Abstract
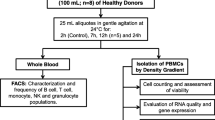
Lymphocyte subset estimations by flow cytometry in population-based studies require transportation of samples from the field site to the laboratory. As samples arrive late in the day they have to wait overnight before being processed. The effect of two possible approaches, sample storage for 24 h before staining and immediate staining with analysis after 24 h and 48 h were evaluated. Two sets of experiments were performed with EDTA (ethylenediamine tetra-acetate) anticoagulated peripheral blood. In the first experiment, after collection, each sample was divided into two portions. One portion was stained at the time of blood collection and the other 24 h later after keeping it at room temperature (38–45°C). In the second experiment, blood samples were stained within 1–2 h. Each sample was analyzed immediately upon completion of staining process and subsequently after 24 h and 48 h of storage at 4°C. Results suggest that blood collected in EDTA can be processed using whole blood lysis method, after storage at room temperature (38–45°C) for 24 h with some but not significant alteration in T-cell subsets. Storage at 4°C after staining for 24 h results in a lesser and insignificant loss of cells or alteration of T-cell subsets and may be the method of choice.
Similar content being viewed by others
References
Ekong, T., Hill, A.M., Gomples, M., Brown, A. and Pinching, A.J. (1992) The effect of the temperature and duration of sample storage on the measurement of lymphocyte subpopulations from HIV-I-Positive and control subjects. J. Immunol. Methods 151: 217–225.
Thornthwaite, J.T., Rosenthal, P.K., Vasquez, D.A. and Seckinger, D. (1984) The effects of anticoagulant and temperature on the measurement of helper and supresser cells. Diagn. Immunol. 2: 167–174.
Reinherz, E.L., Kung, P.Z., Goldstein, G. and Schlossman, S.F. (1979) A monoclonal antibody with selective reactivity with functionally mature human thymocytes and all peripheral human T cells. J. Immunol. 123: 1312–1317.
Thomas, Y., Sosman, J., Irigoyen, O., Friedman, S.M., Kung, P.C., Goldstein, G. and Chess, L. (1980) Expression of cell surface markers after human B lymphocyte activation. Immunol. 125: 2402–2408.
Stashenko P., Nadler, L.M., Hardy, R. and Schlossman, S.F. (1981) Expression of cell surface markers after human B lymphocyte activation. Proc. Natl. Acd. Sci. 78: 3848–3852.
Liu, C., Muirhead, K.A., George, S.P. and Landay, A.L. (1989) Flow cytometric monitoring of HIV-infected patients: Simultaneous enumeration of five lymphocyte subsets. Am. J. Clin. Pathol. 92: 721–728.
Prince, H. and Jensen, E. (1991) Three colour cytofluorometric analysis of Cd8 cell substes in HIV-1 infection. J. Acquir. Immuno. Defic. Synd. 4: 1227–1232.
Ekong, T., Hill, A.M., Clark, C., Alun, D. and Pinching, A.J.P. (1993) Technical influences on immunophenotyping by flow cytometry the effect of time and temperature of storage on the viability of lymphocyte subsets. J. Immunol. Methods. 164: 263–273.
Shield, C.F., Martlett, P. and Smith, A. (1983) Stability of human lymphocyte differentiation antigens when stored at room temperature. J. Immunol. Methods. 62: 347.
Bongers, V. and Bertrams, J. (1984) The influence of common variables on T cell subset analysis by monoclonal antibodies. J. Immunol. Methods. 67 (2): 243–253.
Parker, J.W., Adelsberg, B., Azen, S.P., Boone, D., Fletcher, M.A., Gjerset, G.F.,et al. (1990) Leucocyte immunophenotyping by flowcytometry in a multisite study: standardisation, quality control and normal values in the transfusion safety study. Clin. Immunal. Immunopathol. 55: 187–220.
Nicholson, J.K.A., Jones, B.M. and Cross, D. (1984) Comparison of T and B cell analysis on fresh and aged blood. J. Immunol. Methods. 73: 29–40.
Hensleigh, P.A., Waters, V.B. and Herzenberg, L.A. (1993) Human T lymphocyte differentiation antigens. Effects of blood sample storage of Leu antibody binding. Cytometry. 3: 453–455.
Miller, C.H. and Levy, N.B. (1989) Effects of storage conditions on lymphocyte phenotypes from healthy and diseased persons. J. Clin. Lab. Anal. 3: 296–300.
Nicholson, J.K.A. and Green, T.A. (1993) Selection of anticoagulants for lymphocyte immunophenotyping. J. Immunol. Methods. 165: 31–35.
Paxton, H. and Bendele, T. (1993) Effect of Time, Temperature and Anticoagulant on Flow Cytometry and Hematological values. Annals of New York Academy of Sciences 677: 440–443.
Lloyd, J.B., Gill, H.S. and Husband, A.J. (1995) The effect of storage on immunophenotyping of sheep peripheral blood lymphocytes by flow cytometry. Vet. Immunol. Immunopathol. 47: 135–142.
Ruiz, A.R., Fernandez, I.P., Perea Baena, J.M., de La Torre, F.J. and Ramirez, G.R. (2000) Influence of sample storage time and temperature on lymphocyte subset count using a FACS count system. Haematologica. 85: 550–551.
Jalla, S., Sazawal, S., Deb, S., Black, R.E. and Bhan, M.K. (2002) Modifications in flow cytometric estimation of T cells subsets and B cells in peripheral blood to reduce the cost of investigation. Indian Journal of Clinical Biochemistry. 17 (1): 69–74.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jalla, S., Sazawal, S., Deb, S. et al. Enumeration of lymphocyte subsets using flow cytometry: Effect of storage before and after staining in a developing country setting. Indian J Clin Biochem 19, 95–99 (2004). https://doi.org/10.1007/BF02894264
Issue Date:
DOI: https://doi.org/10.1007/BF02894264