Abstract
Contrasting carbon: nitrogen: phosphorus (C: N: P) stoichiometry between phytoplankton and zooplankton affect consumer growth and phytoplankton nutrient limitation via nutrient recycling by zooplankton. However, no study has assessed how regional differences in terrestrial loadings of organic matter affect plankton N: P stoichiometry and recycling in systems with low N deposition and N-limited phytoplankton. We address this question by using data from 14 unproductive headwater arctic and boreal lakes. We found that boreal lakes had higher lake water- and seston C, N and P concentrations than arctic lakes, whereas seston C: N, C: P and N: P ratios did not differ among regions. Boreal zooplankton were also richer in N and P relative to C, with lower somatic N: P ratios, compared to arctic lakes. Consequently, the estimated N: P imbalances between seston and zooplankton were negative in arctic lakes, indicating zooplankton feeding on phytoplankton of suboptimal N content, resulting in low consumer driven N: P recycling (medians arctic sub-mid and high altitude lakes: 11 and 13). In boreal lakes, estimated N: P imbalance did not differ from zero, with a seston N: P stoichiometry matching the N:P requirements of zooplankton, which resulted in higher consumer driven N: P recycling (median 18). Our results imply that regional climate induced catchment differences, through enhanced terrestrial nutrient inputs, affect plankton stoichiometry by raising consumer N: P recycling ratio and changing zooplankton from being mainly N- (arctic) to NP co-limited (boreal). Browning of lakes, in regions with low N deposition, may therefore promote large-scale regional changes in plankton nutrient limitation with potential feedbacks on pelagic food webs.
Similar content being viewed by others
Introduction
Lakes at northern latitudes are generally nutrient poor, with low productivity, short growing seasons, and can vary in color from clear to humic due to different loadings of terrestrial dissolved organic matter (DOM) (Karlsson et al. 2005; Lewis 2011; Solomon et al. 2015). In northern lakes with low atmospheric nitrogen (N) deposition phytoplankton are primarily N-limited (Bergström et al. 2008, 2013) with low somatic N: phosphorus (P) ratios (i.e. as measured in seston) (Bergström et al. 2015; Deininger et al. 2017). Earlier studies have shown that high inorganic N loadings (mainly nitrate; cf. Hessen 2013) from atmospheric deposition in southern lakes have raised lake water N: P balances, promoting large scale ecosystem shifts from N- to P-limitation of algae and herbivores (Bergström and Jansson 2006; Elser et al. 2009, 2010; Hessen 2013). This change in lake water N: P stoichiometry has increased biomass (Bergström and Jansson 2006) and somatic N: P ratios in phytoplankton (i.e. in seston) (Elser et al. 2010) in many originally N-limited northern hemisphere lakes (Bergström and Jansson 2006). Despite this knowledge we still know little about how differences in catchments properties and terrestrial DOM loadings influence lake water, and in turn, plankton N: P stoichiometry in northern lakes with low N deposition and N-limited phytoplankton. This is indeed timely to assess considering that many northern lakes are impacted by enhanced terrestrial DOM loadings (also refer to as lake browning), attributed to recovery from sulphur deposition (Monteith et al. 2007), as well as to direct and indirect effects of climate warming (Weyhenmeyer and Karlsson 2009; Finstad et al. 2016; de Wit et al. 2016). Hence, earlier comparisons made on plankton stoichiometry among different climate regions (Elser and Hassett 1994; Hassett et al. 1997; Dobberfuhl and Elser 2000) are clearly constrained by assessing lake regions where the N:P stoichiometry has been raised, and where phytoplankton nutrient limitation has been shifted from N- to P-limitation by high N deposition (cf. Elser et al. 2010; Bergström et al. 2015).
Nutrient inputs and N: P stoichiometry affect important ecosystem characteristics such as planktonic nutrient limitation, energy and nutrient turnover, and lake productivity. Here, terrestrial DOM inputs influence light availability for phytoplankton through light absorption, but also affect nutrient availability, as it serves as an important carrier of N and P to lakes (Jones 1992; Karlsson et al. 2005). Since phytoplankton are plastic with high variability in their carbon (C) to N: P ratio depending on light and nutrient availability (Urabe and Sterner 1996; Sterner et al. 1997) differences in lake DOM loadings have the potential to affect not only phytoplankton production through changes in light: nutrient availability (Seekell et al. 2015) but also their somatic C: N: P stoichiometry (Sterner et al. 1997). Crustacean zooplankton, on the other hand, are considered more homeostatic with low variation in their intraspecific C to N: P stoichiometry (Andersen and Hessen 1991; Sterner et al. 1992), with high somatic N: P ratios in copepods and low somatic N: P ratios in cladocerans, especially in Daphnids (Sterner and Elser 2002).
As a result of their different abilities of changing their C: N: P stoichiometry, situations may therefore occur in lakes when there is a mismatch between the dietary N: P stoichiometry (here seston) and the N: P requirements of the consumers (here crustacean zooplankton) (Urabe and Sterner 1996; Sterner et al. 1997). If the dietary N: P stoichiometry is not optimal, this might impair reproduction and buildup of zooplankton biomass (Sterner and Hessen 1994) and have implications for nutrient and energy flows in the pelagic food web (Andersen 1997). When assessing these mismatches in ecological stoichiometry models (Sterner 1990; Hassett et al. 1997), the seston pool (i.e. particles < 50 µm representing edible food for zooplankton; cf. Burns 1968) is often assumed to serve as a proxy for phytoplankton (Elser and Hassett 1994) although proportions of the particles retained on filters may be of non-algal origin (Hessen et al. 2003). If there is a mismatch, ecological stoichiometry theory then predict that any difference in N: P ratios between phytoplankton and the dissolved pool, and between the resources (seston) and the consumers (crustacean zooplankton), result in a nutrient imbalance (the latter expressed as: N: Pseston–N: Pconsumer) (cf. Hassett et al. 1997; Sterner and Elser 2002). A positive nutrient imbalance (N: Pseston > N: Pconsumer) here indicates that zooplankton are feeding on resources with suboptimal P content relative to their N: P demand (i.e. P-limited conditions), whereas a negative imbalance (N:Pseston < N:Pconsumer) indicates that zooplankton are feeding on resources with suboptimal N content relative to their N:P demand (i.e. N-limited conditions).
It then follows that provided that the C:nutrient ratio is sufficiently high in the food diet relative to zooplankton, an N: P imbalance between the food diet and zooplankton, implies that zooplankton growth becomes nutrient (N or P) limited, actively retaining the limiting element while excreting or egesting the element available in excess back into the water column (i.e. the so called consumer driven N: P regeneration). So the N: P imbalance between the diet and the consumer do not only indicate the nutrient limitation condition in zooplankton, but subsequently also determines the consumer driven N: P regeneration (Sterner and Hessen 1994; Sterner and Elser 2002). The consumer driven N: P regeneration (i.e. the N: P ratio of the nutrients recycled by zooplankton) can in turn have important feedback effects on lake ecosystem functioning such as on phytoplankton nutrient limitation (Elser et al. 1988), trophic transfer efficiencies and pelagic food web performance (Hessen and Anderson 2008; Hessen et al. 2013). However, to what extent the N: P stoichiometry of water and phytoplankton is affected by differences in terrestrial DOM loadings, and how this in turn affects nutrient imbalances between the diet (seston) and zooplankton, and the consumer driven N: P regeneration ratios in lake ecosystems with low N deposition and N-limited phytoplankton, is still much of an open question.
Here we present a study on the elemental stoichiometry of water, seston and crustacean zooplankton in arctic and boreal headwater lakes in northern Sweden with low N deposition, where phytoplankton are primarily N- and NP co-limited (Bergström et al. 2008, 2013). Our aims were to assess regional differences in lake water chemistry and seston stoichiometry (boreal and arctic), N: P imbalances between diet (seston) and consumers (herbivorous zooplankton), and consumer driven N: P regeneration ratios. We hypothesize that: (1) boreal lakes have higher lake water and seston C, N and P content than arctic lakes (due to higher terrestrial DOM and nutrient loadings), (2) the N: P imbalances between seston and zooplankton are negative in both arctic and boreal lakes [due to low lake water DIN: TP ratios (cf. Bergström 2010) promoting N-limited phytoplankton that possess low N: P ratios], which results in low consumer-driven N: P regeneration ratios in both arctic and boreal lakes.
Materials and methods
Study area
The 14 study systems represent nutrient poor headwater lakes, with a gradient from clear-water to humic, in northern arctic and boreal Sweden. The arctic lakes (earlier published in Bergström et al. 2015) are situated along an altitudinal and climate gradient from sub- [450–650 m altitude above sea level; (m a.s.l.)], to low-mid (600–990 m a.s.l.), to high altitude vegetation zones (> 990 m a.s.l.) (Table 1). The arctic lakes were divided in two groups: arctic sub-mid- (ASM; 2 + 3 lakes) and arctic high altitude lakes (AH; four lakes) (cf. Table 1). The boreal lakes (B; five lakes) are situated at altitudes between 220 and 359 m a.s.l. (Table 1). Mean annual air temperature at the arctic- and boreal site is − 1 to + 1 °C and + 2 to + 4 °C, respectively (Swedish Meteorological and Hydrological Institute, http://www.smhi.se). Mean air temperatures over the sampling period for the arctic lakes in 2011 were 7.2 ± 0.2 °C and 9.3 ± 0.1 °C (AH and ASM) and for the boreal lakes in 2012 10.8 ± 0.2 °C. Air temperatures in the arctic were gathered from the Abisko Scientific Research Station (http://www.polar.se/ans), and estimated for each lake sites using a lapse rate of 0.57 °C per 100 m elevation (Laaksonen 1976). For boreal lakes, air temperatures were gathered from climate stations located close to the lakes using HOBO U30 USB Weather Station data loggers and HOBO S-THB-M0002 temperature sensors (http://www.onsetcomp.com/products). The length of the ice-free season varies from approximately 3 months in arctic high, 4 months in arctic sub-mid, to 5 months in the boreal lakes. Lake area and maximum lake depth range between 1 and 27 ha, and 5 and 24 m, respectively. The phytoplankton communities are dominated by chrysophytes and cryptophytes, with the exception of lake HA11 and Nästjärn where chlorophytes proportions are somewhat higher (Jansson et al. 2010; Deininger et al. 2017). There are fish in seven of the arctic lakes [dominated by Arctic char, Salvelinus alpinus (ASM3-8 and AH9)] with one lake having populations of Ninespine sticklebacks (SMA5). Four of the boreal lakes have fish (dominated by Eurasian perch, Perca fluviatilis). Three arctic high altitude lakes (AH10-12) and one boreal lake (Nästjärn) are fishless. The lakes are all located on weathering resistant bedrock (mostly granite, gneiss and amphibolites) (Jansson et al. 2001; Karlsson et al. 2005). The boreal lake region is dominated by coniferous forest and mires (Jansson et al. 2001). The arctic sub alpine region is dominated by mountain birch, the low-mid alpine region is dominated by heath and scrub, and the high alpine region is composed of sparsely vegetated grass heath, boulder fields and bedrock outcrops (Jansson et al. 2010). For the arctic lakes, precipitation (mean annual precipitation 500 mm) (Karlsson et al. 2005) and N deposition varies little with altitude (wet DIN deposition < 0.3 kg N ha− 1 year− 1) (Bergström et al. 2013). For the boreal lakes mean annual precipitation ranges between 700 and 800 mm, wet DIN deposition between 1 and 2 kg N ha− 1 year− 1 (Bergström et al. 2008).
Sampling and analyses
Sampling of arctic (cf. Bergström et al. 2015) and boreal lakes occurred over the growing season in 2011 and 2012, respectively. Lake sampling covered the whole ice-free season (depending on altitude and site, ice out and beginning of ice cover takes place in late May/early July and late September/late October, respectively). We used data from three sampling occasions over the growing season and present mean or median seasonal values for each lake, and each lake region. On each sampling occassion, vertical profiles of temperature and light were measured. The vertical light extinction coefficient (kd, m− 1), which is used as a proxy for light availability, was calculated as the slope of the linear regression of the natural logarithm of PAR versus depth. Mean irradiance for the mixed water layer (Im, dimensionless) was calculated from PAR profiles following the procedure in Karlsson et al. (2009).
Composite water samples for analysis of lake water chemistry and chlorophyll a (Chl a) were collected on each occasion within the mixed layer with a Ruttner sampler. These were analysed for dissolved organic carbon (DOC), total nitrogen (TN), ammonium (NH4+-N), nitrite plus nitrate (NO2− + NO3− – N), total phosphorus (TP), and Chl a following the procedure described in Bergström et al. (2008, 2013). Dissolved inorganic nitrogen (DIN) was calculated as the sum of NH4+–N and NO2−+NO3−–N. Composite water samples were also taken from the mixed layer, prescreened through a 50 µm mesh size nylon net, and the filtrate was used for seston particulate C, N, and P analyses (see below). Samples for zooplankton biomass and composition analyses were obtained by vertical hauls with a plankton net (mesh size 100 µm) from 1 m from the bottom to the surface at the deepest point in each lake. Samples were preserved with Lugol´s solution, brought back to the lab and stored dark and cold until counting. Zooplankton samples for stoichiometry analyses (C: N: P) followed the same procedure as for biomass, with the exception that several hauls were used to make sure that sufficient plankton were collected. Samples from the hauls were transferred to plastic 500 ml bottles in the field and brought to the lab. In the lab, zooplankton were then picked and sorted for C, N and P analyses.
For analysis of seston particulate C, N, and P, prescreened lake water was passed through a pre-combusted (450 °C, 4 h) and acid-washed (1.2 M HCl) 25 mm GF/F glass fibre filter (Whatman Arbor technologies, Ann Arbor, MI, USA), until the filter was clogged. One sample for C and N, and one for P analysis was taken on each sampling occasion. The filters were stored frozen until analyses. C and N were analysed using a Costech ECS 4010 elemental analyser (Costech International S. P. A.). Analyses for P were made according to Swedish standard (ISO 15681-1, rev 4) using the molybdate blue method after hydrolysis with persulphate using a FIAstar 5000 (FOSS Inc.). Relative standard deviation (RSD) for seston C/N and P analyses was < 2% and 3.2%, respectively.
Crustacean zooplankton taxa were identified and counted using inverted microscopy, and the dry weight (DW) was estimated from length measurments and length-dry weight regressions (Bottrell et al. 1976). Zooplankton were grouped into cladocerans, calanoid copepods and cyclopoid copepods. In all lakes, calanoid copepods included nauplii, calanoid copepodites and adults, whereas cyclopid copepods (comprising nauplii, cyclopoid copepodites and adults) only were abundant in boreal lakes. For C, N and P determination, zooplankton were pooled into groups of cladocerans, calanoid and cyclopoid copepods, respectively. For analyses of C and N, primarily large specimens from each group were picked and placed onto pre-weighed combusted (450 °C, 4 h) GF/C filter (Whatman Arbor technologies, Ann Arbor, MI, USA). The same procedure was repeated for P, but using burned, pre-weighted and acid-washed (1.2 M HCl) GF/C filters. Filters were then dried (60 °C) over night, and then weighed (Mettler Toledo MT5; resolution ± 1 µg) to determine the dry weigh. For the arctic lakes, C and N contents were analysed on a Costech ECS 4010 elemental analyser (Costech International S. P. A.), whereas for the boreal lakes these were measured on a continuous-flow isotope ratio mass spectrometer (PDZ Europa 20–20). Analyses for P (all lakes) was made according to Swedish standard (ISO 15681-1, rev 4) using a FIAstar 5000 (FOSS Inc.), as described above. Relative standard deviation (RSD) for zooplankton C/N and P analyses was < 2% and 1.1%, respectively. The zooplankton C, N and P content are all reported as percent per unit dry weight (%DW).
Predicted elemental imbalances between resources (seston) and consumers (zooplankton) and the stoichiometry of the nutrients recycled by the zooplankton (all estimated in molar) were based on stoichiometric models according to Sterner (1990) and Hassett et al. (1997), where:
A positive nutrient imbalance (N: Pseston > N: Pconsumer) indicates that zooplankton are feeding on resources with suboptimal P content relative to their nutrient demand, whereas a negative imbalance (N: Pseston < N: Pconsumer) indicates that zooplankton are feeding on resources with suboptimal N content relative to their nutrient demand. The N: P stoichiometry of the nutrients recycled by the zooplankton were then estimated as:
where AE is the maximum accumulation efficiency of the limiting element. For P the AE has been reported to range from 0.5 to 0.9 (most common AEs in the upper range of 0.7 to 0.9) (DeMott 1998; DeMott et al. 1998), whereas values for N are less known. We assume a similar AE (0.75) for both elements (cf. Sterner 1990), but also calculated the N: P recycled using AE equal to 0.5 and 0.9 (cf. Bergström et al. 2015). The N: P elemental imbalances and recycling ratios were calculated for calanoid and cyclopoid copepods and cladocerans separately (in molar), as well as for all zooplankton combined (in molar), the latter by using weighted means based on their relative proportion of the total zooplankton biomass. The nutrient N: P imbalances and regenerations estimates for the arctic lakes have earlier been published in Bergström et al. (2015), but are in this study presented differently as seasonal means for two separate lake categories [i.e. arctic sub-mid- (ASM) and arctic high altitude (AH) lakes]. Importantly, in this study we have then evaluated N: P elemental imbalances, as well as the consumer-driven N: P recycling of zooplankton, and how these were affected by seston and zooplankton stoichiometry in the boreal and arctic sub-mid and high altitude lakes.
Statistical analyses
Relationships and differences in nutrient concentrations, environmental factors, stoichiometry of lake water, seston and zooplankton among regions and lake groups were analyzed using Pearson’s product-moment correlation, and one-way ANOVAs followed by Holm-Sidak method test (normally distributed data) or Kruskal Wallis One Way analyses of Variance on Ranks followed by Dunn’s method test (non-normally distributed data) (SigmaPlot 13.0). Differences in nutrient imbalances and consumer-driven N: P regeneration among lake regions were also analyzed using one-way ANOVAs followed by Holm-Sidak method test (normally distributed data) or with Kruskal Wallis One Way analyses of Variance on Ranks followed by Dunn’s method test (non-normally distributed data) (SigmaPlot 13.0). All statistical test were performed at a significance level of α = 0.05.
Results
Epilimnion temperatures during the ice-free season (June–September) declined with increasing altitude (Supplementary Table S1), with significantly higher temperatures in boreal (15.1 ± 0.6 °C) compared to the arctic lakes (means for AH and ASM: 9.0 ± 0.9 and 10.7 ± 0.8 °C) (one-way ANOVA followed by Holm-Sidak test; df = 2, F = 79.6, P < 0.001). The DOC concentrations in the epilimnion were also higher in boreal (median 14.5 mg L− 1) than in arctic (medians for AH and ASM 1.7 and 3.6 mg L− 1) (Kruskal–Wallis One-way ANOVA followed by Dunn’s test; df = 2, H = 11.6, P < 0.001) (Supplementary Table S1). The kd increased with increasing DOC concentrations (R = 0.93, P < 0.001) and Im ranged between 0.17 and 0.74 (Supplementary Table S2). Epilimnetic TN and TP concentrations were related to DOC concentrations (Supplementary Fig. 1a), with significantly higher concentrations in boreal (means for TN 392 ± 33 µg L− 1; TP 14.1 ± 3.4 µg L− 1) than in arctic lakes (means for AH and ASM TN 100 ± 18 µg L− 1, TP 3.1 ± 1.1 µg L− 1 and TN 161 ± 26 µg L− 1, TP 5.1 ± 2.4 µg L− 1) (One-way ANOVAs followed by Holm-Sidak tests; df = 2, F = 151.5 and 24.7, P < 0.001). The epilimnetic TN: TP ratios (weight) (boreal 29 ± 7; ASM 39.5 ± 23; AH 37 ± 7), did not differ among lake groups (one-way ANOVA; P = 0.579). The DIN concentrations (Fig. 1b; Supplementary Table S1) in the epilimnion were higher in boreal and arctic high (medians 18 and 22 µg L− 1) than in arctic sub-mid lakes (4 µg L− 1) (Kruskal–Wallis One-way ANOVA followed by Dunn’s test; df = 2, H = 8.2, P < 0.001). Only in the arctic high low DOC lakes with poorly developed catchment vegetation cover (cf. Table 1), DIN contributed to more than 10% of the TN concentrations. In all other lakes, DIN proportions were < 6% of TN (Supplementary Fig. 1c). The epilimnetic DIN: TP ratios (molar), followed the same pattern; i.e. were high in arctic high low DOC lakes (range 20–50), and < 7 in all other lakes (Supplementary Fig. 1c). Both DIN: TN and DIN: TP were significantly higher in arctic high (medians: DIN: TN 20.5; DIN: TP 16.9) compared to arctic sub-mid and boreal lakes (medians: DIN: TN 2.5; DIN: TP 2.4 and DIN: TN 4.5; DIN: TP 2.2, respectively) (Kruskal–Wallis one-way ANOVAs followed by Dunn’s tests; df = 2, H = 6.5 and 8.5, P < 0.05). The mean Chl a concentrations ranged between 0.4 and 2.3 µg L− 1 (Supplementary data Table S1), and did not differ among lake regions (One-way ANOVA; P = 0.655).
a Carbon (C), b nitrogen (N), c phosphorus (P) concentrations and d C: P, e C: N, and f N: P ratios in seston in five boreal (B), five arctic sub-mid altitude (ASM) and four arctic high altitude (AH) lakes. The thin and the thick line within the box show median and mean, respectively, the box 25 and 75% percentiles and the whisker 10 and 90% percentiles. Different letters show significant differences. The data for the arctic lakes are from Bergström et al. 2015
The seston C, N and P concentrations were related to DOC concentrations (for seston C, N and P: R = 0.67, 076 and 0.83, respectively; P < 0.05 for all) (Supplementary Fig. S2). Seston N was higher in boreal compared to arctic lakes (Fig. 1b) (Supplementary Table S2 and S3). For seston C and P, concentrations were higher in boreal compared to arctic high, but did not differ between boreal and arctic sub-mid, or between arctic sub-mid and high altitude lakes (Fig. 1a, c) (Supplementary data Table S2 and S3). Seston C: P, C: N and N: P ratios did not differ among the lake groups (Fig. 1d–f) (one way ANOVAs; P = 0.238 or higher), and were not related to mean PAR (Im) in the mixed layer (for all R = 0.41 or lower, P = 0.148 or higher). Median seston N: P ratios in each lake region were: boreal 25; arctic sub-mid 26; and arctic high 30.
The crustacean zooplankton biomass (Table 2) was higher in arctic sub-mid (mean 47.5 ± 2.7 µg L− 1) compared to arctic high altitude lakes (mean 23.9 ± 12 µg L− 1), but did not differ among the other lake groups (mean boreal: 36.7 ± 12.3 µg L− 1) (one-way ANOVA followed by Holm-Sidak test; df = 2, F = 4.0, P < 0.05). Zooplankton biomass was not related to seston C, N or P concentrations (for all: R = 0.4 or lower, P = 0.15 or higher). Calanoid copepods dominated the crustacean zooplankton biomass in all arctic lakes except in two of the high altitude lakes (AH12 and AH10) (Table 2). In the arctic lakes the cladocerans consisted mainly of Bosmina spp., Daphnia spp. and Holopedium gibberum. In one boreal lake (Nästjärn), the crustacean zooplankton biomass was dominated by copepods (calanoids and cyclopoids), whereas in the other boreal lakes proportions of cladocerans were much higher (range 40–58%) (Table 2). In those lakes the cladocerans consisted mainly of small sized Bosmina spp., Ceriodaphnia spp., Diaphanosoma brachyurum and Daphnia spp. with the exception of Övre Björntjärn where Holopedium gibberum was abundant in June.
The C content (Fig. 2a, Supplementary Table S2) of different zooplankton taxa did not differ from each other (range of medians 49.8–53.4%), neither did the weighted C content in all zooplankton (medians boreal 51.8%; ASM 51%; AH 52.4%) (Fig. 2d; Supplementary Table S2). The N content was higher in boreal calanoids (median 11.7%) than in all other taxa in the arctic lakes (medians ranged between 8 and 9.3%), whereas cyclopoids (medians 10.6%) and cladocerans (medians 9.9%) in boreal lakes did not differ from any of the taxa in boreal or arctic lakes (Fig. 2b; Supplementary Table S2). The weighted N content in all zooplankton was significantly higher in boreal (mean 10.6%) than in arctic sub-mid (mean 8.4%) and high altitude (mean 8.4%) lakes (Fig. 2e; Supplementary Table S2). The P content was higher in cyclopoids (mean 1.2%) than in all other taxa except for cladocerans in the boreal lakes (mean 0.9%). Lowest P content was found in calanoids (mean 0.3%) in arctic high altitude lakes. The P content in calanoids in boreal lakes (mean 0.6%) did not differ from cladocerans in arctic sub-mid (mean 0.6%) and high altitude lakes (mean 0.6%), or calanoids in the arctic sub-mid altitude lakes (mean 0.4%) (Fig. 2c; Supplementary Table S2). The weighted P content in all zooplankton was significantly higher in boreal (mean 0.92%) than in arctic sub-mid (mean 0.43%) and high altitude (mean 0.34%) lakes (Fig. 2f; Supplementary Table S2). Hence, crustacean zooplankton communities were overall richer in N and P in boreal compared to arctic lakes.
a Carbon (C), b nitrogen (N) and c phosphorus (P) content (% of DW) in cyclopoid and calanoid copepods, cladocerans, and for the whole zooplankton community in d for (C), in e for (N), and in f for (P) in five boreal (B), five arctic sub-mid altitude (ASM) and four arctic high altitude (AH) lakes, respectively. The thin and the thick line within the box show median and mean, respectively, the box 25 and 75% percentiles and the whisker 10 and 90% percentiles. Different letters show significant differences. The data for the arctic lakes are from Bergström et al. (2015)
There were variations and significant differences in N: P ratios among taxa and among lake regions. For all lake regions, N: P ratios of calanoids were generally higher than for cladocerans and cyclopoids, and N: P ratios of all taxa in boreal lakes were generally lower compared to arctic lakes (Fig. 3a; Supplementary Table S2). Lowest N: P ratios could be seen in cyclopoids (median 19) in boreal lakes. As a result of these differences the N: P ratios were significantly lower in zooplankton (based on mean weighted biomass) in boreal (median 28) than in arctic sub-mid (median 45) and high altitude (median 52) lakes (Fig. 3d; Supplementary Table S2).
a The N: P ratios, the predicted (b) N: P imbalances, and c consumer driven N: P regeneration in cyclopoid and calanoid copepods, cladocerans, and the whole zooplankton community in d for N: P ratios, e for predicted N: P imbalances, and f predicted consumer driven N: P regeneration in five boreal (B), five arctic sub-mid altitude (ASM) and four arctic high altitude (AH) lakes, respectively. The thin and the thick line within the box show median and mean, respectively, the box 25 and 75% percentiles and the whisker 10 and 90% percentiles. Different letters show significant differences. Horizontal hatched lines in c and f shows N: P = 16 (Redfield ratio). The data for the arctic lakes are from Bergström et al. (2015)
There were also differences in N: P imbalances among taxa (Fig. 3b; Supplementary Table S2). The N: P imbalances of all taxa in boreal lakes were overall less negative than in arctic lakes. Highest predicted N: P imbalance (mean 10) could be seen in cyclopoids (Fig. 3b). Since seston N: P ratios did not differ among lake groups (cf. above), the predicted N: P imbalance in all zooplankton (based on mean weighted biomass) (Fig. 3e; Supplementary Table S2) became negative in the arctic lakes (medians: ASM − 22; AH − 30), but did not differ significantly from 0 in the boreal (median − 1) lakes (one-sample two tailed t test; P = 0.77).
The predicted consumer driven N: P regeneration ratio of all zooplankton (based on mean weighted biomass) was in most cases below the Redfield ratio (molar ratio of 16) in the arctic sub-mid and high altitude lakes (medians 11 and 13, respectively) whereas in boreal lakes ratios were higher (median 18) (Fig. 3f). The N: P regeneration for all zooplankton was significantly higher in boreal than in arctic sub-mid lakes, whereas the other lake groups did not differ from each other (Fig. 3f; Supplementary Table S2). There were differences among taxa and lake groups (Fig. 3c; Supplementary Table S2). Cyclopoids (boreal) had higher N: P regeneration ratio (median 35) than crustacean zooplankton in arctic sub-mid (medians 12 and 13 in calanoids and cladocerans, respectively) and calanoids in arctic high altitude lakes (median 13), but did not differ from the other taxa (Fig. 3c). When varying the AE (0.5, 0.75 and 0.9) in the stoichiometry models, the median consumer driven N:P regeneration of all zooplankton (Fig. 4) increased from 15 to 32 in boreal lakes, but declined from 17 to 7 (ASM) and 17 to 8 (AH) in the arctic lakes. Hence, irrespectively of varying the AE, the predicted N: P regeneration was always close to, or below, the Redfield ratio of 16 in the arctic lakes.
Median and mean predicted consumer driven N: P regeneration calculated with accumulation efficiencies (AE) of 0.5, 0.75 and 0.9 for zooplankton (based on weighted mean biomass) in the studied lakes. The thin and the thick line within the box show median and mean, respectively, the box 25 and 75% percentiles and the whisker 10 and 90% percentiles. Horizontal hatched line shows N: P = 16 (Redfield ratio). The data for the arctic lakes are from Bergström et al. (2015)
Discussion
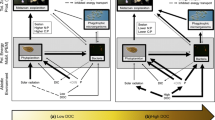
In line with our hypotheses, we found higher C, N and P content in lake water and in seston of boreal compared to arctic lakes. Despite low seston N: P ratios, and primarily N- (boreal) and N- to NP co-limited (arctic) phytoplankton (cf. Bergström et al. 2008, 2013), the estimated median N: P imbalance between seston and crustaceans zooplankton was negative (implying N-limited condition in zooplankton) only for arctic but not for boreal lakes. In boreal lakes the imbalance did not differ significantly from 0, implying that zooplankton were feeding on resources with N: P stoichiometry more or less matching the N: P requirements of the consumers promoting NP co-limitation in zooplankton; i.e. in contradiction to our second hypothesis. This in turn resulted in generally low consumer driven N: P regeneration ratios in all lakes but with somewhat higher ratios in boreal (median 18) compared to the arctic sub-mid (median 11) and high altitude (median 13) lakes. Hence, our results implies regional differences in plankton stoichiometry and herbivore N: P recycling which could not be attributed to differences in N: P ratios of the resources (seston) among lake regions.
We suggest that differences among lake regions largely is a function of climate-mediated differences in catchment properties. Climate differences with higher mean annual temperatures, weathering rates (Humborg et al. 2004) and terrestrial production (Jansson et al. 2008), thus, contribute to significantly higher terrestrial DOM (i.e. DOC) (Jansson et al. 2008; Hessen et al. 2009; Laudon et al. 2012) and N and P loadings to boreal than arctic lakes (cf. Supplementary Fig. S1 and Table S1, Karlsson et al. 2005). It is also evident from our results that these differences in catchment properties influenced the lake water N: P stoichiometry. Despite low N deposition, arctic high altitude lakes with poorly developed catchment vegetation cover and with low lake water TP concentrations, were the only lakes with high DIN: TN and DIN: TP ratios, and where short term bioassays experiments earlier have showed P-limitation in phytoplankton in mid-summer (Bergström et al. 2013). When more vegetated, the catchment efficiently retains inorganic N from deposition (Elser et al. 2009; Bergström 2010; Hessen 2013), but at the same time provides organic N and TP as a structural component, or bound to the terrestrial DOM. Boreal lakes were therefore overall much nutrient richer (DOC, TN, TP), with DIN concentrations comparable to the arctic high altitude lakes. As a result, for lakes situated in more organic rich catchments (arctic sub-mid and boreal), the DIN: TP ratios (and the DIN:TN proportions) of lake water were overall low, which in turn contributed to N- and NP co-limitation in phytoplankton (cf. Bergström 2010; Bergström et al. 2013).
The higher nutrient loadings were seemingly transferred into the pelagic food resource base as indicated by higher seston C, N and P concentrations in boreal than in arctic sub-mid and high altitude lakes. Despite differences in light:nutrient availability (cf. Sterner et al. 1997) among lake regions (cf. Supplementary Table S1) seston C: P, C: N did not differ among lake regions. Neither did the seston N: P ratios, which were low and in the same magnitude as reported from lakes with low N deposition and N-limited phytoplankton (Elser et al. 2010; Deininger et al. 2017). However, if seston composition greatly differed among lake regions this could in turn affect our interpretation of our results in the ecological stoichiometry models (cf. below) since zooplankton are known to preferentially feed upon high quality phytoplankton food (Hessen et al. 2003). We therefore estimated the proportions of phytoplankton and bacteria in seston C using literature data from the lake regions for bacterial biomass (Jansson et al. 2001, 2010) and by converting our Chl-a data to C using a conversion factor of 50 µg C/µg Chl-a (Ahlgren 1983). From these estimates we found that proportions of phytoplankton, bacterial and detritus C were similar among lake regions (31 ± 18%, 30 ± 5%, and 23 ± 10% (phytoplankton), 9 ± 3%, 13 ± 2%, and 8 ± 3% (bacteria) and 60 ± 19%, 57 ± 6% and 68 ± 12% (detritus) for high, sub-mid and boreal lakes, respectively). These proportions are very similar to the ones reported for oligotrophic Norwegian lakes (Hessen et al. 2003), i.e. with a relative large proportion of the seston pool being detritus of autochthonous and allochthonous origin.
Further, in the same study (Hessen et al. 2003) the elemental C: N: P contributions from phytoplankton and detritus in seston was modelled and evaluated. The authors found that the C: P ratios in seston, especially in oligotrophic lakes, greatly overestimated C: P in phytoplankton as caused by large contributions of allochthonous detritus C in seston. However, model-derived estimates for N: P ratios (molar) were nearly equal for phytoplankton (15.4) and allochthonous detritus (16) suggesting that large fractions of the detritus pool is derived from phytoplankton, implying a close link between living and dead cells, and that seston seems to carry the stoichiometric N: P footprint from phytoplankton. This highlights that caution should be made when using seston C: P ratios in ecological stoichiometry models when gauging food quality limitation, whereas seston N: P ratios seem more conservative and more reliable in indicating the N: P ratios in phytoplankton and the food resources (Hessen et al. 2003). Based on this, and that the phytoplankton community composition (primarily dominated by chrysophytes and cryptophytes) (cf. Jansson et al. 2010; Deininger et al. 2017) were similar, we cannot relate differences in zooplankton N and P content (cf. below) to any quality differences in seston composition among lake regions.
We therefore suggest that herbivore consumers seemed to have incorporated larger amounts of more nutrient rich diet in boreal lakes since median zooplankton somatic N and P, but not C, contents were higher in all zooplankton taxa in boreal than in arctic lakes. The zooplankton C, N and P contents were in the same range as those reported in other studies (Andersen and Hessen 1991; Sterner et al. 1992), with the exception of somewhat lower P content in cladocerans in the arctic lakes (cf. Bergström et al. 2015) and higher P content in cyclopoid copepods. The P content of cyclopoid copepods in our study (1.1% P per DW) was substantially higher than those reported (0.7 and 0.5% P per DW) by Hessen and Lyche (1991) and Ventura and Catalan (2005), respectively, but lower than reported (1.5% P per DW) by Bullejos et al. (2014). Hence, these results indicate that there is a substantial variation in C: N: P stoichiometry of zooplankton within taxonomic groups and among regions.
There are several potential explanations for this pattern. Differences in P content among zooplankton have been suggested to be linked to different specific growth rates, where higher growth rates require greater ribosomal RNA which increase the %P per DW (Elser et al. 2000; Vrede et al. 2004; Bullejos et al. 2014). Ingestion rates of filter feeding zooplankton, and, thus, acquisition of C and nutrients, is further negatively affected by lower temperature and increased water viscosity (Loiterton et al. 2004). Differences in nutrient content may also be linked to reproduction mode, where the P content in cladocerans and calanoids, unlike cyclopoids, seems to decline during reproduction (Ventura and Catalan 2005). Ontogeny (interstage variability) in zooplankton has also been shown to affect the elemental (C, N, P) content in calanoid copepods (Villar-Argaiz et al. 2002). Some zooplankton are also capable of lipid storage (Hiltunen et al. 2014; Mariash et al. 2017), taking place in late summer and autumn in order for zooplankton to sustain living and for actively overwintering under the ice (Mariash et al. 2017)—a strategy suggested to be of special importance and common in low productivity alpine and subarctic lakes (Larsson and Wathne 2006; Mariash et al. 2017). However, since our data represent mean seasonal values of primarily large individuals we do not consider ontogeny to have any large impact on our results. In addition, lipid accumulation, potentially affecting C: N: P stoichiometry in zooplankton, could also have taken place among the zooplankton communities in our boreal lakes which are situated far north with substantial ice cover and long winters.
The regional differences, with significantly higher N and P content among zooplankton taxa in boreal compared with arctic lakes, combined with higher proportions of cladocerans and cyclopoids in boreal lakes, contributed to significantly lower weighted zooplankton N: P ratios in boreal than in arctic sub-mid and high altitude lakes (cf. Fig. 3d). Since seston N: P ratios did not differ among lake regions, estimated nutrient imbalances increased from strongly negative values (median − 25 and − 30 for arctic sub-mid and high altitude lakes, respectively) up to levels close to stoichiometric balance (median − 1; boreal). As shown earlier, these results indicate that zooplankton in arctic lakes were feeding on resources (seston) with suboptimal N content (cf. Bergström et al. 2015), whereas in the boreal lakes the N: P stoichiometry of the resources equaled and matched the N: P requirements of zooplankton. The results therefore suggest that in arctic lakes low terrestrial exports of nutrients, and low seston C, N and P concentrations of low N: P ratios, resulted in primarily N-limited zooplankton (cf. Bergström et al. 2015). In boreal lakes, the higher terrestrial nutrient loadings contributed to higher seston amounts with higher N and P content of low N: P ratios, where zooplankton seemingly incorporated more nutrients (became N and P richer), which resulted in co-limitation by N and P in zooplankton (cf. Figs. 2, 3).
The differences in plankton stoichiometry between the arctic and boreal lakes affected the estimated consumer driven N: P regeneration (at AE = 0.75). This was below the Redfield ratio (N: P molar ratio of 16) in the arctic (median 11 and 13 for ASM and AH lakes, respectively) (cf. Bergström et al. 2015), and slightly above this ratio in boreal lakes (median 18). Although the Redfield ratio might not be optimal to use as an indicator of N- and P-limitation (N-limitation < 16 < P-limitation) in phytoplankton, since the ratio might change and vary depending on growth conditions and growth rates (Sterner and Elser 2002; Hillebrand et al. 2013) and phylogenetic groups of phytoplankton (Peltomaa et al. 2017), as earlier suggested in Bergström et al. (2015) the low consumer driven N: P regeneration estimated for the arctic lakes should underpin and sustain N-limitation of phytoplankton in these lakes. In boreal lakes, the slightly higher consumer driven N: P regeneration might, especially during situations of low flow condition in mid-summer when external nutrient loadings to lakes are low (cf. Bergström et al. 2015), to some extent affect nutrient limitation patterns in phytoplankton moving them from primarily N-(Bergström et al. 2008) towards more NP co-limited conditions.
Interestingly, the consumer driven N: P regeneration in boreal lakes was only slightly higher than the ones in arctic lakes, despite the former having higher proportions of P-rich cladocerans and cyclopoids. The cladocerans community N: P ratio depends on the species composition, where for example Bosmina have lower somatic P content than Daphnia (Urabe and Watanabe 1992), and low biomass numbers and small size distributions in zooplankton may further contribute to lower consumer driven N: P regeneration (Elser et al. 1988). Hence, low but significant different consumer driven N: P regeneration ratios among regions can partly be explained by a dominance of calanoid copepods in the arctic lakes (high somatic N: P), and higher proportion of small sized cladocerans taxa and cyclopoid copepods (with low somatic N: P) combined with low biomass in the boreal lakes.
To what extent the community composition influenced the consumer driven N: P regeneration became especially apparent when varying the AE (cf. Fig. 4). As illustrated earlier in Bergström et al. (2015), for the arctic sub-mid and high altitude lakes dominated by calanoid copepods (high somatic N: P) the influence was low, i.e. the N: P regeneration was always close to, or lower than 16, irrespectively of AE, with a declining trend with increased AE. For boreal lakes, however, higher proportions of cladocerans and cyclopoids (low somatic N: P ratios) promoted an opposite trend where the N: P regeneration increased from values below, to slightly above 16 (AE 0.5 and 0.75), to values well > 16 (AE of 0.9) (cf. Fig. 4). Thus, although community differences were apparent among regions, only at the very highest AE used in the model a high N: P regeneration was estimated for the boreal lakes.
The consumer driven N: P regeneration ratio estimates in our study (medians: 18, 11 and 13; AE = 0.75) are on average 5–10 times lower than the estimates made for northern Wisconsin and Michigan lakes (US) (90–100), the Experimental Lake Area lakes (Ontario, Canada) (125) (cf. Hassett et al. 1997; Dobberfuhl and Elser 2000), but similar to those of estuarine (25) and ocean (18) sites (cf. Hassett et al. 1997). The previously documented general differences in elemental stoichiometry between marine and freshwater sites, in which imbalances are positive (P-limited conditions) with high predicted consumer driven N: P regeneration in freshwaters, and negative imbalance (N-limited conditions) with low consumer driven N: P regeneration in marine systems (cf. Elser and Hassett 1994), can therefore be questioned as being a general rule. In addition to catchment properties and climate differences affecting lake nutrient (TN, TP) concentrations and plankton stoichiometry in lakes with low N deposition (this study), lake TN concentrations and seston N: P ratios can also be elevated by N deposition (Bergström et al. 2005; Elser et al. 2009, 2010), thereby promoting an increase in consumer driven N:P regeneration (cf. Bergström et al. 2015). Hence, in the absence of N deposition, lake and ocean N: P stoichiometry seems very similar (cf. this study and Hassett et al. 1997).
Global warming is affecting terrestrial production and export of DOM and nutrients to lakes (Solomon et al. 2015). Our results indicate that these climate driven changes on catchment scales can have consequences for plankton stoichiometry and, hence, ecosystem function in lakes with low N deposition. Our results illustrate that climate mediated differences in catchment properties are unlikely to affect nutrient limitation in phytoplankton qualitatively, which will remain primarily N- and NP co-limited (cf. Bergström et al. 2008, 2013) with low N: P ratios in seston, but that terrestrial nutrient loadings and seston C, N, and P concentrations will increase. Enhanced nutrient and plankton diet availability will promote higher uptake and incorporation of limiting nutrients (especially N but also P) in herbivore zooplankton, thereby lowering their body N: P ratio, raising consumer N: P regeneration and changing zooplankton from being mainly N- (arctic) to NP co-limited (boreal). This change can promote feedbacks on e.g. nutrient limitation in phytoplankton, but also increase zooplankton growth and reproduction since their nutrient requirements [both quantitatively and qualitatively (based on elemental N:P stoichiometry)] are better fulfilled in boreal compared to arctic lakes, which in turn should enhance the trophic transfer of energy and nutrients in pelagic food webs.
References
Ahlgren G (1983) Comparison of methods for estimation of phytoplankton carbon. Arch Hydrobiol 98:489–508
Andersen T (1997) Herbivores as sources and sinks for nutrients. Ecological Series 129. Springer, Berlin
Andersen T, Hessen DO (1991) Carbon, nitrogen, and phosphorus content of freshwater zooplankton. Limnol Oceanogr 36:807–814
Bergström A-K (2010) The use of TN: TP and DIN: TP ratios as indicators for phytoplankton nutrient limitation in oligotrophic lakes affected by N deposition. Aquat Sci 72:277–281
Bergström A-K, Jansson M (2006) Atmospheric nitrogen deposition has caused nitrogen enrichment and eutrophication of lakes in the northern hemisphere. Glob Change Biol 12:635–643
Bergström A-K, Blomqvist P, Jansson M (2005) Effects of nitrogen deposition on nutrient limitation and phytoplankton biomass in unproductive Swedish lakes. Limnol Oceanogr 50:987–994
Bergström A-K, Jonsson A, Jansson M (2008) Phytoplankton responses to nitrogen and phosphorus enrichment in unproductive Swedish lakes along a gradient of atmospheric nitrogen deposition. Aquat Biol 5:55–64
Bergström A-K, Faithfull C, Karlsson D, Karlsson J (2013) Nitrogen deposition and warming—effects on phytoplankton nutrient limitation in subarctic lakes. Glob Change Biol 19:2557–2568
Bergström A-K, Karlsson D, Karlsson J, Vrede T (2015) N-limited consumer growth and low nutrient regeneration in lakes with low N deposition. Ecosphere 6(1):Article 9
Bottrell HH, Duncan A, Gliwicz ZM et al (1976) Review of some problems in zooplankton production studies. Nor J Zool 24:419–456
Bullejos FJ, Carillo P, Gorokhova E, Medina-Sanchez JM, Villar-Argaiz M (2014) Nucleic acid content in crustacean zooplankton: bridging metabolic and stoichiometric predictions. Plos One 9(1):e86493
Burns CW (1968) The relationship between body size of filter feeding Cladocera and the maximum size of particle ingested. Limnol Oceanogr 13:675–678
de Wit HA, Valinia S, Weyhenmeyer GA et al (2016) Surface waters will be further promoted by wetter climate. Environ Sci Technol 3:430–435
Deininger A, Faithfull CL, Bergström A-K (2017) Phytoplankton response to whole lake inorganic N fertilization along a gradient in dissolved organic carbon. Ecology 98:982–994
DeMott WR (1998) Utilization of a cyanobacterium and a phosphorus-deficient green alga as complementary resources by daphnids. Ecology 79:2463–2481
DeMott WR, Gulati RD, Siewertsen K (1998) Effects of phosphorus-deficient diets on the carbon and phosphorus balance of Daphnia magna. Limnol Oceanogr 43:1147–1161
Dobberfuhl DR, Elser JJ (2000) Elemental stoichiometry of lower food web components in arctic and temperate lakes. J Plankton Res 22:1341–1354
Elser JJ, Hassett RP (1994) A stoichiometric analysis of the zooplankton-phytoplankton interaction in marine and freshwater ecosystems. Nature 370:211–213
Elser JJ, Elser MM, MacKay NA, Carpenter SR (1988) Zooplankton-mediated transitions between N- and P-limited algal growth. Limnol Oceanogr 33:1–14
Elser JJ, O´Brien WJ, Dobberfuhl DR, Dowling TE (2000) The evolution of ecosystem processes, growth rate and elemental stoichiometry of key herbivore in temperate and arctic habitats. J Evol Biol 13:845–853
Elser JJ, Andersen T, Baron JS et al (2009) Shifts in lake N.P stoichiometry and nutrient limitation driven by atmospheric nitrogen deposition. Science 326:835–837
Elser JJ, Peace AL, Kyle M et al (2010) Atmospheric nitrogen deposition is associated with elevated phosphorus limitation of lake zooplankton. Ecol Lett 13:1256–1261
Finstad AG, Andersen T, Larsen S et al (2016) From greening to browning: Catchment vegetation development and reduced S-deposition promote organic carbon load on decadal time scales in Nordic lakes. Sci Rep 6:31944. https://doi.org/10.1038/srep31944
Hassett RP, Cardinale B, Stabler LB, Elser JJ (1997) Ecological stoichiometry of N and P in pelagic ecosystems: comparison of lakes and oceans with emphasis on the zooplankton-phytoplankton interaction. Limnol Oceanogr 42:648–662
Hessen DO (2013) Inorganic nitrogen deposition and its impacts on N:P ratios and lake productivity. Water 5:327–341
Hessen DO, Anderson TR (2008) Excess carbon in aquatic organisms and ecosystems: physiological, ecological, and evolutionary implications. Limnol Oceanogr 53:1685–1696
Hessen DO, Lyche A (1991) Interspecific and intraspecific variations in zooplankton element composition. Arch Hydrobiol 121:343–353
Hessen DO, Andersen T, Brettum P, Faafeng BA (2003) Phytoplankton contribution to sestonic mass and elemental ratio in lakes: Implications for zooplankton nutrition. Limnol Oceanogr 48:1289–1296
Hessen DO, Andersen T, Larsen S, Skjelkvåle BL, de Wit HA (2009) Nitrogen deposition, catchment productivity, and climate as determinants of lake stoichiometry. Limnol Oceanogr 54:2520–2528
Hessen DO, Elser JJ, Sterner RW, Urabe J (2013) Ecological stoichiometry: an elementary approach using basic principles. Limnol Oceanogr 58:2219–2236
Hillebrand H, Steinert G, Boersma M, Malzahn A, Meunier CL, Plum C, Ptacnik R (2013) Goldman revisited: faster growing phytoplankton has lower N.P and lower stoichiometric flexibility. Limnol Oceanogr 58:2076–2088
Hiltunen M, Strandberg U, Keinänen M, Taipale S, Kankaala P (2014) Distinctive lipid composition of the copepod Limnocalanus macrurus with a high abundance of polyunsaturated fatty acids. Lipids 49:919–932
Humborg C, Smedberg E, Blomqvist S, Mörth C-L, Brink J, Rahm L, Danielsson Å, Sahlberg J (2004) Nutrient variations in boreal and subarctic Swedish rivers: landscape control of land-sea fluxes. Limnol Oceanogr 49:1871–1883
Jansson M, Bergström A-K, Drakare S, Blomqvist P (2001) Nutrient limitation of bacterioplankton and phytoplankton in humic lakes in northern Sweden. Freshw Biol 46:653–666
Jansson M, Hickler T, Jonsson A, Karlsson J (2008) Links between terrestrial primary production and bacterial production and respiration in lakes in a climate gradient in subarctic Sweden. Ecosystems 11:367–376
Jansson M, Jonsson A, Andersson A, Karlsson J (2010) Biomass and structure of planktonic communities along an air temperature gradient in subarctic Sweden. Freshw Biol 55:691–700
Jones RI (1992) The influence of humic substances of lacustrine food-chains. Hydrobiologia 229:73–91
Karlsson J, Jonsson A, Jansson M (2005) Productivity of high-altitude lakes: climate effect inferred from altitude gradient. Glob Change Biol 11:710–715
Karlsson J, Byström P, Ask J, Ask P, Persson L, Jansson M (2009) Light limitation of nutrient-poor lake ecosystems. Nature 460:506–509
Laaksonen K (1976) The dependence of mean air temperature upon latitude and altitude in Fennoscandia (1921–1950). Ann Acad Sci Fenn 19(Series A):5–19
Larsson P, Wathne I (2006) Swim or rest during the winter – what is best for alpine daphnid? Arch Hydrobiol 167:265–280
Laudon H, Buttle J, Carey SK, McDonnell J, McGuire K, Seibert J, Shanley J, Soulsby C, Tetzlaff D (2012) Cross-regional prediction of long-term trajectory of stream water DOC response to climate change. Geophys Res Lett 39:L18404
Lewis WM (2011) Global primary production of lakes: 19th Baldi Memorial Lecture. Inland Waters 1:1–28
Loiterton B, Sundbom M, Vrede T (2004) Separating physical and physiological effects of temperature on zooplankton feeding rate. Aquat Sci 66:123–129
Mariash HL, Cusson M, Rautio M (2017) Fall composition of storage lipids is associated with the overwintering strategy of Daphnia. Lipids 52:83–91
Monteith DT, Stoddard JL, Evans CD et al (2007) Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature 450: 537–540
Peltomaa EL, Aalto SL, Vuorio KM, Taipale SJ (2017) The importance of phytoplankton biomolecule availability for secondary production. Front Ecol Evol 5:128. https://doi.org/10.3389/fevo.2017.00128
Seekell DA, Lapierre JF, Ask J, Bergström A-K, Deininger A, Rodriques P, Karlsson J (2015) The influence of dissolved organic carbon in primary production in nortehrn lakes lakes. Limnol Oceanogr 60:1276–1285
Solomon CT, Jones SE, Weidel BC et al (2015) Ecosystem consequences of changing inputs of terrestrial dissolved organic matter to lakes: current knowledge and future challenges. Ecosystems 18:376–389
Sterner RW (1990) The ratio of nitrogen to phosphorus resupplied by herbivores: zooplankton and the algal competitive arena. Am Nat 136::209–229
Sterner RW, Elser JJ (2002) Ecological stoichiometry. Princeton University Press, Princeton
Sterner RW, Hessen DO (1994) Algal nutrient limitation and the nutrition of aquatic herbivores. Ann Rev Ecol Syst 25:1–29
Sterner RW, Elser JJ, Hessen DO (1992) Stoichiometric relationships among producers, consumers and nutrient recycling in pelagic systems. Biogeochemistry 17:49–67
Sterner RW, Elser JJ, Fee EJ, Guildford SJ, Chrzanowski TH (1997) The light:nutrient ratio in lakes: the balance of energy and materials affects ecosystem structure and process. Am Nat 150:663–684
Urabe J, Sterner RW (1996) Regulation of herbivore growth by the balance of light and nutrients. PNAS 93:8465–8469
Urabe J, Watanabe Y (1992) Possibility of N and P limitation for planktonic cladocerans: an experimental test. Limnol Oceanogr 37:244–251
Ventura M, Catalan J (2005) Reproduction as one of the main causes of temporal variability in the elemental composition of zooplankton. Limnol Oceanogr 50:2043–2056
Villar-Argaiz M, Medina-Sanchez JM, Carrillo P (2002) Linking life history strategies and ontogeny in crustacean zooplankton: implications for homeostasis. Ecology 83:1899–1914
Vrede T, Dobberfuhl DR, Koojiman SALM, Elser JJ (2004) Fundamental connections among organism C:N:P stoichiometry, macromolecular composition, and growth. Ecology 85:1217–1229
Weyhenmeyer GA, Karlsson J (2009) Nonlinear response of dissolved organic carbon concentrations in boreal lakes to increasing temperature. Limnol Oceanogr 54:2513–2519
Acknowledgements
We thank Jan Johansson, Anders Jonsson and Erik Geibrink for conducting the analyses. Thanks also to Erik Geibrink, Anne Deininger, Jakob Hansson, Marcus Klaus and Sonja Prideuax for field assistance. The study was funded by grants from Oscar and Lili Lamm’s Foundation and the Swedish research councils VR (dnr: 621-2010-4675) and Formas (dnr: 215-2010-992).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Bergström, AK., Karlsson, J., Karlsson, D. et al. Contrasting plankton stoichiometry and nutrient regeneration in northern arctic and boreal lakes. Aquat Sci 80, 24 (2018). https://doi.org/10.1007/s00027-018-0575-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-018-0575-2