rosiglitazone corrected the glucose intolerance. Male Seipin+/− mice displayed a decrease in islet insulin concentration and GSIS with low expression of Pdx-1, Nkx6.1, Glut2 and proinsulin, and a decline in PDX-1 nuclear translocation; these changes were rescued by rosiglitazone administration. Male Seipin−/− mice showed obvious, but rosiglitazone-sensitive, increases in islet insulin concentration, islet number and beta cell mass and proliferation, with a notable decline in GSIS. Ovariectomised female Seipin+/− mice displayed glucose intolerance and deficits in insulin synthesis and secretion, with a decline in islet PPARγ level; these deleterious effects were reversed by administration of oestradiol or rosiglitazone.
Conclusions/interpretation
Heterozygous deletion of Seipin in islet beta cells impacts on insulin synthesis and secretion through reduced PPARγ expression. This leads to glucose intolerance and is relieved by oestradiol, which rescues PPARγ expression.
Similar content being viewed by others

Introduction
Berardinelli–Seip congenital lipodystrophy type 2 (BSCL2) is an autosomal recessive disorder characterised by a near total lack of adipose tissue, together with severe insulin resistance, glucose intolerance, liver steatosis and hypertriacylglycerolaemia [1]. BSCL2 is caused by loss-of-function mutations in the Seipin gene (also known as Bscl2) [2, 3]. Seipin-knockout (Seipin−/−) mice recapitulate many aspects of human BSCL2, such as a dramatic loss of adipose tissue, insulin resistance, glucose intolerance and hepatic steatosis [4].
Depletion of seipin, an exclusive endoplasmic reticulum (ER)-resident N-glycosylated protein, has been reported to decrease the generation of peroxisome proliferator-activated receptor γ (PPARγ) in murine embryonic fibroblasts, stromal vascular cells [5] and adipose tissue [6, 7]. PPARγ is a transcription factor that is involved in insulin sensitivity, adipocyte differentiation, lipid storage and glucose uptake [8]. Knockdown of seipin reduces the differentiation of adipocytes and this reduction is rescued by the PPARγ agonist pioglitazone [9]. In particular, treatment with PPARγ agonists has been reported to improve insulin sensitivity and glucose tolerance in Seipin−/− mice [6].
PPARγ is expressed in islet beta cells, where it is involved in the beta cell proliferation and apoptosis, and is involved in insulin synthesis and secretion [10]. PPARγ, through increased expression of pancreatic duodenal homeobox 1 (PDX-1), regulates insulin transcription [11]. A 75% loss of PPARγ, using RNA interference, can reduce Nkx6.1 (also known as Nkx6-1, encoding beta cell-specific transcription factor NK6 homeobox 1) and Glut2 (also known as Slc2a2) mRNA levels, and affects glucose-stimulated insulin secretion (GSIS) [11]. Therefore, if beta cells express seipin protein, a seipin deficiency would be expected to reduce PPARγ and thereby affect insulin synthesis and secretion. The focus of the present study was to evaluate the influence of seipin deficiency on islet beta cell function.
An earlier study [12] reported sex-related downregulation of PPARγ expression in the brain of Seipin−/− mice, where administration of oestradiol rescued the reduction in PPARγ expression. These findings suggest a correlation between seipin-reduced PPARγ expression and oestrogen. In humans, BSCL2 is a recessive disease. Seipin-deficient homozygous (Seipin−/−) rats exhibit a reduction in body weight but heterozygous (Seipin+/−) rats do not display changes in body weight [13]. Windpassinger et al. [14] reported that heterozygous missense mutations in the Seipin gene led to distal hereditary motor neuropathy and Silver syndrome. However, whether the heterozygous deletion of Seipin affects metabolism and glucose homeostasis remains unclear. In this study, we used 3-month-old male and female Seipin−/− and Seipin+/− mice to examine the influence of the homozygous and heterozygous deletion of Seipin in glucose homeostasis and insulin synthesis and secretion, and explored the underlying molecular mechanisms.
Methods
Generation of Seipin null mice and experimental design
All animal experiments were approved by the Institutional Animal Care and Ethical Committee of the Nanjing Medical University. The Seipin-knockout mice were generated and their genotypes identified as described previously [4]. Eight-week-old animals were used in this study. The mice were maintained under constant environmental conditions (23 ± 2°C, humidity of 55 ± 5%, and a 12:12 h light/dark cycle) with free access to food (a standard laboratory chow) and water. A glucose-lowering drug of the thiazolidinone class, rosiglitazone (Sigma-Aldrich, St Louis, MO, USA) was orally administered daily [15]. Six days after ovariectomy [16], oestradiol (5 μg/kg per day; β-Estradiol, E2758, Sigma-Aldrich) was injected subcutaneously [17]. In this study, male wild-type (WT) mice (n = 20), female WT mice (n = 28), male Seipin−/− mice (n = 26), male Seipin+/− mice (n = 34), female Seipin−/− mice (n = 20) and female Seipin+/− mice (n = 44) were randomly divided into three experimental groups. The first group was used to examine serum leptin and insulin, plasma glucose, and glucose and insulin tolerance, followed by islet immunostaining (n = 6 per experimental group). In the second group, islets were isolated to examine insulin secretion and mRNA expression (n = 8 per experimental group). In the third group, isolated islets were treated with rosiglitazone or oestradiol, and insulin secretion and mRNA expression were examined (n = 8 per experimental group).
Measurement of plasma glucose and insulin
After mice were fasted for 6 h, blood samples were obtained from the tail vein to measure the levels of fasting plasma glucose by the glucose oxidase method (Contour Glucometer; Bayer, Toronto, ON, Canada). Orbital blood was obtained and the level of fasting serum insulin was analysed by an ELISA (Mouse Insulin; Mercodia, Winston-Salem, NC, USA). The intra- and inter-assay CV for insulin was 1.67% and 2.25%, respectively.
ITT
After mice were fasted for 6 h, human recombinant insulin (1 IU/kg; Novolin-R; Novo Nordisk, Plainsboro, NJ, USA) was injected intraperitoneally. Blood (5 μl) was obtained from the tail tip before insulin injection and at 15, 30, 60 and 120 min after insulin injection [18]. ITT curves were constructed for the 0–120 min experimental time window and the AUC was calculated.
GTT and glucose-stimulated insulin secretion
For in vivo determination of GSIS, d-glucose (1 g/kg) was injected intraperitoneally after mice had been fasted for 6 h. Blood samples were collected from the sublingual vein at 0, 2, 5, 10, 30, 60 and 120 min after glucose loading and plasma glucose and insulin levels were measured [19]. For in vitro determination of insulin secretion, islets (isolated from mice as previously described [20]) were perfused with 5 mmol/l glucose for 60 min to obtain the basal insulin release and then treated with 25 mmol/l glucose for another 1 h to determine GSIS [20].
Immunohistochemistry and immunofluorescence
Mice were euthanised with intraperitoneal injection of pentobarbital (3 mg/100 ml) and the image of viscera in each mouse was taken by a stereoscopic microscope (RWD Life Science, Shenzhen, China). The visceral fat (perirenal, mesenteric and epididymal) was removed and weighed to calculate visceral fat to body weight ratio. The pancreases were removed and fixed in Bouin’s fluid for making paraffin sections. Rabbit anti-seipin antibody was kindly provided by J. Sha (Nanjing Medical University); other antibodies (diluted in PBS with 1% BSA) are listed in electronic supplementary material (ESM) Table 1. All antibodies were validated using positive controls. Immunohistochemical staining was observed using a DP70 microscope (Olympus Optical, Tokyo, Japan) and immunofluorescence staining was observed using a laser scanning confocal microscope (FV1000; Olympus). The immunoreactivity of insulin was calculated by integral absorbance (hue: 0–30; saturation: 30–200; intensity: 20–210) corrected for islet area using ImageJ software (https://imagej.nih.gov/ij/, National Institutes of Health, USA). Pancreatic beta cell mass was calculated as (islet area/pancreas area) × pancreas weight. The Ki67-positive ratio was calculated as number of Ki67-positive nuclei per islet area. The PDX-1 nuclear translocation ratio was calculated as the number of PDX-1-positive nuclei divided by total number of islet nuclei.
Primary cultured islets, western blotting and reverse-transcription quantitative PCR
Mice were anaesthetised with chloral hydrate and then their islets were isolated as previously described [20]. Briefly, collagenase V (1 mg/ml concentration, Sigma-Aldrich) was injected into the pancreas through the common bile duct. The pancreas tissue was then quickly removed and digested to isolate islets. Freshly isolated islets were lysed in RIPA buffer (Beyotime, Shanghai, China) to extract protein or in Trizol (Invitrogen, Carlsbad, CA, USA) to extract total RNA, following the manufacturer’s instructions. Western blotting and reverse-transcription quantitative PCR (RT-qPCR) analyses were performed as previously described [21]. For RT-qPCR, after reverse transcription, qPCR analyses were carried out in a LightCycler 480 (Roche Diagnostic, Branchburg, NJ, USA). The primers were synthesised by Invitrogen and the primer sequences are listed in ESM Table 2. The Gadph gene was used as an internal control.
Statistical analysis
All experimenters were blind to group assignment and outcome assessment. Data are expressed as the mean ± SE. All statistical analyses were performed using SPSS software, version 18.0 (SPSS, Chicago, IL, USA). Differences among the means were analysed using Student’s t test or ANOVA with or without repeated measures, followed by the Bonferroni post hoc test where appropriate. Differences were considered statistically significant at p < 0.05.
Results
Seipin deficiency alters insulin sensitivity and glucose homeostasis
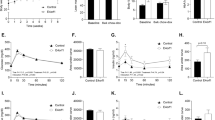
Mean body weight did not differ significantly between the groups of mice at 3 months of age (p > 0.05) (Fig. 1a). Seipin−/− mice of both sexes displayed an obvious reduction in their visceral fat (Fig. 1b) but the Seipin+/− mice did not. When compared with WT mice, the ratio of visceral fat weight to body weight was reduced by approximately 40–50% in male Seipin−/− mice (p < 0.01) and female Seipin−/− mice (p < 0.05) (Fig. 1c), along with a decline in the level of serum leptin (male Seipin−/− mice, p < 0.01; female Seipin−/− mice, p < 0.05) (Fig. 1d), but the ratio of visceral fat weight to body weight was the same in male Seipin+/−, female Seipin+/− and WT mice (p > 0.05). GTT analysis revealed higher plasma glucose levels at 15, 30 and 60 min after the glucose challenge in male Seipin−/− mice (15 min, p < 0.01; 30–60 min, p < 0.05), female Seipin−/− mice (30/120 min, p < 0.05; 60 min, p < 0.01) and male Seipin+/− mice (30–60 min, p < 0.05) than in WT mice but the levels did not differ between female Seipin+/− mice and WT mice (p > 0.05) (Fig. 1e,f). The AUC for the GTT was larger for male Seipin−/− (p < 0.05), female Seipin−/− (p < 0.05) and male Seipin+/− mice (p < 0.05) than for the WT mice (Fig. 1g). The response to an insulin injection during the ITT was significantly reduced in male Seipin−/− (15/60 min, p < 0.01; 30 min, p < 0.05) and female Seipin−/− mice (60 min, p < 0.01) when compared with WT mice, whereas the response in male Seipin+/− and female Seipin+/− mice did not differ from that of the WT mice (p > 0.05) (Fig. 1h,i). The AUC of the ITT was increased in both male and female Seipin−/− mice (p < 0.05) when compared with WT mice (Fig. 1j).
Seipin deficiency alters insulin sensitivity and glucose homeostasis. (a) Body weight of 3-month-old male and female WT, Seipin+/− and Seipin−/− mice (n = 20 per group). (b) Representative images of visceral adipose tissue. (c, d) Ratio of visceral fat weight (VF) to body weight (BW) (c) and level of serum leptin (d). *p < 0.05, **p < 0.01 vs WT mice of the same sex; ††p < 0.01 vs male Seipin+/− mice (one-way ANOVA followed by Bonferroni post hoc test, n = 10 per group). (e–g) IPGTT curve in male (e) and female (f) mice and AUC of GTT (g). *p < 0.05, **p < 0.01 vs WT mice of the same sex; †p < 0.05, ††p < 0.01 vs Seipin+/− mice of the same sex (one-way ANOVA followed by Bonferroni post hoc test, n = 6 per group). (h–j) IPITT curve in male (h) and female (i) mice and AUC of ITT (j). *p < 0.05, **p < 0.01 vs WT mice of the same sex; †p < 0.05, ††p < 0.01 vs Seipin+/− mice of the same sex (one-way ANOVA followed by Bonferroni post hoc test, n = 6 per group)
Seipin is selectively expressed in islet beta cells
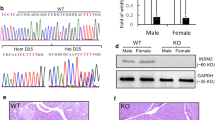
Immunohistochemistry revealed a selective and high level of seipin expression in pancreatic islet cells (Fig. 2a). When compared with WT mice, the seipin immunoreaction in Seipin+/− mice of both sexes was clearly reduced (Fig. 2b). A 30–40% decline was also observed in the level of Seipin mRNA in islets isolated from male Seipin+/− mice of both sexes (p < 0.05) (Fig. 2c). Seipin mRNA was not detected in the islets of male Seipin−/− mice or female Seipin−/− mice (p < 0.01). The seipin protein was localised in the cytoplasm of the islet cells. As shown in Fig. 2d, the immunoreaction of seipin was mostly overlapped in the insulin-positive beta cells, rather than in the glucagon-positive alpha cells, pancreatic polypeptide-positive pancreatic polypeptide cells or somatostatin-positive delta cells. The beta cell marker PDX-1 was found in the seipin-positive cells (Fig. 2d). Moreover, the immunoreaction of seipin was colocalised with the ER marker calnexin (Fig. 2d). The specificity of the immunostaining was confirmed by the lack of seipin in the islet beta cells of male Seipin−/− mice (Fig. 2e).
Seipin is selectively expressed in islet beta cells. (a) Representative images of seipin immunostaining (brown) in the pancreas of WT mice. The magnified inset image shows the cytoplasmic distribution of seipin protein. Scale bar, 50 μm. (b) Immunostaining for seipin in WT, Seipin+/− and Seipin−/− mice. Scale bar, 50 μm. (c) Levels of Seipin mRNA in islets from WT, Seipin+/− and Seipin−/− mice. Data are expressed as fold of levels in WT mice. *p < 0.05, **p < 0.05 vs WT mice of the same sex (Student’s t test, n = 6 per group). (d) Pancreases from male WT mice were used for double immunofluorescence. Representative images of seipin (red) and insulin, glucagon, pancreatic polypeptide (PP), somatostatin, PDX-1 or calnexin (all green) double immunofluorescence staining. Nuclei were stained with Hoechst 33342 dye (blue). Scale bar, 50 μm. (e) Double immunofluorescence staining of seipin (red) and insulin (green) in male Seipin−/− mice. Scale bar, 50 μm. All representative images were selected from 6 mice in each group
Seipin deficiency disrupts insulin secretion
Fasting serum insulin levels were higher in Seipin−/− mice of both sexes (p < 0.01) than in WT mice (p < 0.01) (Fig. 3a). Insulin levels were slightly reduced in male Seipin+/− mice vs WT mice but the difference did not reach statistical significance; levels did not differ between female Seipin+/− mice and WT mice (p > 0.05). When compared with WT mice, the integrated absorbance of the insulin immunoreaction (Fig. 3b) was increased in male and female Seipin−/− mice (both p < 0.05), whereas it was reduced in male Seipin+/− mice (p < 0.05) and was not altered in female Seipin+/− mice (p > 0.05) (Fig. 3c). As shown in Fig. 3d,e, the GSIS was measured at 2–5 min (as the first phase) and 10–120 min after glucose injection (as the second phase), respectively. When compared with WT mice, the AUC of the first-phase GSIS in male Seipin−/− mice was lower (p < 0.05; Fig. 3f), whereas the AUC of the second-phase GSIS was higher (p < 0.05; Fig. 3g). By contrast, female Seipin−/− mice only displayed an increase in the AUC of the second-phase GSIS in comparison with WT mice (p < 0.05). Notably, the AUCs of the first-phase GSIS (p < 0.01) and the second-phase GSIS (p < 0.05) were reduced in male Seipin+/− mice; however, the responses of the female Seipin+/− mice did not differ significantly from those of the WT mice (p > 0.05). In islets isolated from male Seipin−/− mice (p > 0.05) or male Seipin+/− mice (p > 0.05), the basal level of insulin secretion was no different from that in WT mice (Fig. 3h). However, after the isolated islets were treated with 25 mmol/l glucose for 60 min, the levels of in vitro GSIS in male Seipin+/− mice (p < 0.01) and male Seipin−/− mice (p < 0.05) were lower than those in WT mice.
Seipin deficiency disrupts insulin secretion. (a) Fasting serum insulin in WT, Seipin+/− and Seipin−/− mice. **p < 0.01 vs WT mice of the same sex; ††p < 0.01 vs Seipin+/− mice of the same sex (one-way ANOVA followed by Bonferroni post hoc test, n = 6 per group). (b) Representative image of insulin immunostaining (brown). Scale bar, 50 μm. The representative images were selected from 6 mice in each group. (c) Integrated absorbance of insulin immunoreactions corrected for islet area. Data are expressed as fold of absorbance of insulin immunoreactions for WT mice. *p < 0.05 vs WT mice of the same sex; †p < 0.05, ††p < 0.01 vs Seipin+/− mice of the same sex (one-way ANOVA, n = 6 per group). (d, e) GSIS in male (d) and female (e) WT, Seipin+/− and Seipin−/− mice. (f, g) AUC of the first-phase GSIS (f) and second-phase GSIS (g). *p < 0.05, **p < 0.01 vs WT mice of the same sex; ††p < 0.01 vs Seipin+/− mice of the same sex (one-way ANOVA, n = 6 per group). (h) Levels of insulin secretion from isolated islets treated with 5 mmol/l (5G) and 25 mmol/l (25G) glucose. *p < 0.05, **p < 0.01 vs WT mice of the same sex (one-way ANOVA, n = 6 per group)
Seipin deficiency reduces PPARγ expression to alter insulin secretion
When compared with the levels in WT mice, the expression levels of Pparγ (also known as Pparg) mRNA (Fig. 4a) and PPARγ protein (Fig. 4b) were reduced in the islets obtained from male Seipin+/− mice (mRNA/protein p < 0.05) and male Seipin−/− mice (mRNA/protein p < 0.01) but were unchanged in female Seipin−/− and Seipin+/− mice (p > 0.05). Notably, the administration of rosiglitazone was able to correct the glucose intolerance in male Seipin+/− mice (p < 0.05) (Fig. 4c and ESM Fig. 1a) and increased the absorbance of the insulin immunoreaction (p < 0.05) (Fig. 4d), the first-phase GSIS (p < 0.01) (Fig. 4e) and the second-phase GSIS (p < 0.01) (Fig. 4f). In male Seipin−/− mice, treatment with rosiglitazone corrected the level of fasting serum insulin (p < 0.01) (Fig. 4g), the absorbance of insulin immunoreaction (p < 0.01) (Fig. 4d) and the first-phase GSIS (p < 0.05) (Fig. 4e), whereas the treatment with rosiglitazone failed to recover the glucose intolerance (p > 0.05) (Fig. 4c and ESM Fig. 1a), the second-phase GSIS (p > 0.05) (Fig. 4f) or insulin resistance (p > 0.05) (Fig. 4h and ESM Fig. 1b). Moreover, the treatment with rosiglitazone for 24 h corrected GSIS in islets isolated from male Seipin+/− or Seipin−/− mice (both p < 0.05) (Fig. 4i).
Seipin deficiency reduces PPARγ expression to alter insulin secretion. (a, b) Levels of Pparγ mRNA (a) and PPARγ protein (b) in isolated islets. Data are expressed as fold of levels in WT mice. *p < 0.05, **p < 0.01 vs male WT mice; ††p < 0.01 vs male Seipin+/− mice (one-way ANOVA, n = 8 (a) or 6 (b) per group). (c–h) Effects of rosiglitazone (5 mg/kg daily) on AUC of GTT (c), integrated absorbance of insulin immunoreactions (d), AUC of the first-phase GSIS (e) and second-phase GSIS (f), fasting serum insulin (g) and AUC of ITT (h) in male Seipin−/− mice and male Seipin+/− mice. *p < 0.05, **p < 0.01 vs WT mice; †p < 0.05, ††p < 0.01 vs no rosiglitazone treatment (one-way ANOVA followed by Bonferroni post hoc test, n = 6 per group). (i) Insulin secretion from isolated islets from male mice treated with rosiglitazone for 24 h. *p < 0.05, **p < 0.01 vs WT mice; †p < 0.05 vs no rosiglitazone treatment (one-way ANOVA followed by Bonferroni post hoc test, n = 6 per group). Rosi, rosiglitazone
Seipin deficiency, through reduced PPARγ expression, suppresses the expression of regulators of insulin synthesis and secretion
The mRNA levels of Pdx-1 (Fig. 5a), Nkx6.1 (Fig. 5b) and Glut2 (Fig. 5c) were decreased (all p < 0.05) in the islets isolated from male Seipin+/− or Seipin−/− mice. The levels of proinsulin mRNA were reduced in the islets isolated from male Seipin+/− mice (p < 0.05) but not male Seipin−/− mice (p > 0.05) (Fig. 5d). The systemic administration of rosiglitazone for 14 days or treatment of the isolated islets with rosiglitazone for 24 h rescued the decline in the levels of proinsulin (p < 0.05), Pdx-1 (p < 0.05), Nkx6.1 (p < 0.05) and Glut2 mRNA (p < 0.05) in male Seipin+/− and Seipin−/− mice (Fig. 5a–d). Double immunofluorescence staining also confirmed that PDX-1 nuclear translocation in the islets was lower for male Seipin+/− mice than for WT mice (p < 0.01) (Fig. 5e,f) and was sensitive to rosiglitazone treatment (p < 0.05). Similarly, the ratio of PDX-1 nuclear translocation was lower in male Seipin−/− mice than in WT mice (p < 0.01).
Seipin deficiency suppresses the expression of regulators of insulin synthesis and secretion in male mice, through its reduction of PPARγ expression. (a–d) Levels of Pdx-1, Nkx6.1, Glut2 and proinsulin mRNA in islets of WT mice and male Seipin−/− and Seipin+/− mice treated with rosiglitazone (in vivo), or in isolated islets treated with rosiglitazone (in vitro). Data are expressed as fold of WT mice. *p < 0.05 vs WT mice; †p < 0.05 vs no rosiglitazone treatment (two-way ANOVA, n = 8 per group). (e) Representative images of insulin (green) and PDX-1 (red) double immunofluorescence staining. Nuclei were stained with Hoechst 33342 dye (blue). Scale bar, 50 μm. The representative images were selected from 6 mice in each group. (f) Ratio of PDX-1 nuclear translocation in islets quantified from immunofluorescence images as in (e). **p < 0.01 vs WT mice; †p < 0.05 vs no rosiglitazone treatment (two-way ANOVA, n = 6 per group). Rosi, rosiglitazone
Seipin deficiency, through reduced PPARγ expression, enhances beta cell proliferation
Immunohistochemical analyses for insulin (Fig. 6a) revealed increases in the beta cell mass (p < 0.05) (Fig. 6b) and the number of islets (p < 0.05) (Fig. 6c) in male and female Seipin−/− mice but no changes in male or female Seipin+/− mice (p > 0.05). Consistent with changes in the fasting serum insulin and the absorbance of the insulin immunoreaction (Fig. 4e,f), the administration of rosiglitazone for 14 days to male Seipin−/− mice restored the islet beta cell mass (p < 0.05) and number of islets (p < 0.05). A subsequent examination of the beta cell proliferation by double immunohistochemical staining for insulin and Ki67 (Fig. 6d) showed that there was a >30% increase (p < 0.01) in the number of insulin/Ki67-positive cells per islet in male Seipin−/− mice when compared with WT mice, and this was corrected by the administration of rosiglitazone (p < 0.01) (Fig. 6e). By contrast, the number of insulin/Ki67-positive cells did not differ significantly between the male Seipin+/− mice and WT mice (p > 0.05).
Seipin deficiency enhances beta cell proliferation, through reduced PPARγ expression. (a) Representative images of insulin-positive (brown) beta cells in WT mice, Seipin+/− mice and Seipin−/− mice. Scale bar, 300 μm. The representative images were selected from 6 mice in each group. (b, c) Beta cell mass (b) and islet number corrected for tissue area (c) quantified from immunostaining images as in (a). *p < 0.05 vs WT mice of the same sex; †p < 0.05, ††p < 0.01 vs male Seipin+/− mice; §p < 0.05 vs no rosiglitazone treatment (two-way ANOVA, n = 6 per group). (d) Representative images of insulin (green) and Ki67 (red) double immunofluorescence staining in the islets of male mice. Nuclei were stained with Hoechst 33342 dye (blue). Scale bar, 50 μm. (e) Quantification of immunofluorescence images as in (d). Bars indicate the group means of Ki67+ cell numbers per islet. **p < 0.01 vs WT mice; †p < 0.05 vs Seipin+/− mice; §§p < 0.01 vs no rosiglitazone treatment (two-way ANOVA, n = 6 per group). Rosi, rosiglitazone
Oestrogen relieves the reduction in PPARγ expression induced by seipin deficiency in female mice
The level of Pparγ mRNA was reduced in the islets isolated from female Seipin+/− mice on the fourth week after ovariectomy (p < 0.01 vs ovariectomised WT mice) (Fig. 7a); levels in WT mice were not affected by ovariectomy (p > 0.05). The reduction of Pparγ mRNA in Seipin+/− mice was corrected by the replacement of oestrogen with oestradiol (p < 0.01). In addition, ovariectomised Seipin+/− mice, but not ovariectomised WT mice, displayed glucose intolerance (p < 0.05) (Fig. 7b and ESM Fig. 2a) and a decline in first-phase (p < 0.01) (Fig. 7c) and second-phase GSIS (p < 0.01) (Fig. 7d) without insulin resistance (p > 0.05) (Fig. 7e and ESM Fig. 2b). The administration of rosiglitazone or oestradiol for 14 days to ovariectomised Seipin+/− mice improved their glucose tolerance (both p < 0.05) (Fig. 7b and ESM Fig. 2a), first-phase GSIS (both p < 0.01) (Fig. 7c) and second-phase GSIS (both p < 0.05) (Fig. 7d). Similarly, ovariectomy reduced the mRNA levels of Pdx-1 (p < 0.01) (Fig. 7f), Nkx6.1 (p < 0.05) (Fig. 7g), Glut2 (p < 0.05) (Fig. 7h) and proinsulin (p < 0.05) (Fig. 7i) in Seipin+/− mice; levels were restored by the administration of rosiglitazone (Pdx-1, p < 0.01; Nkx6.1, Glut2 and proinsulin, p < 0.05) or oestradiol (p < 0.05 for all).
Oestrogen replacement relieves the reduction in PPARγ expression induced by seipin deficiency in ovariectomised female mice. (a) Levels of Pparγ mRNA in islets from unoperated and ovariectomised WT mice, unoperated and ovariectomised Seipin+/− mice and ovariectomised Seipin+/− mice treated with rosiglitazone or oestradiol. Data are expressed as fold of levels in unoperated WT mice. **p < 0.01 vs unoperated WT mice; ††p < 0.01 vs untreated ovariectomised Seipin+/− mice (two-way ANOVA, n = 8 per group). (b–e) Changes in AUC of GTT (b), AUC of first-phase GSIS (c), AUC of second-phase GSIS (d) and AUC of ITT (e) in mice treated as in (a). *p < 0.05 and **p < 0.01 vs unoperated WT mice; †p < 0.05 and ††p < 0.01 vs untreated ovariectomised Seipin+/− mice (two-way ANOVA, n = 6 per group). (f–i) Levels of Pdx-1 (f), Nkx6.1 (g), Glut2 (h) and proinsulin mRNA (i) in islets from mice treated as in (a). Data are expressed as fold of levels in unoperated WT mice. *p < 0.05 and **p < 0.01 vs unoperated WT mice; †p < 0.05 and ††p < 0.01 vs untreated ovariectomised Seipin+/− mice (two-way ANOVA, n = 8 per group). E2, oestradiol; OVX, ovariectomised mice; Rosi, rosiglitazone
Discussion
In the current study we used the model of adult male and female Seipin−/− mice and male and female Seipin+/− mice and provided, for the first time, the following in vivo and in vitro evidence: (1) seipin is specifically expressed in islet beta cells and (2) heterozygous deletion of Seipin in islet beta cells reduces insulin synthesis and secretion, thereby inducing glucose intolerance.
A critical finding in the present study is that seipin deficiency, through its suppression of PPARγ expression, decreases insulin secretion. This conclusion is deduced mainly from the following observations. First, male Seipin+/− and Seipin−/− mice displayed glucose intolerance with impaired GSIS, and decreased expression of PPARγ in the islets. Second, the decline in first-phase GSIS in male Seipin−/− and Seipin+/− mice was recovered by the administration of rosiglitazone. Third, administration of rosiglitazone for 14 days could relieve glucose intolerance in the male Seipin+/− mice. However, there were conflicting results showing that the treatment with rosiglitazone failed to improve the glucose intolerance and the high second-phase GSIS in male Seipin−/− mice; female Seipin−/− mice also appeared to be glucose intolerant but their expression of PPARγ in islets was not altered. Although this discrepancy is difficult to be reconciled in the present study, the contradictory phenotype may arise from insulin resistance in Seipin−/− mice, because the GTT monitors alteration in blood glucose concentration, so it actually assesses not only insulin secretion but also peripheral glucose disposal over time [22]. Insulin resistance has been reported in adipose tissue Seipin-knockout mice [23]. Lipodystrophy causes the deposition of triacylglycerol in the liver, leading to hepatic steatosis and insulin resistance. In addition, hepatic insulin signalling is impaired in Seipin−/− mice [24]. Low expression of the insulin receptor and insulin receptor substrate 1/2 in Seipin−/− mice might produce the hepatic insulin resistance [25].
Many studies demonstrate the effects of PPARγ on the insulin secretion of islet beta cells [26, 27]. In one, PPARγ was found to regulate the transcriptional activity of target genes by forming a heterodimer with retinoid X receptor and binding to a specific PPARγ response element (PPRE) sequence within the promoter region [28]. PPRE has been identified in the 5′ regulatory region of the Pdx-1 gene, and PPRE mutations dramatically reduce the promoter activity of the Pdx-1 gene [29]. The expression of PDX-1 in the adult pancreas is generally restricted to islet beta cells (~91%) [30], where it enhances the expression of proinsulin and GLUT2, a glucose transporter expressed in beta cells [31]. Moibi et al. [11] reported that deletion of PPARγ decreases the levels of Pdx-1 and Glut2 mRNA. Indeed, the transcript levels of Pdx-1 and Nkx6.1, and ratios of PDX-1 nuclear translocation were significantly reduced in the islets of male Seipin+/− and Seipin−/− mice, associated with a decline in the expression level of Glut2. In addition, seipin is predicted to span the ER membrane twice, with both N- and C-terminals in the cytoplasm and a large luminal loop [32]. Indeed, we observed that the seipin in islet beta cells was colocalised with the ER marker calnexin. Missense mutations (N88S and S90 L) of Seipin have been reported to activate the unfolded protein response and induce ER stress [33]. However, the expression levels of C/EBP homologous protein (CHOP) or GRP78 protein, the crucial ER stress markers, are unchanged in the brains of male Seipin−/− mice [12, 34] or in islets isolated from male Seipin−/− mice (data not shown).
Another important finding in the present study is that seipin deficiency, through reducing PPARγ in islet beta cells, impedes insulin synthesis and leads to glucose intolerance. This idea is supported by the decreased insulin synthesis and secretion seen in male Seipin+/− mice that was recovered by the administration of rosiglitazone. PDX-1, as a predominant binding factor, regulates insulin gene transcription in pancreatic beta cells [35] and augments the expression of proinsulin to enhance beta cell function and survival rate [36]. An earlier study reported that decreased PDX-1 expression level is related to beta cell mass reduction [31]. Inconsistently, male Seipin−/− mice exhibited hyperinsulinaemia with notable increases in insulin content and second-phase GSIS. Furthermore, the islet numbers and the beta cell mass were increased in male Seipin−/− mice, and this increase was accounted for by an increase in the proliferation of beta cells. Previous studies reported that the treatment of male Seipin−/− mice with rosiglitazone (0.3 mg/g diet) or thiazolidinediones for 9–10 weeks improved insulin resistance [6, 37]. We found that although administration of rosiglitazone (5 mg/kg daily) to male Seipin−/− mice for 14 days could correct the beta cell hyperplasia and the excessive synthesis of insulin, it did not reverse the insulin resistance. Mice with a beta cell-specific ablation of PPARγ show beta cell hyperplasia and increased beta cell mass [10]. Thus, it is highly likely that the deficits in the anti-proliferative property of PPARγ [38] in male Seipin−/− mice induce beta cell hyperplasia that in turn causes the hyperinsulinaemic status. The level of PPARγ mRNA in male Seipin−/− mice was reduced by approximately 70%, a greater reduction than that seen in male Seipin+/− mice (−30%). Thus, one simple explanation might be that a serious decline in PPARγ expression is critical for triggering the over-proliferation of islet beta cells. On the other hand, leptin deficiency is associated with hyperinsulinaemia in both mice and humans [39, 40]. The lack of adipose tissue, which leads to reduced serum leptin levels in Seipin−/− mice of both sexes, may enhance insulin synthesis. It was noted that male Seipin−/− mice did not show changes in the level of proinsulin mRNA, although the PDX-1 expression was reduced. One possibility is that the increase in insulin synthesis is a potential contributor to the beta cell compensatory response to insulin resistance [41]. Seipin deficiency may elevate the activity of glycogen synthase kinase-3β (GSK-3β) and the levels of IL-6 and TNF-α by reducing PPARγ expression [42]. Thus, further studies are required to explore the mechanisms underlying seipin deficiency-enhanced proliferation of islet beta cells.
Unlike the male Seipin+/− mice, female Seipin+/− mice did not show glucose intolerance or decreased insulin synthesis and secretion. Notably, oestrogen deprivation following ovariectomy caused glucose intolerance and deficits in insulin synthesis and secretion without insulin resistance in the female Seipin+/− mice but not in WT mice. Oestrogen deprivation has been reported to reduce PPARγ expression in aortic tissue [43]. We observed that ovariectomy induced a decline in PPARγ expression in the islets of Seipin+/− mice but not WT mice; this decline responded to oestrogen replacement therapy. Zhou et al. [12] reported that treatment of male Seipin−/− mice with oestradiol restored the expression of PPARγ. As expected, the treatment of ovariectomised female Seipin+/− mice with rosiglitazone restored their insulin synthesis and secretion, and these responses were accompanied by an improvement in glucose intolerance. When compared with intact female Seipin+/− mice, the levels of Pdx-1, Nkx6.1, Glut2 and proinsulin mRNA in islet beta cells of ovariectomised Seipin+/− mice were markedly reduced; the reduced levels were recovered either by the activation of PPARγ or by oestrogen replacement. In addition, the action of oestradiol in beta cells can enhance insulin secretion by reducing ATP-sensitive potassium channel activity [44]. Therefore, oestrogen, acting through recovered expression of PPARγ, exerts an important protective effect on insulin synthesis and secretion in female Seipin+/− mice.
The elevation of phosphatidic acid has been reported in seipin-knockdown yeast [7] and hippocampal neuronal cells of Seipin-knockout mice [45]. Thus, it has been proposed that absence of seipin may lead to the accumulation of phosphatidic acid [7], which may serve as a strong PPARγ antagonist [32] or cause the decline in PPARγ expression to downregulate the MAPK/ERK-CREB and Wnt3 signalling pathways [45, 46]. In addition, seipin physically interacts with sarco/endoplasmic reticulum Ca2+-ATPase (SERCA) to regulate intracellular calcium homeostasis [47]. Moreover, seipin is required for the maintenance of lipid homeostasis [6]. Therefore, further studies are needed to determine the molecular mechanisms underlying the impaired insulin synthesis and secretion in Seipin-knockout islet beta cells.
In conclusion, heterozygous deletion of Seipin in islet beta cells reduces PPARγ expression, thereby causing a decline in insulin synthesis and secretion leading to glucose intolerance. These effects can be relieved by oestrogen treatment, which restores PPARγ expression. These findings provide new perspectives for the therapy of glucose intolerance in individuals with BSCL2.
Data availability
All data generated or analysed during this study are included in this published article (and its ESM).
Abbreviations
- BSCL2:
-
Berardinelli–Seip congenital lipodystrophy type 2
- ER:
-
Endoplasmic reticulum
- GSIS:
-
Glucose-stimulated insulin secretion
- PDX-1:
-
Pancreatic duodenal homeobox 1
- PPARγ:
-
Peroxisome proliferator-activated receptor γ
- PPRE:
-
Peroxisome proliferator-activated receptor γ response element
- RT-qPCR:
-
Reverse-transcription quantitative PCR
- WT:
-
Wild-type
References
Agarwal AK, Simha V, Oral EA et al (2003) Phenotypic and genetic heterogeneity in congenital generalized lipodystrophy. J Clin Endocrinol Metab 88(10):4840–4847. https://doi.org/10.1210/jc.2003-030855
Agarwal AK, Arioglu E, De Almeida S et al (2002) AGPAT2 is mutated in congenital generalized lipodystrophy linked to chromosome 9q34. Nat Genet 31(1):21–23. https://doi.org/10.1038/ng880
Magre J, Delepine M, Khallouf E et al (2001) Identification of the gene altered in Berardinelli-Seip congenital lipodystrophy on chromosome 11q13. Nat Genet 28(4):365–370. https://doi.org/10.1038/ng585
Cui X, Wang Y, Tang Y et al (2011) Seipin ablation in mice results in severe generalized lipodystrophy. Hum Mol Genet 20(15):3022–3030. https://doi.org/10.1093/hmg/ddr205
Chen W, Chang B, Saha P et al (2012) Berardinelli-seip congenital lipodystrophy 2/seipin is a cell-autonomous regulator of lipolysis essential for adipocyte differentiation. Mol Cell Biol 32(6):1099–1111. https://doi.org/10.1128/MCB.06465-11
Liu L, Jiang Q, Wang X et al (2014) Adipose-specific knockout of Seipin/Bscl2 results in progressive lipodystrophy. Diabetes 63(7):2320–2331. https://doi.org/10.2337/db13-0729
Szymanski KM, Binns D, Bartz R et al (2007) The lipodystrophy protein seipin is found at endoplasmic reticulum lipid droplet junctions and is important for droplet morphology. Proc Natl Acad Sci U S A 104(52):20890–20895. https://doi.org/10.1073/pnas.0704154104
Auwerx J (1999) PPARgamma, the ultimate thrifty gene. Diabetologia 42(9):1033–1049. https://doi.org/10.1007/s001250051268
Chen W, Yechoor VK, Chang BH, Li MV, March KL, Chan L (2009) The human lipodystrophy gene product Berardinelli-Seip congenital lipodystrophy 2/seipin plays a key role in adipocyte differentiation. Endocrinology 150(10):4552–4561. https://doi.org/10.1210/en.2009-0236
Rosen ED, Kulkarni RN, Sarraf P et al (2003) Targeted elimination of peroxisome proliferator-activated receptor γ in beta β leads to abnormalities in islet mass without compromising glucose homeostasis. Mol Cell Biol 23(20):7222–7229. https://doi.org/10.1128/mcb.23.20.7222-7229.2003
Moibi JA, Gupta D, Jetton TL, Peshavaria M, Desai R, Leahy JL (2007) Peroxisome proliferator-activated receptor-γ regulates expression of PDX-1 and NKX6.1 in INS-1 cells. Diabetes 56(1):88–95. https://doi.org/10.2337/db06-0948
Zhou L, Yin J, Wang C, Liao J, Liu G, Chen L (2014) Lack of seipin in neurons results in anxiety- and depression-like behaviors via down regulation of PPARγ. Hum Mol Genet 23(15):4094–4102. https://doi.org/10.1093/hmg/ddu126
Ebihara C, Ebihara K, Aizawa-Abe M et al (2015) Seipin is necessary for normal brain development and spermatogenesis in addition to adipogenesis. Hum Mol Genet 24(15):4238–4249. https://doi.org/10.1093/hmg/ddv156
Windpassinger C, Auer-Grumbach M, Irobi J et al (2004) Heterozygous missense mutations in BSCL2 are associated with distal hereditary motor neuropathy and silver syndrome. Nat Genet 36(3):271–276. https://doi.org/10.1038/ng1313
Anthony J, Kelkar A, Wilankar C et al (2013) Discovery of p1736, a novel antidiabetic compound that improves peripheral insulin sensitivity in mice models. PLoS One 8(10):e77946. https://doi.org/10.1371/journal.pone.0077946
Bai Y, Chang F, Zhou R et al (2011) Increase of anteroventral periventricular kisspeptin neurons and generation of E2-induced LH-surge system in male rats exposed perinatally to environmental dose of bisphenol-a. Endocrinology 152(4):1562–1571. https://doi.org/10.1210/en.2010-1042
Chen HY, Lin YN, Chen WC, Wang SJ, Chen CJ, Chen YH (2017) Urethral proteomic analysis in ovariectomized mice administered 17β-oestradiol replacement therapy. J Obstet Gynaecol 37(6):757–765. https://doi.org/10.1080/01443615.2017.1292225
Hua X, Cao XY, Wang XL, Sun P, Chen L (2017) Exposure of pregnant mice to triclosan causes insulin resistance via thyroxine reduction. Toxicol Sci 160(1):150–160. https://doi.org/10.1093/toxsci/kfx166
Sun P, Wang T, Zhou Y et al (2013) DC260126: a small-molecule antagonist of GPR40 that protects against pancreatic beta-cells dysfunction in db/db mice. PLoS One 8(6):e66744. https://doi.org/10.1371/journal.pone.0066744
Wang T, Sun P, Chen L et al (2014) Cinnamtannin D-1 protects pancreatic beta-cells from palmitic acid-induced apoptosis by attenuating oxidative stress. J Agric Food Chem 62(22):5038–5045. https://doi.org/10.1021/jf500387d
Sun P, Zhu JJ, Wang T et al (2018) Benzbromarone aggravates hepatic steatosis in obese individuals. Biochim Biophys Acta Mol basis Dis 1864(6 Pt A):2067–2077. https://doi.org/10.1016/j.bbadis.2018.03.009
Nagy C, Einwallner E (2018) Study of in vivo glucose metabolism in high-fat diet-fed mice using oral glucose tolerance test (OGTT) and insulin tolerance test (ITT). J Vis Exp (131). https://doi.org/10.3791/56672
Zhou H, Lei X, Benson T et al (2015) Berardinelli-Seip congenital lipodystrophy 2 regulates adipocyte lipolysis, browning, and energy balance in adult animals. J Lipid Res 56(10):1912–1925. https://doi.org/10.1194/jlr.M060244
Chen W, Zhou H, Saha P, Li L, Chan L (2014) Molecular mechanisms underlying fasting modulated liver insulin sensitivity and metabolism in male lipodystrophic Bscl2/Seipin-deficient mice. Endocrinology 155(11):4215–4225. https://doi.org/10.1210/en.2014-1292
Xu P, Wang H, Kayoumu A, Wang M, Huang W, Liu G (2015) Diet rich in docosahexaenoic acid/eicosapentaenoic acid robustly ameliorates hepatic steatosis and insulin resistance in seipin deficient lipodystrophy mice. Nutr Metab (Lond) 12:58. https://doi.org/10.1186/s12986-015-0054-x
Welters HJ, McBain SC, Tadayyon M, Scarpello JH, Smith SA, Morgan NG (2004) Expression and functional activity of PPARγ in pancreatic β cells. Br J Pharmacol 142(7):1162–1170. https://doi.org/10.1038/sj.bjp.0705844
Yang C, Chang TJ, Chang JC et al (2001) Rosiglitazone (BRL 49653) enhances insulin secretory response via phosphatidylinositol 3-kinase pathway. Diabetes 50(11):2598–2602. https://doi.org/10.2337/diabetes.50.11.2598
Gearing KL, Gottlicher M, Teboul M, Widmark E, Gustafsson JA (1993) Interaction of the peroxisome-proliferator-activated receptor and retinoid X receptor. Proc Natl Acad Sci U S A 90(4):1440–1444. https://doi.org/10.1073/pnas.90.4.1440
Gupta D, Jetton TL, Mortensen RM, Duan SZ, Peshavaria M, Leahy JL (2008) In vivo and in vitro studies of a functional peroxisome proliferator-activated receptor γ response element in the mouse pdx-1 promoter. J Biol Chem 283(47):32462–32470. https://doi.org/10.1074/jbc.M801813200
Fu Z, Gilbert ER, Liu D (2013) Regulation of insulin synthesis and secretion and pancreatic β-cell dysfunction in diabetes. Curr Diabetes Rev 9(1):25–53. https://doi.org/10.2174/157339913804143225
Shojima N, Hara K, Fujita H et al (2012) Depletion of homeodomain-interacting protein kinase 3 impairs insulin secretion and glucose tolerance in mice. Diabetologia 55(12):3318–3330. https://doi.org/10.1007/s00125-012-2711-1
Fei W, Du X, Yang H (2011) Seipin, adipogenesis and lipid droplets. Trends Endocrinol Metab 22(6):204–210. https://doi.org/10.1016/j.tem.2011.02.004
Ito D, Suzuki N (2009) Seipinopathy: a novel endoplasmic reticulum stress-associated disease. Brain 132(Pt 1):8–15. https://doi.org/10.1093/brain/awn216
Wang L, Hong J, Wu Y, Liu G, Yu W, Chen L (2018) Seipin deficiency in mice causes loss of dopaminergic neurons via aggregation and phosphorylation of α-synuclein and neuroinflammation. Cell Death Dis 9(5):440. https://doi.org/10.1038/s41419-018-0471-7
Chen F, Sha M, Wang Y et al (2016) Transcription factor Ets-1 links glucotoxicity to pancreatic beta cell dysfunction through inhibiting PDX-1 expression in rodent models. Diabetologia 59(2):316–324. https://doi.org/10.1007/s00125-015-3805-3
Kaneto H, Miyatsuka T, Kawamori D et al (2008) PDX-1 and MafA play a crucial role in pancreatic β-cell differentiation and maintenance of mature β-cell function. Endocr J 55(2):235–252. https://doi.org/10.1507/endocrj.k07e-041
Prieur X, Dollet L, Takahashi M et al (2013) Thiazolidinediones partially reverse the metabolic disturbances observed in Bscl2/seipin-deficient mice. Diabetologia 56(8):1813–1825. https://doi.org/10.1007/s00125-013-2926-9
Frohlich E, Machicao F, Wahl R (2005) Action of thiazolidinediones on differentiation, proliferation and apoptosis of normal and transformed thyrocytes in culture. Endocr Relat Cancer 12(2):291–303. https://doi.org/10.1677/erc.1.00973
Chen NG, Swick AG, Romsos DR (1997) Leptin constrains acetylcholine-induced insulin secretion from pancreatic islets of Ob/Ob mice. J Clin Invest 100(5):1174–1179. https://doi.org/10.1172/JCI119629
Boden G, Chen X, Kolaczynski JW, Polansky M (1997) Effects of prolonged hyperinsulinemia on serum leptin in normal human subjects. J Clin Invest 100(5):1107–1113. https://doi.org/10.1172/JCI119621
Mezza T, Muscogiuri G, Sorice GP et al (2014) Insulin resistance alters islet morphology in nondiabetic humans. Diabetes 63(3):994–1007. https://doi.org/10.2337/db13-1013
Qian Y, Yin J, Hong J et al (2016) Neuronal seipin knockout facilitates Aβ-induced neuroinflammation and neurotoxicity via reduction of PPARγ in hippocampus of mouse. J Neuroinflammation 13(1):145. https://doi.org/10.1186/s12974-016-0598-3
Tiyerili V, Muller CF, Fung S, Panek D, Nickenig G, Becher UM (2012) Estrogen improves vascular function via peroxisome-proliferator-activated-receptor-γ. J Mol Cell Cardiol 53(2):268–276. https://doi.org/10.1016/j.yjmcc.2012.05.008
Nadal A, Rovira JM, Laribi O et al (1998) Rapid insulinotropic effect of 17β-estradiol via a plasma membrane receptor. FASEB J 12(13):1341–1348. https://doi.org/10.1096/fasebj.12.13.1341
Li G, Zhou L, Zhu Y et al (2015) Seipin knockout in mice impairs stem cell proliferation and progenitor cell differentiation in the adult hippocampal dentate gyrus via reduced levels of PPARγ. Dis Model Mech 8(12):1615–1624. https://doi.org/10.1242/dmm.021550
Zhou L, Chen T, Li G et al (2016) Activation of PPARγ ameliorates spatial cognitive deficits through restoring expression of AMPA receptors in seipin knock-out mice. J Neurosci 36(4):1242–1253. https://doi.org/10.1523/JNEUROSCI.3280-15.2016
Bi J, Wang W, Liu Z et al (2014) Seipin promotes adipose tissue fat storage through the ER Ca2+-ATPase SERCA. Cell Metab 19(5):861–871. https://doi.org/10.1016/j.cmet.2014.03.028
Funding
This work was supported by the National Natural Science Foundation of China (81471157; 81671253; 81603169), National 973 Basic Research Program of China (2014CB943303), Jiangsu provincial Natural Science Foundation of China (BE2016765; BK20161027) and Natural Science Research Program for Higher Education in Jiangsu Province (16KJB310007).
Author information
Authors and Affiliations
Contributions
JX, PS, YaW, XH, WS and YanW contributed to acquisition, analysis and interpretation of data. PS and LC wrote the draft of the manuscript. JW, GL, WY and LC contributed to the conception and design of this work. All authors revised the manuscript critically for important intellectual content and approved the final version to be published. LC is the guarantor of this work.
Corresponding authors
Ethics declarations
The authors declare that there is no duality of interest associated with this manuscript.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM
(PDF 312 kb)
Rights and permissions
About this article
Cite this article
Xiong, J., Sun, P., Wang, Y. et al. Heterozygous deletion of Seipin in islet beta cells of male mice has an impact on insulin synthesis and secretion through reduced PPARγ expression. Diabetologia 63, 338–350 (2020). https://doi.org/10.1007/s00125-019-05038-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00125-019-05038-x